Abstract
High salt induces the expression of transcription factor hypoxia-inducible factor (HIF)-1α and its target genes in the renal medulla, which is an important renal adaptive mechanism to high salt intake. HIF prolyl hydroxylase domain-containing proteins (PHDs) have been identified as major enzymes to promote the degradation of HIF-1α. PHD2 is the predominant isoform of PHDs in the kidney and primarily expressed in the renal medulla. The present study tested the hypothesis that PHD2 responds to high salt and mediates high salt-induced increase in HIF-1α levels in the renal medulla. In normotensive rats, high salt intake (4% NaCl, 10 days) significantly inhibited PHD2 expressions and enzyme activities in the renal medulla. Renal medullary overexpression of PHD2 transgene significantly decreased HIF-1α levels. PHD2 transgene also blocked high salt-induced activation of HIF-1α target genes heme oxygenase-1 and nitric oxide synthase-2 in the renal medulla. In Dahl salt-sensitive hypertensive rats, however, high salt intake did not inhibit the expression and activities of PHD2 in the renal medulla. Correspondingly, renal medullary HIF-1α levels were not up-regulated by high salt intake in these rats. After transfection of PHD2 shRNA, HIF-1α and its target genes were significantly up-regulated by high salt intake in Dahl S rats. Overexpression of PHD2 transgene in the renal medulla impaired renal sodium excretion after salt loading. These data suggest that high salt intake inhibits PHD2 in the renal medulla, thereby upregulating the HIF-1α expression. The lack of PHD-mediated response to high salt may represent a pathogenic mechanism producing salt sensitive hypertension.
Keywords: hypertension, transcription factor, gene transfection, Dahl salt-sensitive rats, siRNA
The transcription factor hypoxia inducible factor (HIF)-1α and some of its target genes, such as nitric oxide synthase (NOS), cyclooxygenase-2 (COX-2) and hemeoxygenase-1 (HO-1), are highly expressed in the renal medulla 1-6. The products of these HIF-1α target genes play critical roles in regulating renal medullary blood flow and tubular activity, and thereby maintaining the constancy of body fluid volume and arterial blood pressure. These genes in the renal medulla are up-regulated in response to high salt intake 2,4-7 and inhibition of these genes and/or the enzymes encoded by these genes within the renal medulla reduces sodium excretion and increases salt sensitivity of arterial blood pressure 2-4,6,8-10. We have previously shown that high salt intake increases HIF-1α levels in the renal medulla11. Inhibition of HIF-1α blocks the activation of its target genes in the renal medulla in response to high salt intake and consequently promotes sodium retention, inducing salt-sensitive hypertension11. It is suggested that HIF-1α-mediated gene regulation in the renal medulla represents an important molecular adaptive mechanism in response to high salt intake and plays a crucial role in the maintenance of sodium balance. However, it remains unclear how high salt intake induces increases in HIF-1α level in the renal medulla.
It has been recently demonstrated that HIF prolyl-hydroxylases are the major enzymes to promote the degradation of hypoxia inducible factor (HIF)-1a 12-14. HIF prolyl-hydroxylases catalyze site-specific proline hydroxylation of HIF-1α using oxygen as a cofactor, and prolyl-hydroxylated HIF-1α is recognized and targeted for degradation by the ubiquitin-proteasome pathway. Although HIF prolyl-hydroxylases work as oxygen sensor to regulate the destruction of HIF-1α 12-14, recent evidences have clearly shown that the activities and expressions of HIF prolyl-hydroxylases are also regulated independent of oxygen levels by a variety of factors15-19.
Three isoforms of HIF prolyl-hydroxylase, including prolyl hydroxylase domain-containing proteins 1, 2, and 3 (PHD1, 2, and 3), have been identified 12-13,20. It has been demonstrated that PHDs are present in the kidneys with PHD2 as the predominant isoform of PHDs 21-25 and PHD2 is most abundantly expressed in the renal medulla21,25. We have shown that PHDs participate in the regulation of renal medullary function21. Given the important role of PHDs in the regulation of HIF-1α levels and renal function, we hypothesize that PHD2 responds to high salt intake and mediates high salt-induced increase of HIF-1α in the renal medulla. We examined the effect of high salt intake on the expression of PHD2 and determined the role of PHD2 in high salt-induced activation of HIF-1α by transfection of PHD2 transgenes into the renal medulla. We also revealed that there was an impaired response of HIF-1α to high salt, which was associated with PHD2, in the renal medulla from Dahl salt-sensitive rats by transfection of PHD2 shRNA. Our results demonstrate that PHD2 is the mediator for the adaptive activation of renal medullary HIF-1α and its target genes in response to high salt challenge.
Materials and Methods
Animal
Experiments were performed in male Sprague-Dawley rats (Harlan, Madison, WI), Dahl S and SS13BN rats (Charles River, Wilmington, MA), weighing 250-350 g. Animals were kept on a low salt diet and some of them were fed with a high salt diet (4% NaCl) during experiments as indicated in the results section. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Virginia Commonwealth University.
Plasmids expressing rat full-length PHD2 cDNA and rat PHD2 shRNA
Plasmids encoding rat full-length PHD2 cDNA are generous gifts from Dr. Frank S. Lee (University of Pennsylvania). The expression and function of rat PHD2 protein by the plasmids have been validated by Dr. Lee’s publications 26-27 and in our previous study 21. Rat PHD2 siRNA sequences, sense: GUG UGA CAU GUA UAU AUU A, antisense: UAA UAU AUA CAU GUC ACA C, were designed and synthesized by QIAGEN. The target sequence is ATG TGT GAC ATG TAT ATA TTA (Accession Number: NM_178334). After the confirmation of effective knocking down of PHD2 genes by these siRNAs, the sequences were constructed into a pRNA-CMV3.2 vector (Genscript, Piscataway, NJ) to produce shRNA. The effective gene silencing of renal PHD2 by shRNA in vivo was also verified in preliminary experiments.
Transfection of DNA into the renal medulla
Rats were uninephrectomized one week before, and the remaining left kidney was transfected with designated plasmids (50 μg) into the renal medulla using in vivo-jetPEI™ (Polyplus-transfection, New York, NY), a polyethylenimine derivative, in combination with ultrasound radiation. Previous studies have shown that DNA was successfully delivered into the renal medulla using in vivo-jetPEI™ 28 and that combination of ultrasound significantly enhanced the DNA transfection with different transfection reagents 11,29-30 including polyethylenimine nanoparticles 31. For the details of this and the following methods, see the expanded Materials and Methods section in online data supplement, available at http://hyper.ahajournals.org. Our previous studies using similar technique for DNA delivery into the cells in the renal medulla showed that >90% of cells were transfected with no cell-type selectivity 11. We also showed that the expression of transgene in the kidney peaked on around day 5-7 and gradually decreased thereafter, while the mRNA levels in transfected animals remained 4.5 times higher than that in control animals 4 weeks after transfection32. The in vivo expression time period of transgene in our studies is consistent with reports by others using non-viral vectors and different DNA delivery methods, which have shown that in vivo overexpression of transgenes last for at least 2 or 4 weeks. 33-35.
RNA extraction and quantitative RT-PCR analysis of PHD2, heme oxygenase (HO)-1, nitric oxide synthase (NOS)-2 and cyclooxygenase (COX)-2 mRNA
The relative mRNA levels were measured by Real time RT-PCR using TaqMan® Gene Expression Assays kits (Applied Biosystems) with an iCycler iQ Real-Time PCR Detection System (Bio-Rad).
Preparation of tissue homogenate and nuclear extracts and Western blot analyses for protein levels of HIF-1α and PHD2
Renal tissue homogenates and nuclear protein were prepared, and Western blot analyses were performed as described previously 21. Primary antibodies used in the present study included anti-rat HIF-1α (monoclonal, Novus Biologicals, 1:300 dilution) and PHD2 (rabbit polyclonal, Novus Biologicals, 1:300). The intensities of the blots were determined using an imaging analysis program (ImageJ, free download from http://rsbweb.nih.gov/ij/).
Determination of HIF prolyl hydroxylase activity
HIF-1α peptide-specific conversion of 2-oxoglutarate into succinate provides a hydroxyl group for HIF-1α to be prolyl hydroxylated. This reaction has been widely used for the determination of PHD activity by measuring HIF-1α-dependent conversion rate of 2-oxoglutarate into succinate. 36-37.
Measurement of daily sodium balance. Additional groups of Sprague Dawley rats were uninephrectomized and transfected with control or PHD2 plasmids into the renal medulla of remaining kidney, and then housed in metabolic cages 8 days after PHD2 transfection. Daily indexes of sodium balance were computed by subtracting urinary sodium excretion from total sodium intake. After 2 day control measurements, the animals were switched from tap water to 2% NaCl water and sodium balance measurements were continued for additional 4 days 11,38-39. At the end of experiment, PHD2 mRNA levels in the renal medulla were measured by Real time RT-PCR.
Statistics
Data are presented as means ± SE. The significance of differences in mean values within and between multiple groups was evaluated using an ANOVA followed by a Duncan’s multiple range test. Student’s t-test was used to evaluate statistical significance of differences between two groups. P<0.05 was considered statistically significant.
Results
Effect of high salt intake on PHD2 levels in the renal medulla in Sprague Dawley rats
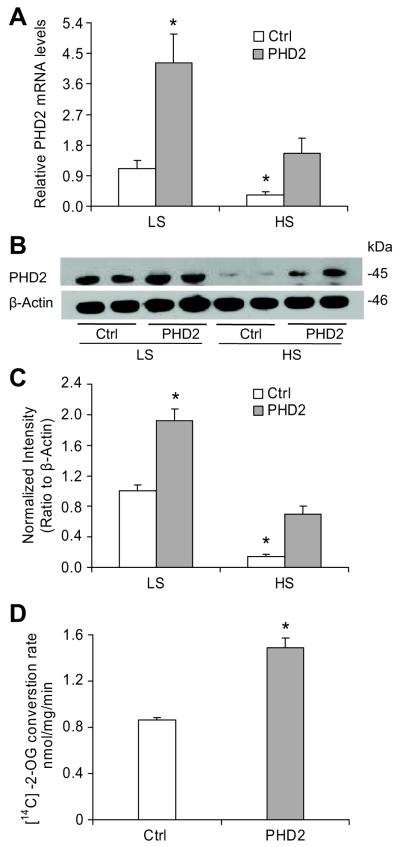
As summarized in figure 1, after 10 day high salt challenge, both mRNA and protein levels of renal medullary PHD2 were remarkably decreased in both control and PHD2 plasmids transfected animals. Overexpression of PHD2 transgene produced a large increase in PHD2 expression compared with control animals. Although a high salt diet also inhibited PHD2 levels in rats transfected with PHD2 plasmids, the PHD2 expression remained at the level shown in control rats on a low salt diet. The HIF-PHD activity in renal medulla, as measured by the conversion of 2-oxoglutarate (2-OG) into succinate, significantly increased in PHD2 plasmids transfected rats on a low salt diet (Figure 1D), suggesting that PHD activities were well correlated with PHD2 levels in the renal medulla.
Figure 1. Effect of high salt intake on PHD2 levels in the renal medulla in Sprague Dawley rats.
A: Real-Time RT-PCR analysis of PHD2 mRNA levels. B: Representative ECL gel documents of Western blot analyses depicting the protein levels of PHD2. C: Summarized intensities of the PHD2 blots normalized to β-actin. D: HIF-PHD activity. * P < 0.05 vs. others (n=6). LS = low salt, HS = high salt, Ctrl = empty vectors, PHD2 = PHD2 expression vectors.
Effect of PHD2 transgene overexpression on high salt-induced increase in HIF-1α levels and transcription of its target genes in the renal medulla in Sprague Dawley rats
As shown in figure 2, high salt intake significantly increased the HIF-1α protein level, which is consistent with our previous finding11. In PHD2 transfected rats, HIF-1α levels were remarkably decreased. Notably, high salt-induced increases in HIF-1α levels were blocked in PHD2-transfected rats to a level similar to that in control rats on a low salt diet. The mRNA levels of two important HIF-1α target genes, HO-1 and NOS2, in the renal medulla were shown in Table 1. Similar to the patterns of HIF-1α protein levels, high salt-induced activation of both HO-1 and NOS2 transcription was remarkably inhibited in PHD2-transfected rats, and the mRNA levels of HO-1 and NOS2 in these rats fed with a high salt diet were decreased to a level similar to that in control rats on a low salt diet.
Figure 2. Effect of PHD2 transgene overexpression on high salt-induced increase in HIF-1α levels in the renal medulla in Sprague Dawley rats.

A: Representative ECL gel documents of Western blot analyses depicting the protein levels of HIF-1α. B: Summarized intensities of the HIF-1α blots normalized to β-actin. * P < 0.05 vs. others (n=6).
Table 1.
Effect of PHD2 transgene on HIF-1α target genes HO-1 and NOS2 mRNA levels in the renal medulla in Sprague Dawley
| Gene | Ctrl + LS | PHD2 + LS | Ctrl + HS | PHD2 + HS |
|---|---|---|---|---|
| HO–1 | 1.05 ± 0.10 | 0.36 ± 0.04* | 3.69 ± 0.55 * | 1.39 ± 0.19 |
| NOS2 | 0.83 ± 0.15 | 0.43 ± 0.12* | 2.90 ± 0.52 * | 1.13 ± 0.22 |
P < 0.05 vs. others (n=6). LS = low salt intake, HS = high salt intake.
Ctrl = control plasmids, PHD2 = PHD2 plasmids.
Data normalized to Ctrl + LS.
Comparison of high salt induced-changes in HIF-1α and PHD2 levels in the renal medulla between Dahl S and SS13BN rats
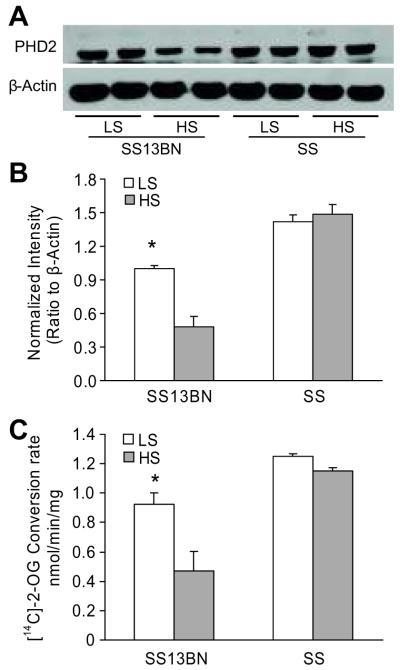
SS13BN rats exhibit minimal differences in genotype from Dahl S rats, and is considered as the best control for hypertension studies in Dahl S rats 40-42. As such, SS13BN rats were used in the present study as control for Dahl S rats. As shown in figure 3, high salt intake increased HIF-1α levels in SS13BN rats as in Sprague-Dawley rats presented above. However, the renal medullary HIF-1α level was significantly lower in Dahl S rats, and high salt-induced increases in HIF-1α levels were absent in this rat strain compared with that in SS13BN rats. Interestingly, the PHD2 levels were significantly higher and high salt-induced inhibition in PHD2 levels in the renal medulla were not observed in Dahl S rats (Figure 4A&B). Biochemical analysis showed that changes in PHD activities in response to a high salt diet ware different between these two rat strains, which followed the similar patterns observed in PHD2 expression (Figure 4C).
Figure 3. Comparison of high salt induced-changes in HIF-1α levels in the renal medulla between Dahl S and SS13BN rats.

A: Representative ECL gel documents of Western blot analyses depicting the protein levels of HIF-1α. B: Summarized intensities of the HIF-1α blots normalized to β-actin. * P < 0.05 vs. others (n=6).
Figure 4. Comparison of high salt induced-changes in the levels and activities of PHD2 in the renal medulla between Dahl S and SS13BN rats.
A: Representative ECL gel documents of Western blot analyses depicting the protein levels of PHD2. B: Summarized intensities of the PHD2 blots normalized to β-actin. C: PHD activities. * P < 0.05 vs. others (n=6).
Comparison of high salt-induced increases in mRNA levels of HIF-1α target genes in the renal medulla between Dahl S and SS13BN rats
The mRNA expressions of HIF-1α target genes HO-1, NOS2 and COX2, which play important roles in regulating renal medullary functions, were evaluated between Dahl S and SS13BN rats. As shown in figure 5, high salt induced dramatic increases in mRNA levels of all three HIF-1α target genes in SS13BN rats. However, these high salt-induced increases in the expressions of HO-1, NOS2 and COX2 gene were almost abolished in Dahl S rats.
Figure 5. Comparison of high salt-induced increases in mRNA levels of HIF-1α target genes HO-1, NOS2 and COX2 in the renal medulla between Dahl S and SS13BN rats.
* P < 0.05 vs. others, # P < 0.05 vs. SS-LS (n=6).
Effect of PHD2 gene silencing on high salt-induced changes in HIF-1α levels and transcriptions of its target genes in the renal medulla from Dahl S rats
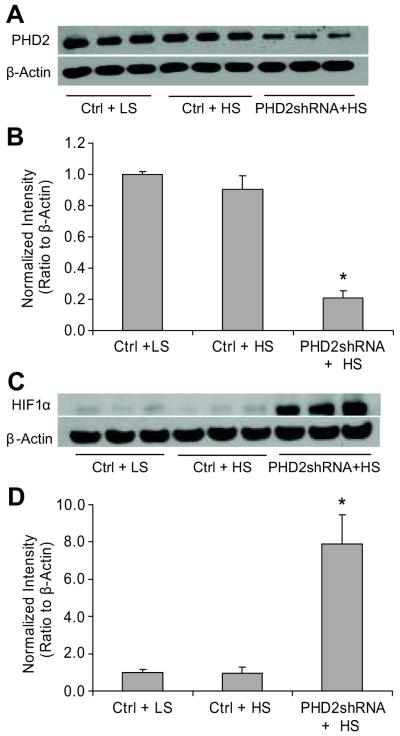
Intra-renal medullary transfection of PHD2 shRNA plasmids knocked down the PHD2 protein expression by 78% in Dahl S rats as shown in Figure 6A&B. This PHD2 gene knocking down was accompanied by a significant up-regulation of HIF-1α in the renal medulla in response to high salt intake (Figure 6C & D). Meanwhile, three important HIF-1α target genes HO-1, NOS2 and COX2, were significantly increased in PHD2 shRNA-transfected rats (Table 2).
Figure 6. Effect of PHD2 gene silencing on high salt-induced changes in HIF-1α levels in the renal medulla from Dahl S rats.
A: Representative ECL gel documents of Western blot analyses depicting the protein levels of PHD2. B: Summarized intensities of the PHD2 blots normalized to β-actin. C and D: Gel documents and summarized intensities of the HIF-1α blots normalized to β-actin. * P < 0.05 vs. others (n=6). Ctrl = vectors expressing scrambled shRNA, PHD2shRNA = vectors expressing PHD2 specific shRNA.
Table 2.
Effect of PHD2 transgene on HIF-1α target genes HO-1, NOS2 and COX2 mRNA levels in the renal medulla in Dahl S rats
| Gene | Ctrl + LS | Ctrl + HS | PHD2shRNA + HS |
|---|---|---|---|
| HO–1 | 1.01 ± 0.15 | 1.41 ± 0.16 | 7.28 ± 1.71* |
| NOS2 | 0.99 ± 0.13 | 1.46 ± 0.14* | 5.81 ± 1.15* |
| COX2 | 1.00 ± 0.13 | 1.52 ± 0.18* | 9.28 ± 2.28* |
P < 0.05 vs. others (n=6)
Ctrl = control plasmids, LS = low salt intake, HS = high salt intake
Data normalized to Ctrl + LS
Effects of renal medullary PHD2 transgene overexpression on salt balance
High salt intake produced a positive daily sodium balance. The positive salt balances were progressively increased in the first two days and started to decrease on the third day of high salt intake. The high salt-induced positive salt balance was significantly greater in PHD2-transfected rats than that in control rats (Figure 7A). The mRNA levels were increased by 3 folds in PHD2-transfected rats (Figure 7B), which confirmed the overexpression of PHD2 transgene in these rats.
Figure 7. Effects of renal medullary transfection of PHD2 plasmids on salt balances in Sprague Dawley rats.
A: daily sodium balance. B: Real-Time RT-PCR analysis of PHD2 mRNA levels in the renal medulla. * P < 0.05 vs. control.
Discussion
The present study showed that high salt intake decreased PHD2 levels in the renal medulla, and that overexpression of PHD2 transgene blocked high salt-induced activation in HIF-1α and its target genes, suggesting that high salt increases HIF-1α level and thereby enhances expression of its target genes through inhibition of PHD2. However, the activation of renal medullary HIF-1α and its target genes in response to a high salt diet was not observed in Dahl S rats, which was associated with an absence of high salt-induced inhibition in PHD2. Gene silencing of PHD2 restored high salt-induced activation of the HIF-1α and its target genes in the renal medulla from Dahl S rats. Moreover, overexpression of PHD2 transgene in the renal medulla impaired renal sodium excretion after salt loading. These results demonstrate that high salt inhibits PHD2 and thereby activates HIF-1α and its target genes in the renal medulla, and that an impairment of this molecular adaptive response to high salt intake may mediate sodium retention in salt sensitive individuals.
HIF-1α target genes such as NOS, COX-2 and HO-1 have been reported as crucial regulators in renal medullary function and sodium excretion, as well as pressure natriuresis 1,43-45. Activation of HIF-1α and its target genes by high salt intake has been proposed to promote the excretion of extra sodium loading through regulating the renal medullary functions, and thereby regulating the sodium balance in the body and salt sensitivity of blood pressure 11. The present study found that high salt-induced activation of HIF-1α and its target genes were associated with PHD2 inhibition and that overexpression of PHD2 transgenes blocked the activation of HIF-1α and its target genes after high salt challenge. These results indicate that PHD2 functions as an upstream signal that initiates HIF-1α-mediated gene activations in the renal medulla in response to high salt. Given the critical roles of the products of HIF-1α target genes HO-1, NOS2 and COX2 in the regulation of renal adaptation to high salt challenge and the maintenance of sodium homeostasis, it is suggested that PHD regulation of HIF-1α in response to high salt in the renal medulla may reveal a novel mechanism involved in renal salt handling.
The above information suggests that PHD/HIF-1α pathway is an important molecular mediator in renal salt adaptation under normal conditions. We wondered whether this PHD/HIF-1α pathway was involved in the pathogenic mechanism of abnormal renal sodium management in salt-sensitive hypertensive individuals. It has been reported that there is a defect in NOS2, one of HIF-1α target genes 46-48, and that the activations of NOS2 by a high salt diet and angiotensin II are diminished 3,46,49-50 in the renal medulla in salt-sensitive hypertensive Dahl S rats. These studies have indicated a possible impairment in renal medullary HIF-1α in this rat strain. We therefore determined whether the renal medullary HIF-1α and PHD2 levels were altered in the renal medulla in response to a high salt diet in Dahl S rats. Our results showed a decreased expression and reduced response to high salt intake in HIF-1α levels in the renal medulla from Dahl S rats, which was accompanied by similar defects in HIF-1α target genes HO-1, NOS2 and COX2. These results indicate that HIF-1α-mediated gene activations in these renal medullary protective factors are impaired in this rat strain. In parallel to these results, a higher level of PHD2 and failed inhibition of PHD2 in response to high salt intake in the renal medulla from Dahl S rats were observed. Additionally, reduction in PHD2 levels by shRNA restored the levels of HIF-1α and its target genes HO-1, NOS2 and COX2 in the renal medulla in Dahl S rats. It is suggested that diminished HIF-1α in Dahl S rats is caused by abnormal PHD2 response to a high salt diet. These data further support the view that PHD2 responds to high salt intake and thus controls HIF-1α-mediated gene activation, consequently maintaining sodium balance. Our results further suggest that deficient PHD/HIF-1α-mediated molecular adaptation in response to high salt intake in the renal medulla may represent a pathogenic mechanism producing salt sensitive hypertension.
To further elucidate the role of renal medullary PHD2 in the regulation of sodium excretion, we compared the sodium balance between control and PHD2-transfected rats after salt loading. It was demonstrated that renal medullary overexpression of PHD2 transgene remarkably impaired the capability of the kidneys to remove extra sodium load, which resulted in sodium retention. These data additionally suggest that renal medullary PHD2 is a crucial mediator in the signaling pathway sensing sodium intake and thereby importantly participates in the regulation of sodium excretion.
The present study did not attempt to explore the mechanisms by which high salt challenge inhibits PHD2 in the renal medulla and what causes the abnormal response of PHD2 in Dahl S rats. In this regard, several pathways might be accountable for it. For example, nitric oxide has been shown to inhibit PHDs in hypoxia 15. One of the possible mechanisms mediating down-regulation of PHD2 by a high salt diet may be that high salt intake initially increases renal tubular activities 51 and decreases renal medullary oxygen levels 52, which inhibits PHD2 activity and activates HIF-1α-mediated adaptive genes.
The proteins encoded by these genes produce medullary protective factors including NO, which in turn inhibit PHD2. In addition, several inflammatory factors have been implicated in the regulation of PHD2 expression and/or HIF-1α 18,53-54, while high salt intake has been shown to cause inflammation in the kidneys 55-56. Therefore, high salt-induced inflammatory factors may be involved in the inhibition of PHD2. The interesting finding that the expression of the PHD2 transgene was decreased by high salt intake in the present study indicated that reduced mRNA stability might be associated with high salt-induced PHD2 inhibition. The exact mechanisms for high salt to inhibit PHD2 require further exploration.
Regarding the mechanism causing impaired PHD2 response to high salt in Dahl rats, increased oxidant stress 57-58 might be one of the mechanisms. It has been shown that inhibition of NAD(P)H oxidase restores the diminished activation of NOS2 in response to high salt intake in the renal medulla in Dahl S rats 50. However, it is not yet clear how inhibition of NAD(P)H oxidase restores NOS2 level in the kidneys in this rat strain. Because O2−. has been demonstrated to stimulate PHDs and thereby inhibit HIF-1α 59-60, it is possible that high salt-induced oxidative stress induces PHD2 and thereby reduces HIF-1α levels in the renal medulla in Dahl S rats. Detailed mechanisms need to be clarified in future investigations.
Perspectives
Our results showed that PHD2-mediated activation of HIF-1α and its target genes in the renal medulla was an important molecular adaptive mechanism in response to high salt intake, which augments the enzymes producing natriuretic factors. It was also demonstrated that there was a defect in this salt adaptive pathway in Dahl S rats and impairment in this adaptive pathway caused sodium retention. It is concluded that high salt-induced inhibition of PHD2 and subsequent HIF-1α-mediated gene activation may play an important role in the maintenance of sodium homeostasis under various sodium loadings. Therefore, modification of PHD/HIF-1α pathway in the renal medulla may serve as a therapeutic approach for the management of salt-sensitive hypertension.
Supplementary Material
Acknowledgments
Source of Funding
National Institutes of Health Grant HL-89563
Footnotes
Conflict of Interest
None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zou A-P, Billington H, Su N, Cowley AW., Jr. Expression and Actions of Heme Oxygenase in the Renal Medulla of Rats. Hypertension. 2000;35:342–347. doi: 10.1161/01.hyp.35.1.342. [DOI] [PubMed] [Google Scholar]
- 2.Mattson DL, Higgins DJ. Influence of Dietary Sodium Intake on Renal Medullary Nitric Oxide Synthase. Hypertension. 1996;27:688–692. doi: 10.1161/01.hyp.27.3.688. [DOI] [PubMed] [Google Scholar]
- 3.Tan DY, Meng S, Cason GW, Manning RD., Jr. Mechanisms of salt-sensitive hypertension: role of inducible nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2297–2303. doi: 10.1152/ajpregu.2000.279.6.R2297. [DOI] [PubMed] [Google Scholar]
- 4.Zewde T, Mattson DL. Inhibition of Cyclooxygenase-2 in the Rat Renal Medulla Leads to Sodium-Sensitive Hypertension. Hypertension. 2004;44:424–428. doi: 10.1161/01.HYP.0000140924.91479.03. [DOI] [PubMed] [Google Scholar]
- 5.Yang T, Singh I, Pham H, Sun D, Smart A, Schnermann JB, Briggs JP. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am J Physiol Renal Physiol. 1998;274:F481–489. doi: 10.1152/ajprenal.1998.274.3.F481. [DOI] [PubMed] [Google Scholar]
- 6.Li N, Yi F, dos Santos EA, Donley DK, Li P-L. Role of Renal Medullary Heme Oxygenase in the Regulation of Pressure Natriuresis and Arterial Blood Pressure. Hypertension. 2007;49:148–154. doi: 10.1161/01.HYP.0000250086.06137.fb. [DOI] [PubMed] [Google Scholar]
- 7.Harris RC, Breyer MD. Physiological regulation of cyclooxygenase-2 in the kidney. Am J Physiol Renal Physiol. 2001;281:F1–11. doi: 10.1152/ajprenal.2001.281.1.F1. [DOI] [PubMed] [Google Scholar]
- 8.Yao B, Harris RC, Zhang M-Z. Interactions between 11{beta}-hydroxysteroid dehydrogenase and COX-2 in kidney. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1767–1773. doi: 10.1152/ajpregu.00786.2004. [DOI] [PubMed] [Google Scholar]
- 9.Mattson DL, Maeda CY, Bachman TD, Cowley AW., Jr. Inducible Nitric Oxide Synthase and Blood Pressure. Hypertension. 1998;31:15–20. doi: 10.1161/01.hyp.31.1.15. [DOI] [PubMed] [Google Scholar]
- 10.Nakanishi K, Hara N, Nagai Y. Salt-sensitive hypertension in conscious rats induced by chronic nitric oxide blockade. American Journal of Hypertension. 2002;15:150–156. doi: 10.1016/s0895-7061(01)02267-1. [DOI] [PubMed] [Google Scholar]
- 11.Li N, Chen L, Yi F, Xia M, Li P-L. Salt-Sensitive Hypertension Induced by Decoy of Transcription Factor Hypoxia-Inducible Factor-1{alpha} in the Renal Medulla. Circ Res. 2008;102:1101–1108. doi: 10.1161/CIRCRESAHA.107.169201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruick RK, McKnight SL. A Conserved Family of Prolyl-4-Hydroxylases That Modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 13.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 14.Jaakkola P, Mole DR, Tian Y-M, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim Av, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 15.Callapina M, Zhou J, Schnitzer S, Metzen E, Lohr C, Deitmer JW, Brune B. Nitric oxide reverses desferrioxamine- and hypoxia-evoked HIF-1[alpha] accumulation--Implications for prolyl hydroxylase activity and iron. Experimental Cell Research. 2005;306:274–284. doi: 10.1016/j.yexcr.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Baek JH, Mahon PC, Oh J, Kelly B, Krishnamachary B, Pearson M, Chan DA, Giaccia AJ, Semenza GL. OS-9 Interacts with Hypoxia-Inducible Factor 1[alpha] and Prolyl Hydroxylases to Promote Oxygen-Dependent Degradation of HIF-1[alpha] Molecular Cell. 2005;17:503–512. doi: 10.1016/j.molcel.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Page EL, Chan DA, Giaccia AJ, Levine M, Richard DE. Hypoxia-inducible Factor-1{alpha} Stabilization in Nonhypoxic Conditions: Role of Oxidation and Intracellular Ascorbate Depletion. Mol Biol Cell. 2008;19:86–94. doi: 10.1091/mbc.E07-06-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM. Transforming Growth Factor beta1 Induces Hypoxia-inducible Factor-1 Stabilization through Selective Inhibition of PHD2 Expression. J Biol Chem. 2006;281:24171–24181. doi: 10.1074/jbc.M604507200. [DOI] [PubMed] [Google Scholar]
- 19.Tug S, Reyes BD, Fandrey J, Berchner-Pfannschmidt U. Non-hypoxic activation of the negative regulatory feedback loop of prolyl-hydroxylase oxygen sensors. Biochemical and Biophysical Research Communications. 2009;384:519–523. doi: 10.1016/j.bbrc.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 21.Li N, Yi F, Sundy CM, Chen L, Hilliker ML, Donley DK, Muldoon DB, Li P-L. Expression and actions of HIF prolyl-4-hydroxylase in the rat kidneys. Am J Physiol Renal Physiol. 2007;292:F207–216. doi: 10.1152/ajprenal.00457.2005. [DOI] [PubMed] [Google Scholar]
- 22.Takeda K, Cowan A, Fong G-H. Essential Role for Prolyl Hydroxylase Domain Protein 2 in Oxygen Homeostasis of the Adult Vascular System. Circulation. 2007;116:774–781. doi: 10.1161/CIRCULATIONAHA.107.701516. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberger C, Rosen S, Shina A, Frei U, Eckardt K-U, Flippin LA, Arend M, Klaus SJ, Heyman SN. Activation of hypoxia-inducible factors ameliorates hypoxic distal tubular injury in the isolated perfused rat kidney. Nephrol Dial Transplant. 2008;23:3472–3478. doi: 10.1093/ndt/gfn276. [DOI] [PubMed] [Google Scholar]
- 24.Takeda K, Aguila HL, Parikh NS, Li X, Lamothe K, Duan L-J, Takeda H, Lee FS, Fong G-H. Regulation of adult erythropoiesis by prolyl hydroxylase domain proteins. Blood. 2008;111:3229–3235. doi: 10.1182/blood-2007-09-114561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schodel J, Klanke B, Weidemann A, Buchholz B, Bernhardt W, Bertog M, Amann K, Korbmacher C, Wiesener M, Warnecke C, Kurtz A, Eckardt K-U, Willam C. HIF-Prolyl Hydroxylases in the Rat Kidney: Physiologic Expression Patterns and Regulation in Acute Kidney Injury. Am J Pathol. 2009;174:1663–1674. doi: 10.2353/ajpath.2009.080687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang J, Zhao Q, Mooney SM, Lee FS. Sequence determinants in hypoxia-inducible factor-1alpha for hydroxylation by the prolyl hydroxylases PHD1, PHD2, and PHD3. J Biol Chem. 2002;277:39792–39800. doi: 10.1074/jbc.M206955200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Percy MJ, Zhao Q, Flores A, Harrison C, Lappin TR, Maxwell PH, McMullin MF, Lee FS. A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc Natl Acad Sci U S A. 2006;103:654–659. doi: 10.1073/pnas.0508423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Singh RJ, Usa K, Netzel BC, Liang M. Renal medullary 11{beta}-hydroxysteroid dehydrogenase type 1 in Dahl salt-sensitive hypertension. Physiol Genomics. 2008;36:52–58. doi: 10.1152/physiolgenomics.90283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosseinkhani H, Aoyama T, Ogawa O, Tabata Y. Ultrasound enhances the transfection of plasmid DNA by non-viral vectors. Curr Pharm Biotechnol. 2003;4:109–122. doi: 10.2174/1389201033489883. [DOI] [PubMed] [Google Scholar]
- 30.Newman CMH, Bettinger T. Gene therapy progress and prospects: Ultrasound for gene transfer. Gene Ther. 2007;14:465–475. doi: 10.1038/sj.gt.3302925. [DOI] [PubMed] [Google Scholar]
- 31.Chumakova OV, Liopo AV, Andreev VG, Cicenaite I, Evers BM, Chakrabarty S, Pappas TC, Esenaliev RO. Composition of PLGA and PEI/DNA nanoparticles improves ultrasound-mediated gene delivery in solid tumors in vivo. Cancer Letters. 2008;261:215–225. doi: 10.1016/j.canlet.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 32.Yi F, Xia M, Li N, Zhang C, Tang L, Li P-L. Contribution of Guanine Nucleotide Exchange Factor Vav2 to Hyperhomocysteinemic Glomerulosclerosis in Rats. Hypertension. 2009;53:90–96. doi: 10.1161/HYPERTENSIONAHA.108.115675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richard-Fiardo P, Payen E, Chevre R, Zuber J, Letrou-Bonneval E, Beuzard Y, Pitard B. Therapy of anemia in kidney failure, using plasmid encoding erythropoietin. Hum Gene Ther. 2008;19:331–342. doi: 10.1089/hum.2006.0101. [DOI] [PubMed] [Google Scholar]
- 34.Ng Y-Y, Hou C-C, Wang W, Huang XR, Lan HY. Blockade of NF[kappa]B activation and renal inflammation by ultrasound-mediated gene transfer of Smad7 in rat remnant kidney. Kidney Int. 2005;67:S83–S91. doi: 10.1111/j.1523-1755.2005.09421.x. [DOI] [PubMed] [Google Scholar]
- 35.Yang J, Dai C, Liu Y. Systemic administration of naked plasmid encoding hepatocyte growth factor ameliorates chronic renal fibrosis in mice. Gene Ther. 2001;8:1470–1479. doi: 10.1038/sj.gt.3301545. [DOI] [PubMed] [Google Scholar]
- 36.D’Angelo G, Duplan E, Boyer N, Vigne P, Frelin C. Hypoxia Up-regulates Prolyl Hydroxylase Activity: A FEEDBACK MECHANSIM THAT LIMITS HIF-1 RESPONSES DURING REOXYGENATION. J Biol Chem. 2003;278:38183–38187. doi: 10.1074/jbc.M302244200. [DOI] [PubMed] [Google Scholar]
- 37.Salnikow K, Donald SP, Bruick RK, Zhitkovich A, Phang JM, Kasprzak KS. Depletion of Intracellular Ascorbate by the Carcinogenic Metals Nickel and Cobalt Results in the Induction of Hypoxic Stress. J Biol Chem. 2004;279:40337–40344. doi: 10.1074/jbc.M403057200. [DOI] [PubMed] [Google Scholar]
- 38.Dibona GF, Jones SY, Sawin LL. Angiotensin receptor antagonist improves cardiac reflex control of renal sodium handling in heart failure. Am J Physiol Heart Circ Physiol. 1998;274:H636–641. doi: 10.1152/ajpheart.1998.274.2.H636. [DOI] [PubMed] [Google Scholar]
- 39.Li P, Morris M, Ferrario CM, Barrett C, Ganten D, Callahan MF. Cardiovascular, endocrine, and body fluid-electrolyte responses to salt loading in mRen-2 transgenic rats. Am J Physiol Heart Circ Physiol. 1998;275:H1130–1137. doi: 10.1152/ajpheart.1998.275.4.H1130. [DOI] [PubMed] [Google Scholar]
- 40.Cowley AW., Jr. Genomics and homeostasis. Am J Physiol Regul Integr Comp Physiol. 2003;284:R611–627. doi: 10.1152/ajpregu.00567.2002. [DOI] [PubMed] [Google Scholar]
- 41.Liang M, Yuan B, Rute E, Greene AS, Olivier M, Cowley AW., Jr. Insights into Dahl salt-sensitive hypertension revealed by temporal patterns of renal medullary gene expression. Physiol Genomics. 2003;12:229–237. doi: 10.1152/physiolgenomics.00089.2002. [DOI] [PubMed] [Google Scholar]
- 42.Cowley AW, Jr., Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension. 2001;37:456–461. doi: 10.1161/01.hyp.37.2.456. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez F, Kemp R, Balazy M, Nasjletti A. Effects of Exogenous Heme on Renal Function: Role of Heme Oxygenase and Cyclooxygenase. Hypertension. 2003;42:680–684. doi: 10.1161/01.HYP.0000085785.40581.1A. [DOI] [PubMed] [Google Scholar]
- 44.Gross JM, Dwyer JE, Knox FG. Natriuretic Response to Increased Pressure Is Preserved With COX-2 Inhibitors. Hypertension. 1999;34:1163–1167. doi: 10.1161/01.hyp.34.5.1163. [DOI] [PubMed] [Google Scholar]
- 45.Majid DS, Navar LG. Nitric oxide in the mediation of pressure natriuresis. Clin Exp Pharmacol Physiol. 1997;24:595–599. doi: 10.1111/j.1440-1681.1997.tb02098.x. [DOI] [PubMed] [Google Scholar]
- 46.Szentivanyi M, Jr., Zou A-P, Mattson DL, Soares P, Moreno C, Roman RJ, Cowley AW., Jr. Renal medullary nitric oxide deficit of Dahl S rats enhances hypertensive actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2002;283:R266–272. doi: 10.1152/ajpregu.00461.2001. [DOI] [PubMed] [Google Scholar]
- 47.Ikeda Y, Saito K, Kim J-I, Yokoyama M. Nitric Oxide Synthase Isoform Activities in Kidney of Dahl Salt-Sensitive Rats. Hypertension. 1995;26:1030–1034. doi: 10.1161/01.hyp.26.6.1030. [DOI] [PubMed] [Google Scholar]
- 48.Cowley AW, Jr., Mori T, Mattson D, Zou A-P. Role of renal NO production in the regulation of medullary blood flow. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1355–1369. doi: 10.1152/ajpregu.00701.2002. [DOI] [PubMed] [Google Scholar]
- 49.Yoshihara F, Suga S, Yasui N, Horio T, Tokudome T, Nishikimi T, Kawano Y, Kangawa K. Chronic administration of adrenomedullin attenuates the hypertension and increases renal nitric oxide synthase in Dahl salt-sensitive rats. Regul Pept. 2005;128:7–13. doi: 10.1016/j.regpep.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 50.Tojo A, Onozato ML, Kobayashi N, Goto A, Matsuoka H, Fujita T. Antioxidative effect of p38 mitogen-activated protein kinase inhibitor in the kidney of hypertensive rat. J Hypertens. 2005;23:165–174. doi: 10.1097/00004872-200501000-00027. [DOI] [PubMed] [Google Scholar]
- 51.Scherzer P, Wald H, Czaczkes JW. Na-K-ATPase in isolated rabbit tubules after unilateral nephrectomy and Na+ loading. Am J Physiol Renal Physiol. 1985;248:F565–573. doi: 10.1152/ajprenal.1985.248.4.F565. [DOI] [PubMed] [Google Scholar]
- 52.Brezis M, Agmon Y, Epstein FH. Determinants of intrarenal oxygenation. I. Effects of diuretics. Am J Physiol Renal Physiol. 1994;267:F1059–1062. doi: 10.1152/ajprenal.1994.267.6.F1059. [DOI] [PubMed] [Google Scholar]
- 53.Frede S, Berchner[hyphen (true graphic)]Pfannschmidt U, Fandrey J. Regulation of Hypoxia Inducible Factors During Inflammation Methods in Enzymology. In: Brüne HSaB., editor. Oxygen Biology and Hypoxia. Vol. 435. Academic Press; 2007. pp. 403pp. 405–419. [DOI] [PubMed] [Google Scholar]
- 54.Fan JM, Huang XR, Ng YY, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY. Interleukin-1 induces tubular epithelial-myofibroblast transdifferentiation through a transforming growth factor-beta1-dependent mechanism in vitro. Am J Kidney Dis. 2001;37:820–831. doi: 10.1016/s0272-6386(01)80132-3. [DOI] [PubMed] [Google Scholar]
- 55.Roson MI, Cavallero S, Della Penna S, Cao G, Gorzalczany S, Pandolfo M, Kuprewicz A, Canessa O, Toblli JE, Fernandez BE. Acute sodium overload produces renal tubulointerstitial inflammation in normal rats. Kidney Int. 2006;70:1439–1446. doi: 10.1038/sj.ki.5001831. [DOI] [PubMed] [Google Scholar]
- 56.Mattar AL, Machado FG, Fujihara CK, Malheiros DM, Zatz R. Persistent hypertension and progressive renal injury induced by salt overload after short term nitric oxide inhibition. Clinics (Sao Paulo) 2007;62:749–756. doi: 10.1590/s1807-59322007000600015. [DOI] [PubMed] [Google Scholar]
- 57.Taylor NE, Glocka P, Liang M, Cowley AW., Jr. NADPH Oxidase in the Renal Medulla Causes Oxidative Stress and Contributes to Salt-Sensitive Hypertension in Dahl S Rats. Hypertension. 2006;47:692–698. doi: 10.1161/01.HYP.0000203161.02046.8d. [DOI] [PubMed] [Google Scholar]
- 58.Tian N, Moore RS, Phillips WE, Lin L, Braddy S, Pryor JS, Stockstill RL, Hughson MD, Manning RD., Jr. NADPH oxidase contributes to renal damage and dysfunction in Dahl salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1858–1865. doi: 10.1152/ajpregu.90650.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Callapina M, Zhou J, Schmid T, Kohl R, Brune B. NO restores HIF-1alpha hydroxylation during hypoxia: role of reactive oxygen species. Free Radic Biol Med. 2005;39:925–936. doi: 10.1016/j.freeradbiomed.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 60.EMORI Y, MIZUSHIMA T, MATSUMURA N, OCHI K, TANIOKA H, SHIRAHIGE A, ICHIMURA M, SHINJI T, KOIDE N, TANIMOTO M. Camostat, an oral trypsin inhibitor, reduces pancreatic fibrosis induced by repeated administration of a superoxide dismutase inhibitor in rats. Journal of Gastroenterology and Hepatology. 2005;20:895–899. doi: 10.1111/j.1440-1746.2005.03826.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.