Abstract
Irinotecan has radiosensitizing effects and shows synergism with nitrosoureas. We performed a Phase II study of RT and irinotecan, followed by BCNU plus irinotecan in newly-diagnosed GBM. The MTD for patients receiving enzyme-inducing anticonvulsants (EIAC) was as follows: irinotecan 400 mg/m2/week on Days 1, 8, 22 and 29 during RT, followed by BCNU 100 mg/m2 Day 1, and irinotecan, 400 mg/m2 on Days 1, 8, 22 and 29, every 6 weeks. The MTD for non-EIAC patients was as follows: irinotecan 125 mg/m2/week on Days 1, 8, 22 and 29 during RT, followed by BCNU 100 mg/m2 Day 1 and irinotecan 75 mg/m2 Days 1, 8, 22 and 29, every 6 weeks. Median OS was 10.8 mos. (95% CI: 7.7–14.9); OS at 12 months was 44.6% (95% CI: 33.3–59.8) and PFS 6 was 28.6% (95% CI: 18.9–43.2). Patients went off treatment due to adverse events (7%), refusal (11%), progressive disease (48%), death (9%), and other (9%); 16% completed protocol treatment. Survival was similar in patients with variant (6/7 or 7/7) and wild-type (6/6) UGT1A1*28 genotypic alleles. Grade 3–4 toxicity was more common in non-EIAC patients with variant alleles. SN-38 Cmax and AUC in EIAC patients receiving 400 mg/m2 irinotecan were 20.9 ng/ml and 212 ng/ml h, and in non-EIAC patients receiving 125 mg/m2, 15.5 ng/ml and 207 ng/ml h. SN-38 AUC varied by UGT1A1*28 status in non-EIAC patients. This regimen was not significantly active and radiosensitization was not observed. Non-EIAC patients with UGT1A1*28 variant alleles appear particularly sensitive to toxicity from irinotecan.
Keywords: Glioblastoma, BCNU, Nitrosourea, Irinotecan, NCCTG, UGT1A1, Enzyme-inducing anticonvulsant
Introduction
In preclinical models, irinotecan has radiosensitizing effects, and produces additive cytotoxicity when combined with nitrosoureas [1–5]. This Phase II study (NCCTG N997D) evaluated the safety and efficacy of radiotherapy (RT) and concomitant irinotecan, followed by adjuvant BCNU plus irinotecan in patients with newly diagnosed glioblastoma (GBM). The primary goals were to determine the maximum tolerated dose in patients receiving and not receiving enzyme-inducing anticonvulsants (EIAC), and to evaluate the efficacy of the regimen utilizing a primary endpoint of overall survival at 12 months (OS12).
Methods
An initial pilot study was performed order to determine the MTD for patients not receiving (Arm A) and receiving (Arm B) enzyme-inducing anticonvulsants (EIAC). The Phase II study (Arm C) included all patients (EIAC and non-EIAC) treated at the MTD. All patients signed an informed, written consent to participate in the study, and the protocol was reviewed and approved by the Institutional Review Boards of each participating institution.
Radiotherapy (5000 cGy) was administered in 200 cGy/day fractions to the initial fields, consisting of the area of localized contrast enhancement or mass and surrounding edema as estimated from the postoperative MRI or CT scan plus a 2 cm margin. A final boost of 1000 cGy, administered in 200 cGy/day fractions, was then administered to the area of contrast enhancement plus a 2 cm margin. Radiotherapy was administered in 5 fractions per week for 6 weeks.
Eligibility criteria included a histologic diagnosis of newly diagnosed glioblastoma (WHO Grade 4 astrocytoma) confirmed by pre-registration central review by an NCCTG neuropathologist; recovery from effects of surgery; age ≥ 18; ECOG performance status 0–2; absolute neutrophil count ≥1500/mm3, platelet count ≥130000/mm3, serum creatinine ≤0.5 mg/dl above the upper limit of normal (ULN), total bilirubin ≤1.5 times the ULN; SGOT (AST) ≤2 times ULN. Patients were ineligible if they were or had received prior RT or chemotherapy; pregnant or nursing; unwilling to use adequate contraception if of childbearing potential; Grade 4 oligodendroglioma or mixed oligoastrocytoma; uncontrolled infection; co-existent malignant disease (other than superficial skin cancers); or significant other medical illnesses.
Dose limiting toxicity (DLT) was defined as the inability to deliver at least three of four planned doses of irinotecan during RT due to drug-related grade ≥4 diarrhea or myelosuppression, or severe neurological deterioration not responsive to corticosteroids. Dose de-escalations (but not escalations) were allowed. Toxicity was graded by the NCI Clinical Toxicity Criteria (CTC version 2.0).
The primary endpoint for the pilot study was to determine the maximum tolerated doses (MTD) for non-EIAC and EIAC patients. The primary endpoint for the Phase II study was overall survival at 12 months (OS12). The Phase II study was a one-stage design, and included two interim efficacy analyses after 35 and 60 eligible patients were followed for one year. The a priori decision rule required that 18 patients met criteria for success, which was defined as being alive one year after the start of therapy. The planned target accrual for the Phase II trial was 84 eligible patients. The a priori decision rules used for the interim and final analyses were based on a 3-stage Fleming version of Simon’s Optimum 2-Stage design for testing the null hypothesis. The survival experience was compared by log-rank test with that previously observed utilizing a set of patients pooled from five previous NCCTG trials involving newly-diagnosed GBM (79–72–51, 85–72–51, 88–72–52, 93– 72–52, and 98–72–52). The proportion of patients alive at 12 months from this historical database was 0.50. The sample size was determined to achieve an overall one-sided significance level of 0.10 with power of 0.90 for detecting a proportion of patients alive at 12 months of 0.65 or greater. The overall time-to-event distributions (survival and progression-free survival) were estimated using the method of Kaplan–Meier [6]. Overall survival (OS) was defined as time from start of study therapy to death from any cause. Progression-free survival (PFS) was defined as time from start of study therapy to documentation of disease progression. Patients who died without documented progression were considered as having progressed at time of death. Patients who had not died or progressed at the time of analysis were censored at the date of last follow-up.
Response was defined by NCCTG criteria as previously described [7]. Response had to be sustained on two consecutive MRI or CT scans at least 4 weeks apart. Patients progressing on two consecutive neurological exams ≥4 weeks apart were considered as having progressed, regardless of scan findings.
UGT1A1*28 genotyping (6/6, wild type; 6/7, heterozygote; 7/7, dual variant allele) was performed on tumor tissues obtained at initial surgery. Statistical comparisons were made between genotype (6/6, 6/7, 7/7) and outcome categories of response (complete or partial response, regression, stable, progression, unknown); survival (OS12, PFS6); time to event (survival and progression); and drug tolerability (reasons for off treatment; number of cycles; frequency of Grade 3+ adverse events). Two analyses were performed, one comparing all three genotypes, and one comparing wild type (6/6) versus mutated (6/7 + 7/7) genotypes.
For the pharmacokinetic analyses, blood samples were obtained prior to and at the end of initial infusion, and at 1, 2, 4 and 24 h following the end of infusion, placed on ice, and plasma was separated and stored at −70°C until assay. Plasma samples were assayed by high performance liquid chromatography as previously described [8]. Irinotecan, APC and unconjugated SN-38 concentrations were determined by direct analysis of plasma. Total SN-38 concentrations were determined after hydrolysis of SN-38G to SN-38 by incubation with β-glucuronidase and represent the sum of unconjugated SN-38 and conjugated SN-38. SN-38G concentrations estimated by the difference between total SN-38 concentration and the unconjugated SN-38 concentration.
Irinotecan, SN-38, SN-38G and APC plasma concentration data were analyzed by non-compartmental methods (WINNONLIN v4.1, Pharsight, Mountain View, CA). The apparent terminal elimination rate constants (λz) were determined by linear least-squares regression through the 4 and 24 h plasma-concentration time points. The apparent elimination half-life (t1/2) was calculated as 0.693/λz. Area under the plasma concentration–time curves (AUC0–24 h) was determined using the linear trapezoidal rule from time zero to the 24 h sample time. Area under the plasma concentration–time curves through infinite time (AUC0-∞) was calculated by adding CT/λz to AUC0–24 h. The clearance of irinotecan was calculated as dose/AUC0-∞, where dose is the administered dose of irinotecan expressed in free base equivalents.
Results
The baseline characteristics of the 56 treated patients (Arm A-20; Arm B-12; Arm C-24) are presented in Table 1. In the pilot study (Arms A and B), the first six non-EIAC patients (Arm A) tolerated irinotecan 125 mg/m2 on Days 1, 8, 22 and 29 during RT (dose level 0). However, five required hospitalization for drug-related adverse events (AE) during the post-RT adjuvant cycles of therapy (BCNU (100 mg/m2 IV over 2 h on Day 1, plus irinotecan 125 mg/m2 IV over 90 min Days 1, 8, 22 and 29, repeated every 6 weeks for up to 4 cycles). Consequently, the protocol was amended to reduce the adjuvant irinotecan dose to 75 mg/m2 (Days 1, 8, 22, and 29). This dose was tolerated and became the Phase II dose for non-EIAC patients. The MTD for patients receiving EIAC (Arm B) was RT + irinotecan (400 mg/m2 IV per week on Days 1, 8, 22 and 29), followed by adjuvant BCNU (100 mg/m2 IV every 6 weeks) + irinotecan (400 mg/m2 IV Days 1, 8, 22 and 29) every 6 weeks, for up to 4 cycles. All patients in the pilot studies were included in the Phase II cohort (Arm C) for the purposes of analysis, since all received drug at or above the established MTD (Table 2).
Table 1.
Baseline patient characteristics (N = 56)
| Baseline variable | Summary |
|---|---|
| Gender, n (%) | |
| Male | 36 (64%) |
| Female | 20 (36%) |
| Age, years | |
| Median (min, max) | 52 (21, 80) |
| Mean ± SD | 54.2 ± 14.7 |
| ECOG PS, n (%) | |
| 0 | 23 (41%) |
| 1 | 28 (50%) |
| 2 | 5 (9%) |
| Extent of surgery, n (%) | |
| Total resection | 18 (32%) |
| Subtotal resection | 25 (45%) |
| Biopsy only | 13 (23%) |
| Baseline steroid, n (%) | |
| Yes | 46 (82%) |
| No | 10 (18%) |
| Baseline EIAC, n (%) | |
| Yes | 14 (25%) |
| No | 42 (75%) |
| Patients per study, n | |
| Study 1: Phase I for non-EIACs | 20 (14 at Phase II dose, 6 above |
| Study 2: Phase I for EIACs | 12 (all at Phase II dose) |
| Study 3: Phase II | 24 |
Table 2.
Frequency of Grade ≥ 3 drug-related adverse events as a function of UGT1A1*28 genotype and EIAC status
| Patient group | UGT 1A1*28 genotype | P value | |
|---|---|---|---|
| 6/6 | 6/7 or 7/7 | ||
| Non-EIAC, no Grade 3+ AE | 5 | 0 | 0.011 |
| Non-EIAC, ≥1 Grade 3+ AE | 8 | 16 | |
| EIAC, no Grade 3+ AE | 0 | 3 | 1.0 |
| EIAC, at least one Grade 3+ AE | 3 | 10 | |
EIAC enzyme-inducing anticonvulsants; AE adverse events (CTC version 2.0)
Phase II study (Arm C)
Efficacy analysis
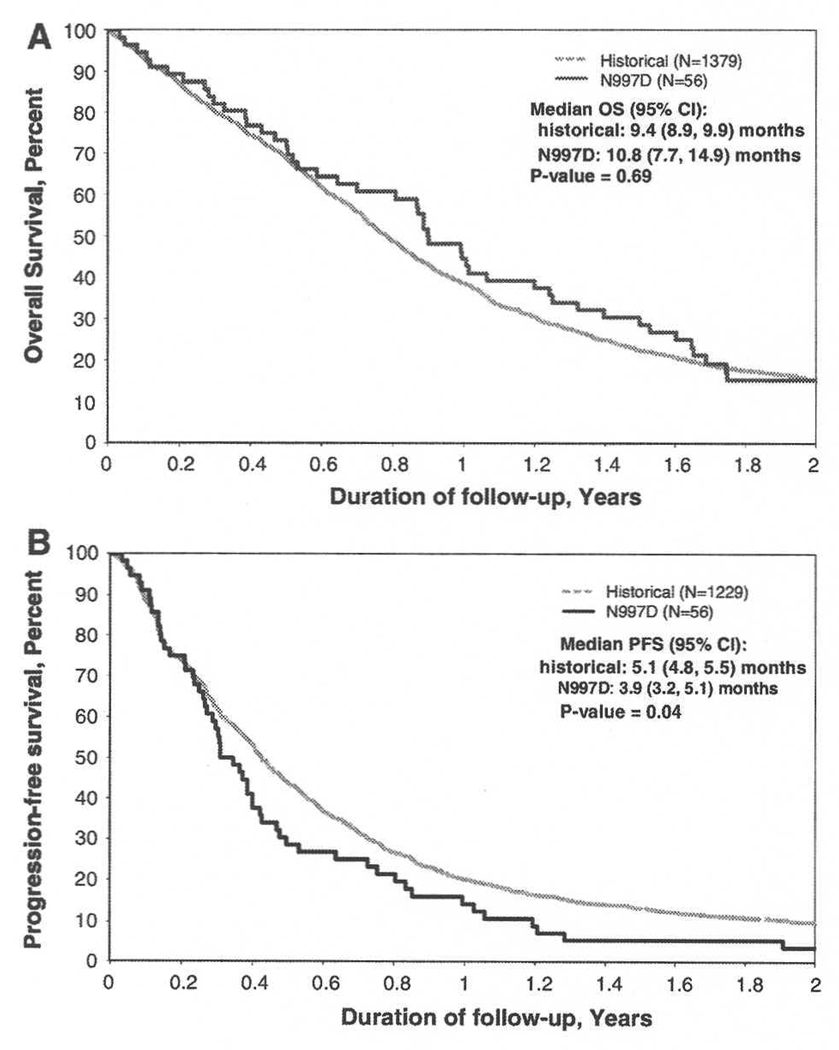
At the first interim analysis, the decision rule (18 successes) was not met (14 successes, 19 failures and 2 with incomplete data); thus, further accrual was stopped. In the 56 treated patients, the OS12 was 44.6% (95% CI: 33.3– 59.8). Median OS was 10.8 months (95% CI: 7.7–14.9) and was not superior to that observed in our historical database (Fig. 1a; P-value = 0.69). The PFS6 was 28.6% (95% CI: 18.9–43.2); PFS-survival rate at 12 months was 14.3% (95% CI: 7.5–27.1), and median PFS was 3.9 months (95% CI: 3.2–5.1). PFS was inferior to that observed in our historical database (Fig. 1b; P-value = 0.04). Responses in 38 patients with measurable post-op disease included 1 PR (3%), 7 with regression not meeting PR criteria, (18%) and 12 stable (32%). Response was centrally confirmed in 5/8 of those with PR or regression; scans were not available for review in the 3 others.
Fig. 1.
Outcome of GBM patients treated on N997D: comparison with the historical NCCTG database; a Overall survival. b Progression-free survival
Toxicity analysis
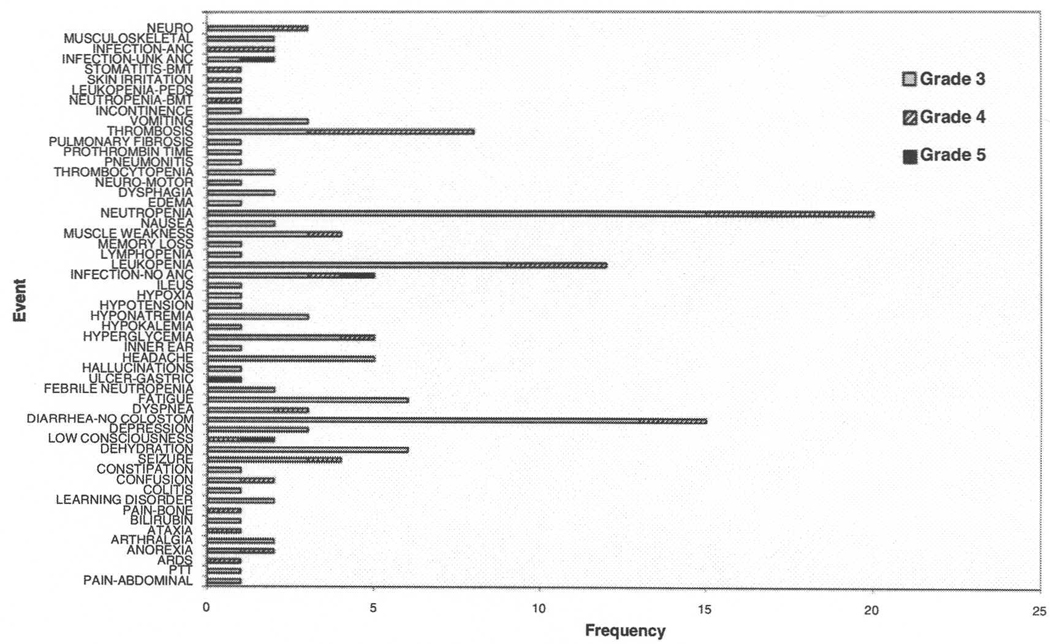
Nine patients (16%) completed all cycles of protocol therapy. Treatment was not completed in 27 patients (48%) with disease progression, 4 (7%) withdrawing due to adverse events, 6 (11%) who otherwise refused further treatment, 5(9%) who died on study, and 5 (9%) for other medical reasons felt unrelated to therapy. Fifty-five patients (98%) received at least one cycle of treatment (one cycle, 39%; 2 cycles, 20%; 3 cycles, 18%; 4 cycles 2%; and 5 cycles 3%; all cycles, 16%). Regardless of attribution, 44/56 patients (79%) had at least one Grade 3+ adverse event (Fig. 2). The most common Grade 3+ adverse events were neutropenia, leukopenia, fatigue, diarrhea, and dehydration. There was one Grade 5 event, a patient who died from complications of a gastric ulcer. Twenty-two of the 44 (50%) patients experienced the Grade 3 event prior to disease progression, and 17 (39%) were able to restart active treatment.
Fig. 2.
Treatment-related Grade 3+ adverse events, all cycles; ARDS, acute respiratory distress syndrome
Correlation of UGT1A1*28 status with outcome
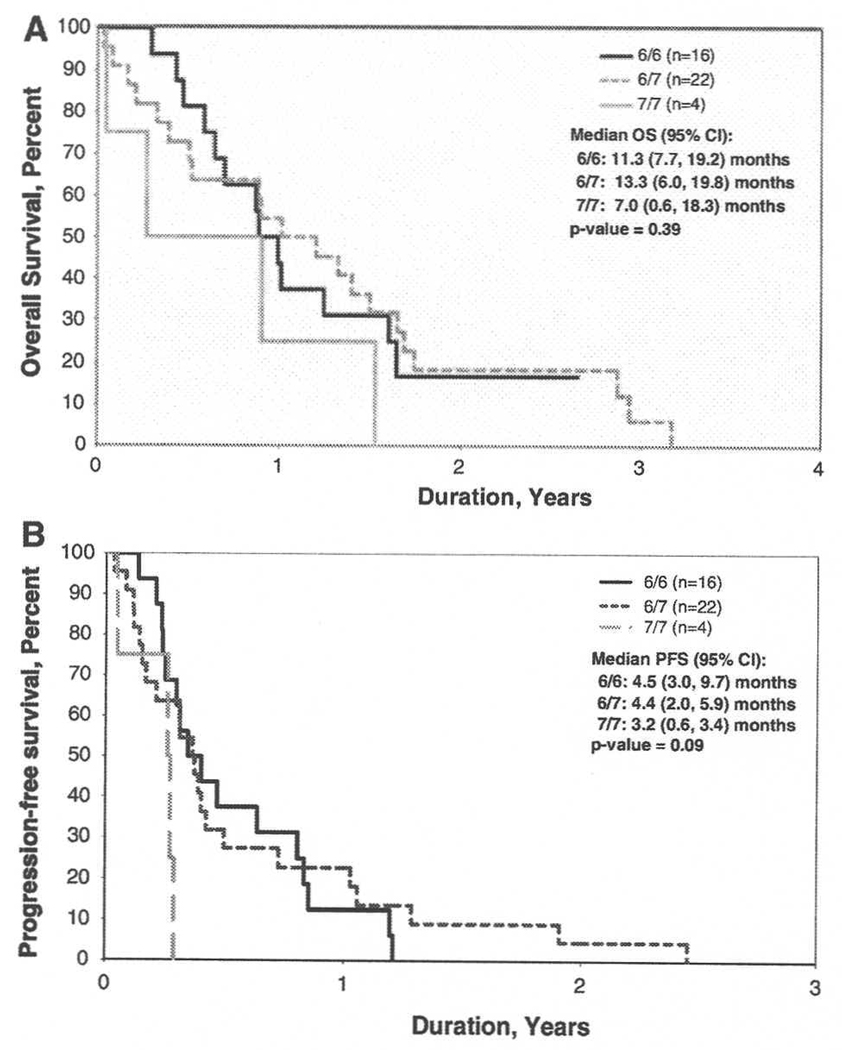
Specimens were available for UGT1A1*28 genotyping from 42 patients. Of these, both alleles were wild type (6/6) in 16 (38%) patients; one variant allele (6/7) was detected in 22 (52%), and both variant alleles (7/7) detected in 4 (10%). Two analyses were performed, first comparing all three groups, and second, comparing the group of wild-type-allele patients with the group of patients with either one or both variant alleles. There were no significant differences in age, gender, ECOG performance status (0, 1, 2), EIAC use, steroid use, or extent of resection between 6/6, 7/7 or 7/7 patients. No significant differences were observed in best response, OS12, PFS6, progression status, or vital status as a function of genotype (Fig. 3). Overall, no differences in drug tolerability were observed as a function of genotype. However, in the subgroup of non-EIAC patients with 6/7 or 7/7 genotypes, Grade 3+ adverse events were more frequent, as compared to the wild type 6/6 patients (P = 0.011). Also, the time to development of the first Grade 3+ event, regardless of attribution, varied as a function of UGT1A1*28 genotype (6/6 vs. 6/7 vs. 7/7, P = 0.005); 6/6 vs. 6/7 + 7/7 P = 0.03). This difference was mostly accounted for by the non-EIAC patients (6/6 vs. 6/7 vs. 7/7, P = 0.05; 6/6 vs. 6/7 + 7/7, P = 0.02). The frequency of Grade 3 adverse events in EIAC patients did not vary by genotype (6/6 vs. 6/7 vs. 7/7, P = 0.15; 6/6 vs. 6/7 + 7/7, P = 0.82).
Fig. 3.
Outcome as a function of UGT1A1*28 variant allele status, a Overall Survival. b Progression-free survival
The pharmacokinetics of irinotecan and its metabolites were characterized in 27 patients from both cohorts to establish the effect of anticonvulsant usage and UGT1A1*28 genotype (Table 3). Irinotecan plasma clearance for all patients varied over a 4.5-fold range (10.8–46.2 l/h/m2). Concurrent treatment with EIAC did not affect the clearance of irinotecan. The mean clearance value of 20.8 l/h/m2 for non-EIAC patients receiving 125 mg/m2 irinotecan was identical to the mean clearance value of 20.9 l/h/m2 for EIAC patients, receiving 400 mg/m2 irinotecan. Similarly, concurrent treatment with EIAC did not appear to substantially affect the formation and elimination of SN-38G. SN-38G Cmax and AUC values were only threefold greater for patients receiving EIAC as compared to those not receiving EIAC. In contrast, concurrent treatment with EIAC had a greater effect on formation of the metabolite carbonyloxycamptothecin (APC). The APC Cmax and AUC values were eightfold greater in EIAC patients as compared to patients not receiving EIAC. Concurrent treatment with EIAC also had a pronounced affect on formation and elimination of the active metabolite, SN-38 (7-ethyl-10-hydroxy camptothecin). Mean Cmax and AUC values of 20.9 ng/ml and 212 ng/ml h, respectively, for EIAC patients were not much greater than the mean Cmax and AUC values of 15.5 ng/ml and 207 ng/ml h, respectively, for non-EIAC patients. The presence of the UGT1A1 variant allele (6/7 or 7/7) did not substantially alter irinotecan clearance or SN-38 and SN-38G levels. A modest effect of genotype on SN-38 AUC was observed in non-EIAC patients. SN-38 AUC was higher and SN-38G AUC was lower in patients homozygous for the UGT1A1 variant allele (7/7) as compared with patients homozygous for the wild type allele (6/6).
Table 3.
Effect of EIAC treatment and UGT1A1*28 genotype on the pharmacokinetics of CPT-11 and its metabolites
| Cohort A (non-EIAC), 125 mg/m2 | Cohort B (EIAC), 400 mg/m2 | |||||||
|---|---|---|---|---|---|---|---|---|
| All patients (n = 15) |
6/6 (n = 5) | 6/7 (n = 8) | 7/7 (n = 2a) |
All patients (n = 12) |
6/6 (n = 3) | 6/7 (n = 7) | 7/7 (n = 2a) |
|
| CPT-11 | ||||||||
| Cmax (ng/ml) | 1035 ± 272 | 1038 ± 314 | 901 ± 285 | 1318 | 3361 ± 943 | 3837 ± 1001 | 3378 ± 438 | 2589 |
| t1/2 (h) | 6.27 ± 2.38 | 7.32 ± 4.26 | 6.05 ± 0.80 | 5.51 | 5.61 ± 0.91 | 5.33 ± 0.16 | 5.52 ± 1.00 | 6.34 |
| AUC (ng/ml h) | 6890 ± 2448 | 5458 ± 2030 | 7322 ± 2885 | 9431 | 19449 ± 2966 | 21296 ± 5499 | 18697 ± 1484 | 19170 |
| Cl (1/h/m2) | 20.8 ± 8.9 | 26.4 ± 12.1 | 19.0 ± 5.8 | 13.9 | 20.9 ± 2.6 | 19.6 ± 4.8 | 21.5 ± 1.7 | 20.9 |
| APC | ||||||||
| Cmax (ng/ml) | 104 ± 30 | 112 ± 39 | 101 ± 28 | 92 | 857 ± 312 | 796 ± 324 | 901 ± 336 | 794 |
| t1/2 (h) | 6.3 ± 1.5 | 5.7 ± 0.5 | 7.0 ± 1.9 | 5.3 | 6.4 ± 1.7 | 6.4 ± 1.6 | 5.9 ± 1.0 | 8.4 |
| AUC (ng/ml h) | 1327 ± 538 | 1063 ± 220 | 1549 ± 656 | 1095 | 10073 ± 3581 | 8466 ± 5095 | 10398 ± 3459 | 11345 |
| SN-38 | ||||||||
| Cmax (ng/ml) | 15.5 ± 10.0 | 14.9 ± 3.2 | 15.2 ± 13.7 | 18.2 | 20.9 ± 12.3 | 18.7 ± 5.3 | 21.8 ± 15.9 | 21.2 |
| t1/2 (h) | 18.8 ± 19.2 | 16.3 ± 5.2 | 21.9 ± 26.4 | 12.7 | 17.3 ± 10.4 | 16.9 ± 4.1 | 14.4 ± 7.4 | 28.0 |
| AUC (ng/ml h) | 207 ± 141 | 141 ± 22 | 248 ± 184 | 207 | 212 ± 89 | 180 ± 57 | 203 ± 94 | 291 |
| SN-38G | ||||||||
| Cmax (ng/ml) | 43.7 ± 22.3 | 56.6 ± 20.4 | 38.6 ± 23.8 | 31.9 | 133 ± 62 | 109 ± 80 | 145 ± 65 | 126 |
| t1/2 (h) | 11.7 ± 2.8 | 11.0 ± 2.5 | 12.5 ± 3.2 | 10.4 | 12.7 ± 5.2 | 14.0 ± 3.4 | 11.2 ± 4.9 | 16.0 |
| AUC (ng/ml h) | 580 ± 377 | 550 ± 221 | 658 ± 482 | 340 | 1314 ± 604 | 1119 ± 1054 | 1305 ± 487 | 1635 |
Mean value only is listed for 7/7 variant, as n = 2
Discussion
Irinotecan is a pro-drug that exerts its action after conversion by a carboxyl esterase to an active metabolite, SN-38 (7-ethyl-10-hydroxy camptothecin). Irinotecan is also metabolized to an inactive metabolite, carbonyloxycamptothecin (APC), via the hepatic p450—microsomal enzyme CYP3A4. SN-38 is inactivated by glucuronidation, via the uridine-diphosphate glucuronyltransferase isoenzyme (UGT1A1) [9]. An inherited gene polymorphism of UDP-glucuronosyltransferase (UGT1A1*28) may result in reduced metabolism of SN-38, and subsequent increase in drug-related toxicity at conventional doses [10, 11].
Prior studies of glioma patients on EIACs have reported significant reductions in SN-38 exposure (AUC) following irinotecan administration [12–15]. Patients receiving EIACs often require 2–4 times greater dosage than non-EIAC patients to achieve the same SN-38 exposure. The MTD of irinotecan was determined to be 117 mg/m2 in non-EIAC patients, and 419 mg/m2 weekly in patients receiving EIACs [16]. Using an every 3-week regimen, the MTD was established at 750 mg/m2 for EIAC patients, and 350 mg/m2 for non-EIAC patients [17].
We previously evaluated two different dose schedules of irinotecan in recurrent glioma patients [8]. The first tested 100–125 mg/m2 weekly for 4 weeks, repeated every 6 weeks; the second tested 250–300 mg/m2 every 3 weeks. Tumor response (PR) was observed in 6.5% (2/32) in each cohort. Lower mean concentrations and AUC of both irinotecan and its metabolites were observed in EIAC patients. In other studies, response rates to irinotecan have been 0–15%, with OS varying from 4 to 8.5 months [18–21].
There are limited clinical data regarding co-administration of RT and irinotecan. In a study of newly-diagnosed locally advanced non-small cell lung cancer patients, the response rate to RT plus concurrent irinotecan and cisplatin was 83%, with a median survival of 20.1 months [22]. In a prior Phase II study involving newly-diagnosed GBM patients, RT plus irinotecan followed by adjuvant BCNU plus irinotecan (adjusted for EIAC status) produced minor responses in 3/25 (12%) of patients; toxicities included neutropenia (26%), asthenia (13%) and diarrhea (8%) [23]. In another study, GBM patients receiving RT and concurrent temozolomide and irinotecan had a median PFS and OS of 7.7 and 12.8 months [20].
Pre-clinical studies have reported radiation sensitizing effects of irinotecan in lung and colon carcinoma cell lines and xenografts [1, 4, 5]. Irinotecan and carmustine have also shown synergism in human glioma xenografts [2, 3].
In the correlative analysis, patients not receiving EIAC with UGT1A1*28 6/7 or 7/7 variant alleles more frequently developed Grade 3 or greater toxicity, and experienced a shorter time to development of such toxicity than EIAC patients. We suspect that the lack of toxicity in the variant patients receiving EIAC may result from significant induction of CYP3A4 with enhanced metabolism of irinotecan. These results suggest that consideration be given to testing for the presence of UGT1 Al *28 variant alleles, if not receiving EIAC.
The pharmacokinetics of irinotecan and its metabolites for both EIAC and non-EIAC patients were similar to those observed in a prior Phase I study [16]. Plasma concentrations of SN-38 achieved in both cohorts were equal to or greater than the concentrations used in vitro to enhance radiation-induced cytotoxicity [1, 4]. However, SN-38 exposure achieved in both cohorts were lower than the exposure in mice (AUC 534 ng/ml h) administered a single dose of 10 mg/kg irinotecan that was associated with radiosensitization [1, 24]. The impact of the UGT1A1*28 polymorphism on irinotecan pharmacokinetics and toxicity is highly dependent on dose, with lower toxicity and AUC following administration in a weekly schedule [25, 26].
Data from this study were not mature until after the EORTC randomized Phase III study was reported, which showed a survival benefit for the combination of RT plus concomitant and adjuvant temozolomide (TMZ) over RT alone [27]. At the time of design of the current study, nitrosourea-based regimens were considered standard for front line therapy for newly-diagnosed GBM patients. BCNU had been shown to produce superior survival when combined with RT as compared to RT + methylprednisolone alone [28], and evidence of combined efficacy with RT was reported in a meta-analysis of existing prospective studies [29]. There has been some resurgence of discussion regarding nitrosoureas, largely due to unanticipated findings resulting from follow-up analyses involving long term glioma survivors. Although needing validation, recent analyses have hinted at a beneficial effect in long-term survivors when comparing RT with nitrosourea-based regimens (largely procarbazine, CCNU and vincristine, PCV) to RT alone for low-grade glioma [30], and even when compared with RT + TMZ for anaplastic oligodendroglioma [31]. Furthermore, the outcome of anaplastic glioma patients, as measured by time to second relapse, does not differ between patients receiving RT followed by chemotherapy (either temozolomide or CCNU) at relapse, or with temozolomide or CCNU initially, followed by RT at relapse [32]. Despite these interesting data, the results from our current study do not support replacement of current therapy with the nitrosourea-based regimen that we utilized in this study.
There has been longstanding interest in the potential radiosensitizing effects of irinotecan. In addition, recent studies have combined irinotecan with bevacizumab for recurrent GBM [33], and RT + bevacizumab for newly diagnosed GBM. To date, it is not clear that irinotecan produces additive benefit to bevacizumab alone; however, should this prove to be the case, the MTD of the irinotecan + RT, as determined in this study, may be helpful in construct of future trials involving these agents in combination.
We conclude that in treatment of patients with newly diagnosed GBM, concomitant RT and irinotecan and adjuvant irinotecan plus BCNU does not show greater efficacy than prior NCCTG regimens or current TMZ-containing regimens, and is more toxic. We did not observe clinically relevant radiosensitizing effects. Finally, patients who exhibit marked toxicity with irinotecan may harbor UGT1A1*28 variant alleles, and in particular, caution is advised when utilizing these agents in patients not receiving EIACs.
Acknowledgments
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-35269, CA-35101, CA-35103, CA-35113, CA-35431, CA-35267, CA-52352, CA-37417, CA-63848, and by grants from Pharmacia and Upjohn. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institute of Health.
Appendix
Additional participating institutions include: Rapid City Regional Oncology Group, Rapid City, SD 59709 (Richard Tenglin, M.D.); Mayo Clinic Arizona, Scottsdale, AZ 85259 (Tom R. Fitch, M.D.); CentraCare Clinic, St. Cloud, MN 56301 (Donald Jurgens, M.D.); Illinois Oncology Research Assn. CCOP, Peoria, IL 61602 (John W. Kugler, M.D.); Cedar Rapids Oncology Project CCOP, Cedar Rapids, IA 52403 (Martin Wiesenfeld, M.D.); Michigan Cancer Research Consortium, Ann Arbor, MI 48106 (Philip J. Stella, M.D.); Metro-Minnesota Community Clinical Oncology Program, St. Louis Park, MN 55416 (Patrick J. Flynn, M.D.); Sioux Community Cancer Consortium, Sioux Falls, SD 57105 (Loren K. Tschetter, M.D.); Wichita Community Clinical Oncology Program, Wichita, KS 67214-3882 (Shaker R. Dakhil, M.D.).
Footnotes
Additional participating institutions include in Appendix.
Contributor Information
Kurt A. Jaeckle, Mayo Clinic Florida, 4500 San Pablo Road, Jacksonville, FL 32224, USA, jaeckle.kurt@mayo.edu
Karla V. Ballman, Mayo Clinic Rochester, Rochester, MN 55905, USA
Caterina Giannini, Mayo Clinic Rochester, Rochester, MN 55905, USA.
Paula J. Schomberg, Mayo Clinic Rochester, Rochester, MN 55905, USA
Matthew M. Ames, Mayo Clinic Rochester, Rochester, MN 55905, USA
Joel M. Reid, Mayo Clinic Rochester, Rochester, MN 55905, USA
Renee M. McGovern, Mayo Clinic Rochester, Rochester, MN 55905, USA
Stephanie L. Safgren, Mayo Clinic Rochester, Rochester, MN 55905, USA
Evanthia Galanis, Mayo Clinic Rochester, Rochester, MN 55905, USA.
Joon H. Uhm, Mayo Clinic Rochester, Rochester, MN 55905, USA
Paul D. Brown, Mayo Clinic Rochester, Rochester, MN 55905, USA
Julie E. Hammack, Mayo Clinic Rochester, Rochester, MN 55905, USA
Robert Arusell, Meritcare Hospital CCOP, Fargo, ND 58122, USA.
Daniel A. Nikcevich, Duluth CCOP, Duluth, MN 55805, USA
Roscoe F. Morton, Iowa Oncology Research Association CCOP, Des Moines, IA 50309-1014, USA
Donald B. Wender, Siouxland Hematology-Oncology Associates, Sioux City, IA 51105, USA
Jan C. Buckner, Mayo Clinic Rochester, Rochester, MN 55905, USA
References
- 1.Tamura K, Takada M, Kawase I, et al. Enhancement of tumor radio-response by irinotecan in human lung tumor xenografts. Jpn J Cancer Res. 1997;88:218–223. doi: 10.1111/j.1349-7006.1997.tb00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coggins CA, Elion GB, Houghton PJ, et al. Enhancement of irinotecan activity against central nervous system tumor xenografts by alkylating agents. Cancer Chemother Pharmacol. 1998;41:485–490. doi: 10.1007/s002800050771. [DOI] [PubMed] [Google Scholar]
- 3.Castellino RC, Elion GB, Keir ST, et al. Schedule-dependent activity of irinotecan plus BCNU against human malignant glioma xenografts. Cancer Chemother Pharmacol. 2000;45:345–349. doi: 10.1007/s002800050050. [DOI] [PubMed] [Google Scholar]
- 4.Sasai K, Guo GZ, Shibuya K, et al. Effects of SN-38 (an active metabolite of irinotecan) on responses of human and rodent cells to irradiation. Int J Radiat Oncol Biol Phys. 1998;42:785–788. doi: 10.1016/s0360-3016(98)00326-5. [DOI] [PubMed] [Google Scholar]
- 5.Omura M, Torigoe S, Kubota N. SN-38, a metabolite of the camptothecin derivative irinotecan, potentiates of cytotoxic effect of radiation in human colon adenocarcinoma cells grown as spheroids. Radiother Oncol. 1997;43:197–201. doi: 10.1016/s0167-8140(97)01924-5. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan E, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 7.Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolomus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23:5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 8.Santisteban M, Buckner JC, Reid JM, et al. Phase II trial of two different irinotecan schedules with pharmacokinetic analysis in patients with recurrent glioma: North Central Cancer Treatment Group results. J Neurooncol. 2009;92:165–175. doi: 10.1007/s11060-008-9749-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckner JC, Reid JM, Wright K, et al. Irinotecan in the treatment of glioma patients: current and future studies of the North Central Cancer Treatment Group. Cancer. 2003;97 Suppl:2352–2358. doi: 10.1002/cncr.11304. [DOI] [PubMed] [Google Scholar]
- 10.Ando Y, Saka H, Asai G, et al. UGT1A1 genotypes and glucuronidation of SN-38, the active metabolite of irinotecan. Ann Oncol. 1998;9:845–847. doi: 10.1023/a:1008438109725. [DOI] [PubMed] [Google Scholar]
- 11.Iyer L, Hall D, Mortell MA, Ramirez J, et al. Phenotypegenotype correlation of in vitro SN-38 (active metabolite of irinotecan) and bilirubin glucuronidation in human liver tissue with UGT1 Al promoter polymorphism. Clin Pharmacol Ther. 1998;65:576–582. doi: 10.1016/S0009-9236(99)70078-0. [DOI] [PubMed] [Google Scholar]
- 12.Reid JM, Buckner JC, Schaaf LF, et al. Anticonvulsants alter the pharmacokinetics of irinotecan in patients with recurrent glioma. Proc Am Soc Clin Oncol. 2000;19:160a. (Abstr) [Google Scholar]
- 13.Gajjar AJ, Radomski KM, Bowers DC, et al. Pharmacokinetics of irinotecan and metabolites in pediatric high-grade glioma patients with and without co-administration of enzyme-inducing anticonvulsants. Proc Am Soc Clin Oncol. 2000;19 Suppl:162a. (Abstr) [Google Scholar]
- 14.Odani A, Hashimoto Y, Otsuki Y, et al. Genetic polymorphism of the CYP2C subfamily and its effect on the pharmacokinetics of phenytoin in Japanese patients with epilepsy. Clin Pharmacol Ther. 1997;62:287–292. doi: 10.1016/S0009-9236(97)90031-X. [DOI] [PubMed] [Google Scholar]
- 15.Slatter JG, Su R, Sams JP, et al. Bioactivation of the anticancer agent irinotecan to SN-38 by human hepatic microsomal carboxylesterases and the in vitro assessment of potential drug interactions. Drug Metab Dispos. 1997;25:1157–1164. [PubMed] [Google Scholar]
- 16.Gilbert M, Supko J, Batchelor T, et al. Phase I clinical and pharmacokinetic study of irinotecan in adults with recurrent malignant glioma. Clin Cancer Res. 2003;9:2940–2949. [PubMed] [Google Scholar]
- 17.Kuhn JG. Influence of anticonvulsants on the metabolism and elimination of irinotecan. A North American Brain Tumor Consortium preliminary report. Oncology. 2002;16 suppl:33–40. [PubMed] [Google Scholar]
- 18.Cloughesy TF, Filka E, Nelson G. Irinotecan treatment for recurrent malignant glioma using an every 3-week regimen. Am J Clin Oncol. 2002;25:204–208. doi: 10.1097/00000421-200204000-00022. [DOI] [PubMed] [Google Scholar]
- 19.Cloughesy TF, Filka E, Kuhn J. Two studies evaluating irinotecan treatment for recurrent malignant glioma using an every-3-week regimen. Cancer. 2003;97:2381–2386. doi: 10.1002/cncr.11306. [DOI] [PubMed] [Google Scholar]
- 20.Friedman HS, Petros WP, Friedman AH, et al. Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol. 1999;17:1516–1525. doi: 10.1200/JCO.1999.17.5.1516. [DOI] [PubMed] [Google Scholar]
- 21.Chamberlain MD. Salvage chemotherapy with irinotecan for recurrent glioblastoma multiforme. J Neurooncol. 2002;56:183–188. doi: 10.1023/a:1014532202188. [DOI] [PubMed] [Google Scholar]
- 22.Fukuda M, Soda H, Fukuda M, et al. Irinotecan and cisplatin with concurrent split-course radiotherapy in locally advanced non small-cell lung cancer: a multi-institutional phase 2 study. Cancer. 2007;110:606–613. doi: 10.1002/cncr.22817. [DOI] [PubMed] [Google Scholar]
- 23.Raymond E, Fabbro M, Goige V, et al. Multicentre phase II study and pharmacokinetic analysis of irinotecan in chemotherapy—naïve patients with glioblastoma. Ann Oncol. 2003;14:604–614. doi: 10.1093/annonc/mdg159. [DOI] [PubMed] [Google Scholar]
- 24.Fountzilas G, Karkavelas G, Kalogera-Fountzila A, et al. Post-operative combined radiation and chemotherapy with temozolomide and irinotecan in patients with high-grade astrocytic tumors. A phase II study with biomarker evaluation. Anticancer Res. 2006;26:4675–4686. [PubMed] [Google Scholar]
- 25.Stewart CF, Zamboni WC, Crom WR, et al. Disposition of irinotecan and SN-38 following oral and intravenous irinotecan dosing in mice. Cancer Chemother Pharmacol. 1997;40:259–265. doi: 10.1007/s002800050656. [DOI] [PubMed] [Google Scholar]
- 26.Hoskins JM, Goldberg RM, Qu R, et al. UGT1A1*28 genotype and irinotecan-induced neutropenia: dose matters. J Natl Cancer Inst. 2007;99:1290–1295. doi: 10.1093/jnci/djm115. [DOI] [PubMed] [Google Scholar]
- 27.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 28.Green SB, Byar DP, Walker MD, et al. Comparisons of carmustine, procarbazine, and high-dose methylprednisolone as additions to surgery and radiotherapy for the treatment of malignant glioma. Cancer Treat Rep. 1983;67:121–132. [PubMed] [Google Scholar]
- 29.Fine HA, Dear KB, Loeffler JS. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71:2585–2597. doi: 10.1002/1097-0142(19930415)71:8<2585::aid-cncr2820710825>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 30.Shaw EG, Wang M, Coons S, et al. Final report of Radiation Therapy Oncology Group (RTOG) protocol 9802: radiation therapy (RT) versus RT + procarbazine, CCNU and vincristine (PCV) chemotherapy for adult low-grade glioma (LGG) J Clin Oncol. 2008;26 15S:90s. (Abstr) [Google Scholar]
- 31.Lassman AB, Panageas KS, Iwamoto FM, et al. International retrospective study of 1001 adults with anaplastic Oligodendroglial tumors. Neurooncol. 2009;11:629. doi: 10.1093/neuonc/nor040. (Abstr) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolfgang WW, Weller M. NOA-4 randomized phase IIII study of sequential radiochemothearpy of anaplastic glioma with PCV or temozolomide. J Clin Oncol. 2008;26(Part II) 18S:1008s. (Abstr) [Google Scholar]
- 33.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]