Abstract
Many components of resting-state (RS) FMRI show non-random structure that has little to do with neural connectivity but can covary over multiple brain structures. Some of these signals originate in physiology and others are hardware-related. One artifact discussed herein may be caused by defects in the receive coil array or the RF amplifiers powering it. During a scan, this artifact results in small image intensity shifts in parts of the brain imaged by the affected array components. These shifts introduce artifactual correlations in RS time series on the spatial scale of the coil’s sensitivity profile, and can markedly bias RS connectivity results. We show that such a transient artifact can be substantially removed from RS time series by using locally formed regressors from white matter tissue. This is particularly important in arrays with larger numbers of coils, which may generate smaller artifact zones. In such a case, brain-wide average noise estimates would fail to capture the artifact. We also examine the anatomical structure of artifactual variance in RS FMRI time series, by identifying sources that contribute to these signals and where in the brain are they manifested. We consider current methods for reducing confounding sources (or noises) and their effects on connectivity maps, and offer an improved approach (ANATICOR) that can also reduce hardware artifacts. The methods described herein are currently available with AFNI, in addition to tools for rapid, interactive generation of seed based correlation maps at single-subject and group levels.
Keywords: Resting State, Functional Magnetic Resonance Imaging, Noise Reduction, Artifact Correction, Human Brain
1. Introduction
Connectivity analysis of human brain using resting-state (RS) functional magnetic resonance imaging (FMRI) has gained wide use since its discovery, reporting brain regions fluctuating together with a significant degree of temporal correlation (Biswal et al., 1995). RS connectivity has been used for observing functional networks covarying (Biswal et al., 1995; Greicius et al., 2003; Raichle and Snyder, 2007), segregating functionally distinguishable brain regions (Kim et al., 2010; Mezer et al., 2009), and in comparative studies between typical and abnormal brains (Cherkassky et al., 2006; Greicius et al., 2004). To make these applications possible, the most commonly used approach uses the cross correlation of residual time-series to estimate the strength of connection between a pair of voxels or regions of interest after possible artifacts are removed by linear regression from the original echo planar imaging (EPI) time series data.
For artifact removal, there are many existing methods, and we consider several types of non-random artifacts or noise sources that bias the connectivity results of blood-oxygen-level dependent (BOLD) FMRI data; these are (i) global artifacts (e.g. head motion, hardware drift signals) (Lund et al., 2006), (ii) physiological “noises” (true NMR signals with noise-like properties, caused by respiration and the heartbeat), and (iii) acute hardware malfunctions or instability. Given the nature of FMRI noise and the sampling constraints on FMRI time series, many components of RS FMRI show temporal structure that covaries over long spatial distances, but have little to do with the notion of neural connectedness (Birn et al., 2008). Especially, respiration and the heartbeat have long been targeted as a source of physiological noises in FMRI, and these sources are more problematic in RS FMRI than in task-induced FMRI (Birn et al., 2006; Chang et al., 2009; Shmueli et al., 2007; Wise et al., 2004). Retrospective image correction (RETROICOR) is a common approach to reduce the effects of heartbeat- and respiration-related changes (Glover et al., 2000; Jones et al., 2008).
However, there could be more artifact sources in residual signals after these physiological noises are modeled out. One considerable artifact source is the draining vessel system. The BOLD signal originates in the venous bed on the capillary side and moves though venules, and then veins, to the sinuses in sulcal cerebrospinal fluid (CSF) (Nencka and Rowe, 2007). With task FMRI, the temporal signature of the stimulus allows the localization of activation. Large vessels that show a significant fit to the task activation can be discounted. But in RS FMRI, this is less practicable. Large vessels contain signal sources that carry over long distances. Some of these signals are legitimate, gray matter (GM) BOLD signal, and some of these signals are also noise. Given the general orientation of venous vasculature draining in the direction of the pial surface and then coursing on the outer hull of the brain, there should be little reflection of GM-based RS signal changes in white matter (WM). Even if the above noise sources are not directly reflected in GM tissue, whole volume blurring operations can spread these noise signals into GM voxels, producing artifactual spatial coherence.
The large-scale effect of hardware drifts can largely be removed by baseline modeling. However, there can be remaining artifacts induced by hardware issues. One possible hardware artifact results in signals that fluctuate locally and are spatially correlated. An illustration of this can be seen in RS data obtained from a multi-channel array head coil. In this instance, one of the RF amplifier channels was intermittently slightly faulty, and these fluctuations in the coil’s input to the image reconstruction software caused small fluctuations in the RS EPI data that were spatially correlated over the spatial scale of the coil's sensitivity profile. However, visual image quality was unimpaired. As a result, a seed placed anywhere in the coil's sensitive zone results in a relatively high degree of correlation measurements elsewhere in that zone. In the most benign case, this spatially correlated noise would mask out true underlying connectivity. But unless accounted for, it could also bias the results of group comparisons where data acquisition could not be fully randomized because of subject population constraints. This artifact would have gone unnoticed, were it not for the interactive seed-based correlation maps that can be generated with AFNI’s InstaCorr and GrpInCorr tools, both in the volume and on cortical surfaces with SUMA (Argall et al., 2006; Saad, 2004). These tools greatly facilitate RS data exploration by allowing immediate generation of single-subject (∼100ms) or group-level (∼500–1500ms) seed-based correlation statistics at the click of a mouse button.
In this work, we examine the anatomy of RS FMRI time-series, identifying noise sources that contribute to RS FMRI signals and where in the brain are they manifested. In the process, we examine current methods for reducing noise contributions and their effects on connectivity maps, and offer improvements to some of these approaches. We attempt to reduce the effect of spurious sources of correlation that may bias RS connectivity studies. To control the effects of spatially correlated noise, we examine the spatiotemporal structure of RS FMRI data using high spatial resolution EPI data and anatomically segmented masks generated from T1-weighted images. Finally, we suggest a well-designed artifact correction method using anatomically modeled signals (ANATICOR), which can remove both local and global artifacts. The idea here is to model about as much of the artifactual signals as the anatomical masks are capable of, and use what left in the residual of the regression model to be used for functional connectivity analysis.
2. Methods
2.1. Data Acquisition
Fifteen healthy volunteers (males, 18.27±2.46 years) were instructed to fixate on a black cross in the center of the screen while keeping their mind clear, and were scanned using an 8 channel head coil array equipped GE 3 Tesla scanner. RS FMRI time-series were acquired using a T2*-weighted gradient echo pulse sequence with high spatial resolution (1.719×1.719×3.000 mm3, TR=3.5 s, TE=27 ms, flip angle=90°). Each of the RS FMRI scans lasted for a duration of 490s or 140 volumes. A respiration belt was used to measure respiration volume and a pulse oximeter to monitor heart rhythm. Belt diameter and pulse oximeter readings were sampled at 50 Hz with recording onset triggered by the scanner's slice selection trigger pulse. Two High-resolution (0.938×0.938×1.200 mm3) T1-weighted anatomical images were also acquired with an MPRAGE sequence during the scanning session.
2.2. Pre-processing
2.2.1. Anatomical Segmentation
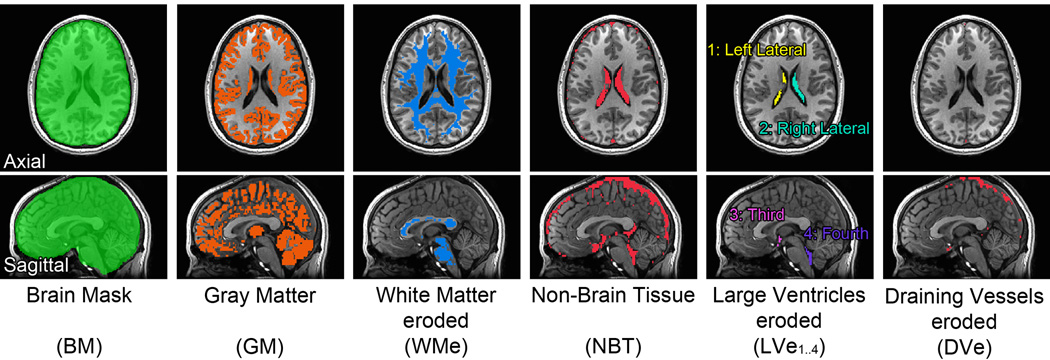
Prior to segmentation, the high-resolution anatomical T1 was aligned to the fifth EPI volume of the EPI time series. This alignment optimization was performed using the Local Pearson Correlation (LPC) cost function (AFNI’s align_epi_anat.py). This cross-modal registration cost function is optimized for EPI and T1 images, and has been shown to be superior for this purpose to more general multi-modal cost functions (Saad et al., 2009). Aligned anatomical images were then processed with FreeSurfer’s automated pipeline for generating surface models of the cortical surface (Fischl et al., 2002), and whole brain and surface-based parcellation. From this, mask volumes of the whole brain (BM), gray matter (GM), white matter (WM), and the four large ventricles (LV1..4) were generated for each individual subject. In addition, we generated a Non-Brain-Tissue mask [NBT = BM \ (GM ∪ WM)], defined as the set difference between BM and its subsets GM and WM. All masks were then nearest-neighbor resampled to EPI resolution. To reduce Partial Voluming Effects (PVE) from GM on tissue masks WM, LVi, and NBT, we eroded these masks by one voxel along each of the three axes. Fig. 1 shows masks from one subject overlaid on a high-resolution T1 that was aligned to the EPI data. The first and second columns show the whole brain mask in green, and the gray matter mask in brown, respectively. Neither of these masks were eroded. The third column shows in blue the eroded white matter mask. The resultant NBT mask shown in the fourth column, consists largely of large draining vessels, ventricles, and sinuses. Subtracting the four large ventricles in the fifth column from NBT, followed by a one step erosion, results in the draining vessels (DVe) mask shown in the last column. The DVe mask was labeled thus largely because of the mask’s overlap with the vascular sinuses. The erosion operation removed small sulcal CSF and most of the fifth ventricle. Note that this erosion step requires EPI data of a resolution comparable to or higher than that of the data used here. At lower-resolutions, such as 3.5 mm isocubic voxel, little remains of WM or LV voxels after the erosion operation.
Fig. 1.
Anatomically segmented masks for one subjects. The masks (colored voxels) were defined on the T1 images, and then resampled to EPI’s grid space. See the text for the details.
2.2.2. EPI Time-Series Preprocessing
The following procedures were carried out using AFNI’s suite of programs (Cox, 1996). The first 4 volumes of the RS time-series were removed to ensure that all remaining volumes in the time-series were at magnetization steady state. Rigid body registration (3dvolreg) was used to estimate subject movement during RS EPI scans and correct for slice time acquisition (Cox and Jesmanowicz, 1999). At this stage, the truncated and motion-corrected EPI time series are in good alignment with the high-resolution anatomical volume. In all subjects, estimated displacement due to head motion was less than 1 mm between successive time-series volumes.
In the next section, we examine the various sources of signal variations in RS EPI data. The overall data processing procedures are presented in Fig. 2.
Fig. 2.
Diagram for data processing procedures.
2.3. Regression Models in Resting-State Analysis
In one typical correlation-based resting state analysis, a seed time series is chosen from a particular location in the brain, and correlated with the remaining time series in the brain. Correlations between seed and other voxels are used as an indication of the connectedness. However, from the earliest explorations of the approach (Biswal et al., 1995), it has been recognized that noise in RS FMRI time series can covary between distant voxels. Such noise-induced correlations are not reflective of functional brain connectivity and should be minimized before inferences are made about brain connections. Separating noise from signal in RS FMRI is more complicated than in task-based FMRI, where the temporal signature of the task-induced signal is known. With resting state analysis, RS noise sources are of comparable magnitude to the RS signal and we have no a priori temporal signature to help dissociate signal from noise. In practice, noise suppression is carried out in three steps: bandpass filtering, regressing out components of no interest, and spatial smoothing. In this work, we do not bandpass the time series. Our aim is to determine the contributions of various components of the noise to RS FMRI time series. Bandpass filtering would restrict such analysis to a subset of the data’s full bandwidth. In any case, bandpass filtering does not significantly alter our final group connectivity maps. We also avoid any of the typical Gaussian spatial blurring at this initial level of the analysis. Blurring would compound the PVE, mixing noise from WM and other non-GM compartments together with the signal in GM. Instead, we perform a blurring operation within GM only, and within WM only, after all identifiable noise sources are projected out of the EPI time series. We begin by focusing on the second step, where certain components deemed of no interest (i.e., noise) are regressed out of RS FMRI signals prior to correlation analysis. We seek to develop an approach to reduce the contribution of spatially coherent fluctuations that emanate from non-GM sources while minimizing the bias that such reductions can introduce into resultant resting state correlation maps (Murphy et al., 2009).
2.3.1. Global Nuisance Estimates
In this section, we briefly summarize the various models to be analyzed in detail. Each model is given a name for convenient reference. Some motivating details are left to later sections. We begin with a basic regression model for RS FMRI defined as follows:
| (eq. 1; Model RETROTS) |
where, yi is the RS EPI time series N-vector at voxel i. For our data, we use a set of second order Legendre polynomials to fit quadratic temporal trends, and XMO is the N×9 matrix of these polynomials plus six regressors containing rigid-body motion parameter estimates (three translations and three rotations) intended to model any residual effects of movement after registration. XRI is the N×13 matrix of eight slice-wise RETROICOR (Glover et al., 2000; Jones et al., 2008) regressors that model the effect of respiration and heart cycle, and five respiration volume per time (RVT) regressors that model slow blood oxygenation level fluctuations (Birn et al., 2008; Chang et al., 2009). The five RVT regressors are time shifted versions of the RVT function, with delays of 0, 5, 10, 15, and 20 seconds, as proposed by Birn et al. (Birn et al., 2008). RETROICOR and RVT, henceforth referred to as XRI, are generated by AFNI’s “RetroTS.m”, which takes as input cardiac and respiratory time-series sampled at 50 Hz and initially synchronized with the EPI data. In Eq. 1 and subsequent similar equations, is the desired residual signal referring to the RS FMRI signal with unwanted noise components projected out. Another common approach, defined in Eq. 2, and the subject of recent debate, concerns the use an additional nuisance component that consists of the average RS time-series averaged across the whole brain mask (BM) (Desjardins et al., 2001; Fox et al., 1988). This component, termed Global Signal (XGS), is intended to remove brain-wide, and consequently irrelevant, covariance which would include noise.
| (eq. 2) |
where XGS is the N×1 matrix containing the average RS time series over all BM voxels.
Combining both model RETROTS and Eq. 2, we have:
| (eq. 3; Model GS) |
The effect of global signal regression on correlation based analysis is better understood if the signal is broken up in terms of anatomically labeled components. Using the anatomical masks, we break up XGS into three components as shown in Eq. 4:
| (eq. 4; Model 3GS) |
where, XGM, XWM, and XNBT are the N×1 matrices comprising the average signals across GM, WM, and NBT voxels.
To observe the contributions of non-GM signals in each voxel more precisely, NBT should be broken up into four large ventricles and sulcal sinuses. We removed the XGM component from the model for reasons that we will discuss in later sections. In an effort to avoid GM signal contamination by PVE to the signals derived from the other classes, we eroded the non-GM tissue classes before calculating average time series. For the eroded set, the model becomes:
| (eq. 5; Model 5NBT) |
with WMe, and DVe referring to the eroded WM, and draining vessel masks, respectively.
We also removed the XDV component from the model 5NBT for reasons that we will discuss in later sections. However, this omission does not affect the conclusions about regression analyses. We refer to the resulting model in Eq. 6 as the anatomically segmented global signal (ASGS) model. (eq. 6; Model ASGS)
| (eq. 6; Model ASGS) |
2.3.2. Local Nuisance Estimates
Underlying the use of averaged signals to model a noise or source component is the assumption that one source covaries coherently at a spatial scale comparable to that of the mask. To the degree that this assumption is violated, this regression approach can have unintended consequences that we present in the results and discussions sections ahead. In this section, we detail our method for building a local model for estimating nuisance components in a particular region of the brain.
White matter masks have been used to create estimates of noise time series that are then regressed out of RS FMRI time series (Weissenbacher et al., 2009). However, it is possible that some noise is not coherent over the entire span of the WM mask, and an average over the whole mask would fail to capture this noise’s temporal structure. To allow for this possibility, we compute estimates of noise time series from tissue masks restricted to a small neighborhood around the voxel of interest. In this work, a spherical mask of radius 15 mm centered at each GM voxel was used; the average of the WMe voxel time series within that mask was used as a noise component to be filtered out. The radius of the spherical mask should be smaller than the spatial scale of the noise to be removed, and large enough to contain WM voxels wherever the sphere was centered on a GM voxel. Empirically, we found that 15 mm satisfied both criteria. With this approach, a new regression model is needed at each voxel i because at least one noise component is a function of voxel location. The local model, which we term ‘ANATICOR’ for anatomy-based correlation corrections, is defined in Eq. 7, where XWMe is replaced by the average local WM signal for each voxel i (created with AFNI’s 3dLocalStat).
| (eq. 7; Model ANATICOR) |
Voxel-dependent regression modeling of RETROTS, GS, 3GS, 5NBT, ASGS, and ANATICOR were carried out with the program 3dTfitter, which is also used to calculate the marginal explained variations (R2) discussed in the next section. The appellations and attributes of the models used in this work are summarized in Table 1.
Table 1.
The appellations and attributes of the regression models used in this work.
| Equation | Model | Meaning |
|---|---|---|
| Eq. 1 | RETROTS | A model to remove motion and physiological noise by including MO and RI (RETROICOR and RVT) regressors. |
| Eq. 3 | GS |
GS regressor is added to the above model to regress out global signal. |
| Eq. 4 | 3GS | GS regressor is split into three regressors (GM, WM, NBT). |
| Eq. 5 | 5NBT |
NBT is split into five regressors (LVe1..4, and DVe). GM- related signals are avoided by taking GM regressor out of the above model and using eroded tissue masks (WMe, LVe1..4, and DVe). |
| Eq. 6 | ASGS | Anatomically segmented GS model including only non-GM- related regressors (MO, RI, WMe, and LVe1..4). |
| Eq. 7 | ANATICOR | A model to correct anatomy-based unwanted signals with minimizing the effect of globally averaged regressors. WMeLOCAL regressor replaces WMe of the above model. |
2.3.3. Explained Variances of Regression Models
The marginal R2 values for a model component represent the proportion of variance it explains compared to the variance explained by all other remaining components. R2 is defined as:
| (eq. 8) |
where, SStotal is the total sum of squares for all regressors in a model, and SScomponent is the sum of squares for a model with all regressors except those belonging to a certain component, to measure the component's portion of variance explained. To convey how much of the RS signal variance our model components explain, we calculated the mean R2 values in GM voxels across all subjects.
Additionally, we calculated adjusted R2, which adjusts R2 based on the number of degrees of freedom (DOF) “used up” by a component (Montgomery et al., 2006). The adjusted R2 is defined as:
| (eq. 9) |
where, n is the DOF of sample (i.e., n=136 in this work), and p is the number of regressors which are contained in a component (i.e., p=4 for the component LVe1..4). The adjusted R2 is a modification of R2 that adjusts for the number of regressors in a model. Unlike R2, the adjusted R2 increases only if the new regressor improves the model more than would be expected by chance. The adjusted R2 can be negative if little additional variance is explained relative to the cost of degrees of freedom lost.
2.4. Smoothing Effects
RS time series are often smoothed prior to correlation to reduce the effects of noise (the presumption being that the signals of interest are spatially smooth) and improve overlap across subjects at the group level analysis. For approaches where some of the nuisance regressor are derived from the data themselves (as in all but the RETROTS model), we propose that smoothing should be carried out after regressors of no interest are estimated and regressed out of the data. Linear regression in time and smoothing in space would commute if the nuisance regressors are independent of the data, but not necessarily if the regressors are derived from the data. Furthermore, we propose that smoothing be restricted to the brain’s gray matter mask to reduce the inclusion of unwanted BOLD and other physiological signals that will occur if large draining vessels, which tend to course on the outer surface of gray matter, are included in the blurring mask. We carry out two types of blurring, a typical Gaussian blur of 6 mm full width at half maximum (FWHM) over the entire volume (with 3dmerge), and equivalent blurring restricted to each of the gray matter, and non-brain masks (using a finite difference approach, with 3dBlurInMask). With spatially restricted blurring, signal from non-GM tissue does not leak into GM.
2.5. Correlation Analysis On Residual Signals
To compare the results of different regression models, and smoothing approaches, we created resting state correlation maps for each of the individual subjects and for the whole group of subjects. Pearson’s correlation coefficient (ρ-value) maps were created by choosing a seed location and correlating the residual time-series at the seed with the time-series in the rest of the brain. The seed location was chosen to reflect established RS networks such as the Default Mode Network (DMN). The Talairach-Tournoux coordinates of the seed voxel was in posterior cingulate cortex (PCC), which is known as the PCC peak (Greicius et al., 2003). Considering individual variance of PCC peaks, we averaged the time-series of GM voxels using a 5 mm radius spherical mask at the peak voxel in addition to the smoothing of the entire volume carried out earlier. The correlation maps were converted to z-scores by the Fisher tanh−1 transform. The z-score maps of regression models were spatially transformed to the Talrairach N27 brain template (Holmes et al., 1998) via affine transformation matrices estimated by registering T1-images to the N27 template. The seed point for each individual subject were transformed from [2R, 51A, 27S] in Talairach space to individual native spaces. We obtained a group map by performing a one-sample t-test on the z-score maps versus the null hypothesis of zero mean. To highlight particular artifacts in the data, in addition, we perform the same correlation analysis with an additional seed voxel which will be detailed in the Results section.
3. Results
3.1. Explained variances for regressors
3.1.1. Group average R2 maps
Fig. 3 shows group average R2 maps, thresholded at R2 > 0.04, for regression models GS, 3GS, 5NBT, ASGS, and ANATICOR from eq. 3–eq. 7, respectively. These maps show that some components, such as MO (column 1), and RI (column 2), explain variance throughout the brain., while other components, such as GS (4th column, row 1), and NBT (4th column, row 2), model variance in anatomically restricted regions.
Fig. 3.
Group average explained variance (R2) maps, thresholded at R2 > 0.04, for regression models GS, 3GS, 5NBT, ASGS, and ANATICOR from eq.3–eq.7, respectively. The blue tint to the background is just to increase the contrast with the color overlay. Blue arrows indicate the splitting of a mask into subdivisions. See the text for details.
In general, MO regressors (column 1, all rows) explained most of the variance in the RS time series, throughout the brain. MO R2 was largest at the perimeter of the brain where motion tends to have the largest effect on voxel intensity. RI regressors (column 2, all rows) also explain variance throughout the brain; however, R2 values were on average less than half of those of MO. R2 values for the global signal regressor GS are shown in the fourth column of row 1. Note how GS explains signals that are largely confined to the gray matter area and the vascular sinuses. Splitting the brain mask BM by tissue types WM, NBT, and GM (row 2, columns 3, 4, and 5) provides more detail about the contributions to the GS regressor. On average, we found that white matter tissue regressors model little in the data (row 2, column 3). NBT models signal in the sinuses and the midsagittal plane. GM regressors model signal in gray matter, and in the sinuses (but to a lesser degree than NBT). In row 3, we drop the GM regressors, and replace WM with WMe, the regressor from eroded WM. Also, NBT from row 2 is split into components from the eroded four large ventricles and eroded DV. WMe in column 3 explains less variance than before erosion. However, the LVe1..4 component (column 4) of NBT now explains variance throughout most of the brain. DVe regressor (column 5) explains the most variance in the sinuses, and to a lesser degree in peripheral gray matter. In row 4, we further drop regressor DVe, in the absence of which WMe explains some of the variance in the sinuses (column 3). LVe1..4 R2(column 4) remained essentially unchanged. In row 5, locally averaged WM (WMeLOCAL) replaced WMe from row 4. WMeLOCAL explained less of the variance in the midsagittal plane.
3.1.2.R2 values in Gray Matter
Fig. 4 summarizes the proportions of variance, in gray matter voxels only, accounted for by the components of the different models presented above. In addition to average R2, we also show the average adjusted R2 values in the lower half of the graphs. For all models, MO component had the highest R2, while WM-related regressors showed the smallest. RI, and LVe1..4 components had R2 values in between MO and WM. However, when considering adjusted R2, we find that MO ranked highest, followed by GS, GM, DVe, LVe1..4, WM, and RI. The last regressors had negative adjusted R2, suggesting that on average across subjects, and gray matter, the inclusion of this many regressors does not model enough variance to improve statistical power in single subject (or level 1) regression analysis. To examine this more closely, we broke up the RI component in three components of four respiration, four cardiac, and five RVT regressors per the model RETROTS. Panel RETROTS shows that Respiration, and Cardiac regressors had higher R2 than RVT; however, all three components had negative adjusted R2. Splitting GS (Panel GS) into its three tissue-based components: GM has the highest R2, followed by NBT, and WM (Panel 3GS). Removing GM from the model, and splitting NBT into five regressors (four LVes and one DVe, Panel 5NBT), the proportional variances of those regressors increased for both R2 and adjusted R2. Removing regressors from tissue types that can reflect GM signals (i.e. GS, GM, and DVe), we have models ASGS and ANATICOR, which exhibit comparable average R2 and adjusted R2values.
Fig. 4.
The explained variances (R2) of regressors and adjusted R2 for the regression models RETROTS, GS, 3GS, 5NBT, ASGS, and ANATICOR in GM band. The upper and lower groups of bars in each graph are R2 and adjusted R2, respectively. The method appellations are same as Fig. 1 and Fig. 3. The R2 or adjusted R2 values are averaged in GM masks for each subject, and the crosses and error bars are the grand means of the individually averaged R2 or adjusted R2 values of each regressor and their standard deviations, respectively. To compare the proportional explained variances of the regressors with the full regression models, the R2 and R2 adjusted values of full regression models (FULL) are also shown in the graphs. See the text for details.
The mean adjusted R2 values across subjects of those physiological-noise-involved regressors also showed negative values.
3.1.3.R2 maps for individual subjects
R2 maps are presented in Fig. 5 for a representative subject in column Subject 1, and an outlier subject in column Subject 2. For every regressor, the group characteristics shown in Fig. 3 were also reflected in the results of subject 1. For example, the pattern of fit for the GS regressor (row GS) was most similar to that of the GM only regressor (row 3GS), exhibiting the highest fit in gray matter voxels. Also, for all, except the ANATICOR model, at least one of the regressors showed a fit across most gray matter voxels. This was the case even for WMe regressor which still showed higher R2 in peripheral GM, and sinuses, compared to WMeLOCAL. Most intriguing, is the observation that almost none of the white matter regressors fit many white matter voxels, although this was less the case for WMeLOCAL in ANATICOR.
Fig. 5.
The individual explained variance (R2) maps of each regressor for a representative subject in column Subject 1, and an outlier subject in column Subject 2. The blue tint to the background is just to increase the contrast with the color overlay. See the text for details.
In subject 2, the R2 patterns in white matter were different than the norm. In contrast to subject 1, rows 3GS, 5NBT, ASGS, and ANATICOR show a concentration of high R2 values in both white and gray matter tissues in the right middle frontal cortical region. Also in subject 2, WM had higher fit across GM voxels throughout the brain, compared to WMe and WMeLOCAL, which were more restricted to the right middle frontal region.
3.2. Individual connectivity maps of RMFG
3.2.1. WM-related regressors in Subject 2
To further examine the localized high R2 clusters in subject 2, and its effects on correlation maps, we examined the resting state time series and correlation maps computed with a reference time series at a seed within right middle frontal gyrus (RMFG) under the different models considered. The residuals of each regression model were smoothed with a 6 mm isotropic Gaussian kernel, and a seed point was selected at an arbitrary location [35R, 42A, 10S] within RMFG and in the large cluster with high R2 values of Subject 2. The reference time series was the average residual time-series of GM voxels within a spherical mask with radius 5 mm centered at the seed point. We calculate correlation coefficients (ρ-values) between the reference and every voxel’s residual time-series. The correlation maps shown in Fig. 6 were created at a correlation coefficient threshold of | ρ |>0.28 (voxelwise p<0.001, FDR q < 0.05).
Fig. 6.
The connectivity maps of the residual time-series derived from GS, 3GS, 5NBT, ASGS, and ANATICOR. See the text for details.
Time series plots show in gray the time course of EPI data, after motion correction, averaged over a sphere of 5 mm from GM only. For each correlation map, the top graph was obtained with the sphere centered on the RMFG seed, and the bottom graph was obtained with the sphere centered on the PCC seed. Colored time series show the model fit according to the different models in RMFG (red), and PCC (blue). The reference time series, not shown, would be the difference between the gray, and colored time series.
For Subject 1, the correlation maps were quite similar for all the models considered, except for the negative correlations, which were much reduced in in extent with ASGS and ANATICOR. This is expected given that each of models GS, 3GS, and 5NBT contains one or more regressors that are correlated with the average gray matter signal (Murphy et al., 2009). The seed time course graphs show no remarkable traits. Correlation maps in the first row (model GS) show very different patterns between the two subjects. The difference in the correlation maps is greatly reduced in subsequent models. Models 3GS, 5NBT, ASGS, and ANATICOR show improved matching between subjects 1, and 2.
For Subject 2, correlation maps of model GS model showed very different patterns from those of the other models, and for the corresponding model in subject 1. Note the huge cluster in and around RMFG, and which extends medially to posterior locations, and the large clusters with negative correlation coefficients distributed throughout the rest of the brain. For all other models, each of which includes a WM-related regressor, the correlation maps were more similar both within subject 2, and in comparison to maps from corresponding models in subject 1. A closer comparison of corresponding maps between subjects 1 and 2 reveals that the bilateral connectivity pattern in all maps from subject 1 was mostly absent in all but the ANATICOR model of subject 2. Examining the TS plots in subject 2 model GS, we find that a segment (highlighted in green), where the mismatch was greatest between data and model fit when compared to the other models. This observation, coupled with the considerable difference in correlation maps, led us to examine the spatial distribution of these fit differences. We found that the temporal pattern in the highlighted section of the motion corrected TS to be quite uniform over a large spatial extent (see Fig. S1). Such differences, which are expressed in the residual TS, are not remarkable when confined to a few voxels. However, when these differences are coherent over space, as in subject 2, then they will result in extensive areas of high artifactual correlation.
3.2.2. Effect of WM-related regressors
In Fig. 7, we examine the change in R2 over time at the seed location showing the large cluster of high correlation in RMFG in Fig. 6, subject 2. The graphs show in gray are the motion corrected EPI, and in color, the WMe seed regressors for ASGS (blue), and WMeLOCAL for ANATICOR (red). The images below show the R2 maps calculated over a 31 TR (108.5 sec) windows centered at volume numbers 30, 45, 65, 75, 85, 95, and 105. The window size was the smallest with enough DOF for the ASGS and ANATICOR models. The largest increase in R2occurs between the volume numbers 65 and 95, which coincides with the segment highlighted in green on the graphs of Fig. 6. Both ASGS and ANATICOR show strongly time dependent R2maps; however, ANATICOR appears more spatially specific, with a more focal and earlier rise in R2 at the volume number 30 and an earlier decrease at the volume number 95.
Fig. 7.
The temporal changes of explained variance maps (R2>0.3) of WMe and WMeLOCAL of ASGS and ANATICOR, respectively (Subject 2). The R2 maps were blurred by an isotropic Gaussian smoothing kernel (FWHM = 6 mm), and the time series were plotted after remove the quadratic polynomial trends. See the text for details.
3.3. Group results for Default Mode Network
Fig. 8 shows group RS correlation maps obtained under various modeling scenarios and spatial smoothing at the preprocessing stage. The results were thresholded at uncorrected p<0.001 (|t| <4.14, FDR q<0.05). In the left column, the first row shows DMN results with the GS model. Under isotropic smoothing, the network fits the patterns reported in the literature (Raichle and Snyder, 2007). Using the smoothing within tissue type method, the regions are somewhat more circumscribed. The results of 3GS showed the most widespread regions having negative t values. The models GS, 3GS, and 5NBT are similar to each other in their connectivity patterns with PCC and the distribution of negative t values. However, the bilateral patterns of the regions marked inside green squares and circles more clearly appear in ASGS or ANATICOR than the GS, 3GS, and 5NBT.
Fig. 8.
The spatial patterns of Default Mode Network (DMN) for different regression models and smoothing techniques. The results were thresholded at p<0.001 (|t|<4.14, FDR q<0.05). See the text for details.
Using blurring within brain tissue types, the results showed more smaller supra-threshold regions than the results of isotropic Gaussian smoothing. For ASGS and ANATICOR, the draining sinuses correlations were removed by masking NBT regions out of the masks prior to blurring.
4. Discussion
4.1. Heart and Respiration Noise Correction
Several physiological noise correction methods have been used to predict the effect of heart rate and respiration phase on BOLD signal (Birn et al., 2008; Glover et al., 2000; Shmueli et al., 2007). Averaged over gray matter, both RETROICOR and RVT explained little variance, and had negative adjusted R2 values for every regression model (see Fig. 4). This is largely due to the number of regressors involved compared to the number of samples (100 to 200) commonly used in RS experiments. The number of regressors containing physiological signals should be reduced because they do not explain much variance in comparison to their DOFs. Appropriate reduction of the number of regressors can increase R2 and improve the efficiency of the full regression model. For example regressors from LVe1 and LVe2 are highly correlated, as shown in supplemental figure S4, and can be replaced by their average. Despite the low R2 values for RETROICOR and RVT regressors, we do recommend their inclusion in the model. While regressors’ overall adjusted R2 is low, they may still reduce the impact of physiological noise in certain brain areas. RVT regressors would have higher R2 for subjects whose breathing depth and rate varies during the scan. Accounting for physiological noise is of greatest importance when comparing resting state correlations across groups where breathing and heart rhythms can differ. More sophisticated approaches for modeling them and reducing the number of regressors (Beall, 2010) would greatly benefit resting state based analyses.
4.2. Global Signal, Gray Matter, and White Matter regressors
By segmenting high-resolution EPI data into anatomical tissue classes, we were able to examine the amount of variance modeled by a variety of regressors, some of which are commonly used (Greicius et al., 2003; Noonan et al., 2009; Raichle and Snyder, 2007) to remove unwanted components in resting state data. Global signal (GS) regressors, formed by averaging over space all brain tissue classes, explain variance mostly in gray matter (GM) tissue. Separating the GS component into three tissue subclasses, we find that GM regressors capture most of the variance in gray matter, as the GS does. Non Brain Tissue (NBT) regressors modeled variance mostly in NBT tissue. Most notably however, in the absence of hardware-induced artifacts, White Matter (WM) regressors explained little marginal variance whether in white or gray matter voxels. In the absence of NBT and GM regressors, WM regressors can account for gray matter variance, but mostly because of the partial volume effect with GM (Fig. S2, row 1). Regressors from eroded white matter capture much less variance in GM tissue (Fig. S2, row 2). Taken together, these results indicate that the majority of spatial coherence originates in gray matter tissue, and that signals in WM tissue, eroded to minimize PVE with GM, shows little correlation across the brain. Conversely, regressors formed from either GS, GM, or WM with GM partial voluming, produce similar correlation maps (Weissenbacher et al., 2009). Therefore, retaining such regressors would produce the correlation bias described by Murphy et al (Murphy et al., 2009). To use WM for capturing components of no interest, while avoiding GM signal, it is necessary to have accurate segmentation models, and erode WM to avoid GM PVE.
4.3. Hardware-Related Artifacts
In some of our datasets, we found synchronous shifts in signal intensity that were localized to a portion of the brain. This artifact does not result in visible degradation in any single image’s quality. It is only evident once time series from a particular neighborhood are examined simultaneously (see Fig. S1). Two aspects of this artifact led us to suspect that it may be caused by defects in the coil array or the RF amplifiers powering it: 1- Its spatial extent suggests a local coil’s sensitivity profile in a multi-channel array. 2- The intermittent intensity shifts (see Fig. 7) are common across tissue types and span multiple slices. Small variations in RF reception in a subset of a coil array would result in image intensity shifts in those parts of the brain most coupled to the affected array components (see Fig. 5 and Fig. 6). These intensity shifts introduce artifactual correlations in RS time series on the spatial scale of the coils’ sensitivity profile, and can markedly bias RS connectivity results (see the negative correlations of Subject 2 in Fig. 6). This issue will become more and more salient as RF coils arrays (transmit and receive) become more complex. Since coil array positioning is likely to be similar across subjects, the artifactual correlations can appear to be significant across subjects, even if the total amount of variance across the whole brain attributed to the artifact is small.
However, because these artifacts are uniform across tissue types (see Fig. S1), they can be modeled from eroded non-gray matter masks, and regressed out of the RS time series without biasing correlations in gray matter. We only used eroded WM to model them because little remains of non-white matter tissue types after the erosion step. In our data, both the WMe and WMeLOCAL approaches successfully reduced this artifact in gray matter, with the local approach appearing to better recover contralateral correlations. Additionally, the local approach including WMeLOCAL is expected to outperform WMe when multiple coils exhibit independent artifacts in their sensitivity zones.
4.4. Smoothing and Draining Vessels
RS signal in sinuses, and presumably in large draining vessels, is correlated with signal in gray matter tissue (see Fig. 3). Regressing it out of the data would be comparable to regressing out GM signal, and therefore bias the correlation maps. Isotropic Gaussian smoothing over the entire brain can introduce artificial activations between remote areas in brain (Jo et al., 2008; Jo et al., 2007) because of signal blending from draining vessels with nearby underlying tissue (see Fig. 8, column “Isotropic Gaussian Smoothing”). This problem can be reduced by masking DV and blurring the RS data within remaining tissue types (see Fig. 8, column “Mask Blurring within Brain Tissue”).
On the topic of smoothing, it is important to note again that spatial smoothing should be performed on the residuals of resting state data, only after regressors of no interest have been formed and regressed out of the original resting state time series.
4.5. Group effect of the Hardware-Induced Artifact
We perform an additional computation to investigate whether data with coil artifacts can be safely used after correction by ANATICOR and GS, or if these subjects should be excluded from the group analysis (see Fig. S3). Both ANATICOR and GS seem to successfully reduce the difference between two groups with or without the artifact being present. For GS, however, the pattern of the group difference is spatially biased to the region where the coil artifacts occurred. For ANATICOR, the spatial pattern of the group difference is more randomized and the biased pattern in the artifactual region is absent. Therefore, the data as corrected by ANATICOR can be included in group analysis.
5. Conclusion
We found most RS correlation in the brain to be positive, and largely restricted to gray matter and venous vasculature draining it. To the extent that signal correlation exists between other tissue types and gray matter, it appears due to PVE or hardware artifacts. In other terms, a regressor formed from a non-eroded white matter mask models largely the same variance as a regressor from gray matter, or a global signal regressor. We identified transient hardware artifacts specific to multi-channel coil arrays that can introduce strong correlation over portions of the volume.
We also found that the techniques currently used for modeling physiological noise (RETROICOR and RVT) are generally weak. We do not claim that physiological noise is unimportant or unremovable, but we do believe that more work is needed to parsimoniously model such effects.
We present two approaches (ASGS, and ANATICOR) that can model and remove components of no interest from RS time series, without introducing correlation bias in the resultant maps. ANATICOR was found to be superior because it can more effectively model local transient hardware artifacts. In addition, restricting spatial smoothing to occur only within tissue class is important, to avoid spreading noise sources from non-GM to GM voxels. Similarly, using eroded white matter and ventricle masks in ANATICOR is important to avoid introducing gray matter signals into the regressors of no interest.
Interactive tools for generating seed-based correlation maps at the single-subject and group levels are currently available within AFNI (http://afni.nimh.nih.gov), in addition to implementations of the processing methods described above.
Supplementary Material
Acknowledgment
The authors appreciate Marta Bianciardi and Jongho Lee for useful discussions about hardware-related and physiological noises, and Catie E. Chang and Gary H. Glover for code and assistance with RETROICOR. We especially wish to acknowledge the continuing encouragement and aid from Alex Martin. This research was supported by the NIMH and NINDS Intramural Research Programs of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Argall BD, Saad ZS, Beauchamp MS. Simplified intersubject averaging on the cortical surface using SUMA. Hum Brain Mapp. 2006;27:14–27. doi: 10.1002/hbm.20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall EB. Adaptive cyclic physiologic noise modeling and correction in functional MRI. J Neurosci Methods. 2010;187:216–228. doi: 10.1016/j.jneumeth.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Birn RM, Smith MA, Jones TB, Bandettini PA. The respiration response function: the temporal dynamics of fMRI signal fluctuations related to changes in respiration. Neuroimage. 2008;40:644–654. doi: 10.1016/j.neuroimage.2007.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Chang C, Cunningham JP, Glover GH. Influence of heart rate on the BOLD signal: the cardiac response function. Neuroimage. 2009;44:857–869. doi: 10.1016/j.neuroimage.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Desjardins AE, Kiehl KA, Liddle PF. Removal of confounding effects of global signal in functional MRI analyses. Neuroimage. 2001;13:751–758. doi: 10.1006/nimg.2000.0719. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fox PT, Mintun MA, Reiman EM, Raichle ME. Enhanced detection of focal brain responses using intersubject averaging and change-distribution analysis of subtracted PET images. J Cereb Blood Flow Metab. 1988;8:642–653. doi: 10.1038/jcbfm.1988.111. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Jo HJ, Lee JM, Kim JH, Choi CH, Gu BM, Kang DH, Ku J, Kwon JS, Kim SI. Artificial shifting of fMRI activation localized by volume- and surface-based analyses. Neuroimage. 2008;40:1077–1089. doi: 10.1016/j.neuroimage.2007.12.036. [DOI] [PubMed] [Google Scholar]
- Jo HJ, Lee JM, Kim JH, Shin YW, Kim IY, Kwon JS, Kim SI. Spatial accuracy of fMRI activation influenced by volume- and surface-based spatial smoothing techniques. Neuroimage. 2007;34:550–564. doi: 10.1016/j.neuroimage.2006.09.047. [DOI] [PubMed] [Google Scholar]
- Jones TB, Bandettini PA, Birn RM. Integration of motion correction and physiological noise regression in fMRI. Neuroimage. 2008;42:582–590. doi: 10.1016/j.neuroimage.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee JM, Jo HJ, Kim SH, Lee JH, Kim ST, Seo SW, Cox RW, Na DL, Kim SI, Saad ZS. Defining functional SMA and pre-SMA subregions in human MFC using resting state fMRI: functional connectivity-based parcellation method. Neuroimage. 2010;49:2375–2386. doi: 10.1016/j.neuroimage.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TE, Madsen KH, Sidaros K, Luo WL, Nichols TE. Non-white noise in fMRI: does modelling have an impact? Neuroimage. 2006;29:54–66. doi: 10.1016/j.neuroimage.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Mezer A, Yovel Y, Pasternak O, Gorfine T, Assaf Y. Cluster analysis of resting-state fMRI time series. Neuroimage. 2009;45:1117–1125. doi: 10.1016/j.neuroimage.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Montgomery DC, Peck EA, Vining GG. Introduction to linear regression analysis. 4th ed. Hoboken N.J.: Wiley-Interscience; 2006. [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nencka AS, Rowe DB. Reducing the unwanted draining vein BOLD contribution in fMRI with statistical post-processing methods. Neuroimage. 2007;37:177–188. doi: 10.1016/j.neuroimage.2007.03.075. [DOI] [PubMed] [Google Scholar]
- Noonan SK, Haist F, Muller RA. Aberrant functional connectivity in autism: evidence from low-frequency BOLD signal fluctuations. Brain Res. 2009;1262:48–63. doi: 10.1016/j.brainres.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–1089. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage. 2009;44:839–848. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZSRRC, Argall B, Japee S, Cox RW. SUMA: An interface for surface-based intra- and inter-subject analysis with AFNI. Biomedical Imaging: Macro to Nano, 2004. IEEE International Symposium on. 2004:1510–1513. [Google Scholar]
- Shmueli K, van Gelderen P, de Zwart JA, Horovitz SG, Fukunaga M, Jansma JM, Duyn JH. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. Neuroimage. 2007;38:306–320. doi: 10.1016/j.neuroimage.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Wise RG, Ide K, Poulin MJ, Tracey I. Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage. 2004;21:1652–1664. doi: 10.1016/j.neuroimage.2003.11.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.