Abstract
Acute exposure to ionizing radiation can cause lethal damage to the gastrointestinal (GI) tract, a condition called the GI syndrome. Whether the target cells mediating the GI syndrome are derived from the epithelium or endothelium, and whether the target cells die by apoptosis or other mechanisms, are controversial issues. Studying mouse models, we found that selective deletion of the pro-apoptotic genes Bak1 and Bax from the GI epithelium or from endothelial cells did not protect mice from developing the GI syndrome after subtotal body gamma irradiation. In contrast, selective deletion of p53 from the GI epithelium, but not endothelial cells, sensitized irradiated mice to the GI syndrome. Transgenic mice overexpressing p53 in all tissues were protected from the GI syndrome after irradiation. These results suggest that the GI syndrome is caused by death of GI epithelial cells by a mechanism that is regulated by p53 but independent of apoptosis.
Concern over the potential exposure of civilians to radiation has increased interest in understanding the mechanisms underlying radiation injury. Acute Radiation Syndrome (also called “radiation sickness”) occurs when the body is exposed to a high dose of penetrating radiation in a short period of time. The constellation of symptoms that define Acute Radiation Syndrome include effects related to bone marrow toxicity (hematopoietic syndrome) and gastrointestinal toxicity (GI syndrome). The hematopoietic syndrome can occur after whole-body irradiation (WBI) at a dose of 6 Gy. The resultant reduction in lymphocytes and platelets leads to infection or hemorrhage, respectively, and death can occur within 30 days of exposure (1). In certain situations, death from the hematopoietic syndrome can be prevented (e.g., by shielding part of the bone marrow or by a subsequent bone marrow transplant) (2-4). At higher doses of radiation, death occurs within 10 days from damage to the GI tract (3, 4). In contrast to the hematopoietic syndrome, no medical countermeasures are approved to prevent or treat the GI syndrome.
Despite decades of study, the critical cellular targets of radiation-induced toxicity in the GI tract and the molecular mechanism of cell death underlying the GI syndrome remain controversial (5). For example, the role of p53 in the GI syndrome is unclear. Radiation activates p53 in the GI epithelium (6), which induces PUMA to kill cells via the intrinsic pathway of apoptosis in 4 hours (7-9). Deletion of either p53 or PUMA blocks radiation-induced apoptosis of most intestinal epithelial cells. Because death after WBI is delayed in mice lacking PUMA, p53-mediated apoptosis has been implicated in regulating the GI syndrome (9) . Other research groups have reported that the absence of p53 does not alter the GI syndrome and proposed that it is instead regulated by radiation-induced apoptosis of endothelial cells (10). Although radiation-induced endothelial cell apoptosis can proceed in the absence of p53 or PUMA (9, 11), the intrinsic pathway is still believed to be involved because Bax−/− and Bak1−/− mice have reduced endothelial cell apoptosis following irradiation (12, 13).
Others have implicated p53 in protecting mice from the GI syndrome (14). Although radiation-induced apoptosis is suppressed in p53 null mice (6), other modes of cell death, such as mitotic catastrophe, can still occur (15). For example, 24 hours after radiation at doses of 8 Gy or more, reproductive or clonogenic cell death (hereafter referred to as “mitotic death”), occurs in the small intestine (16). The molecular mechanism of this form of death remains unclear, but at this dose of radiation, a cell cycle arrest is followed by proliferation where some cells may undergo an aberrant mitosis and die by mitotic catastrophe (15, 17, 18).
To investigate the cellular target and the molecular mechanism of cell death in the acute radiation syndrome, we have utilized the Cre-loxP system (19). Mice in which Cre is expressed under the control of a tissue-specific promoter can selectively delete DNA flanked by loxP sites, which is termed a floxed (FL) allele, in a cell type-specific manner. Therefore, we utilized mice (VillinCre) that express Cre specifically throughout the GI epithelium under the control of the villin promoter (20) and mice (Tie2Cre) in which Cre is expressed specifically in endothelial cells and the hematopoietic system under the control of the Tie2 promoter (21). To investigate the role of the intrinsic pathway of apoptosis in the acute radiation syndrome, VillinCre or Tie2Cre mice were crossed with mice carrying floxed alleles for Bax (22). In some settings, BAK can mediate BAX-independent apoptosis via the intrinsic pathway (23). Therefore, experiments were carried out on a Bak1 null genetic background (24).
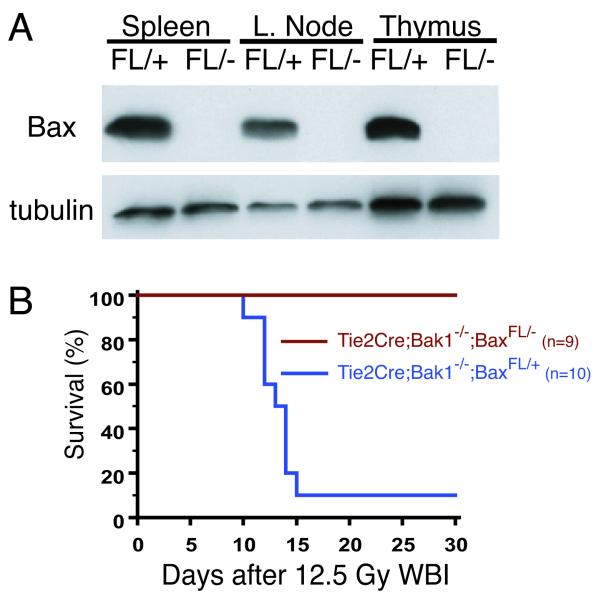
To determine if Bax was deleted in the hematopoietic system in Tie2Cre; Bak1−/−; BaxFL/− mice, we subjected cell lysates from the spleen, thymus, and lymph nodes to Western blot analysis. In these tissues, the levels of BAX were significantly reduced when compared to Tie2Cre; Bak1−/−; BaxFL/+ littermate controls (Fig. 1A). Deleting the intrinsic pathway of apoptosis from the hematopoietic system in Tie2Cre; Bak1−/−; BaxFL/− mice protected the bone marrow (fig. S1) and improved survival of the mice after exposure to 12.5 Gy WBI (Fig. 1B). Therefore, the intrinsic pathway of apoptosis is required for death from the hematopoietic syndrome. Because Cre is also expressed in endothelial cells in Tie2Cre mice, it is possible that protection of the vascular niche (25) within the bone marrow contributed to increased survival from WBI.
Fig. 1.
Deletion of the intrinsic pathway of apoptosis in the hematopoietic system protects mice from death after whole-body gamma irradiation (WBI). A. Western blot for BAX and tubulin in spleen, lymph node, and thymus from Tie2Cre; Bak1−/−; BaxFL/− mice (FL/−) demonstrates deletion of Bax in the hematopoietic system. Tie2Cre; Bak1−/−; BaxFL/+ mice (FL/+) retain Bax and serve as a control. B. Kaplan-Meier survival analysis of Tie2Cre; Bak1−/−; BaxFL/+ mice and Tie2Cre; Bak1−/−; BaxFL/− litter mates after 12.5 Gy WBI. By log-rank comparison, p = 0.0001.
We next examined the role of the intrinsic pathway of apoptosis in the GI syndrome. For these experiments, the bone marrow of the front legs was shielded for subtotal-body irradiation (SBI) to avoid the hematopoietic syndrome (fig. S2). Initial experiments were performed with mice in which either Bak1 or Bax was deleted. No difference in survival from the GI syndrome was observed for Bak1−/− mice vs. Bak1+/+ littermate controls or between Bax−/− mice vs. Bax+/+ littermate controls after SBI (fig. S3). Because Bak1−/−; Bax−/− mice are generally not viable (23), we studied mice in which Bak1 and Bax were deleted from either endothelial cells or GI epithelial cells.
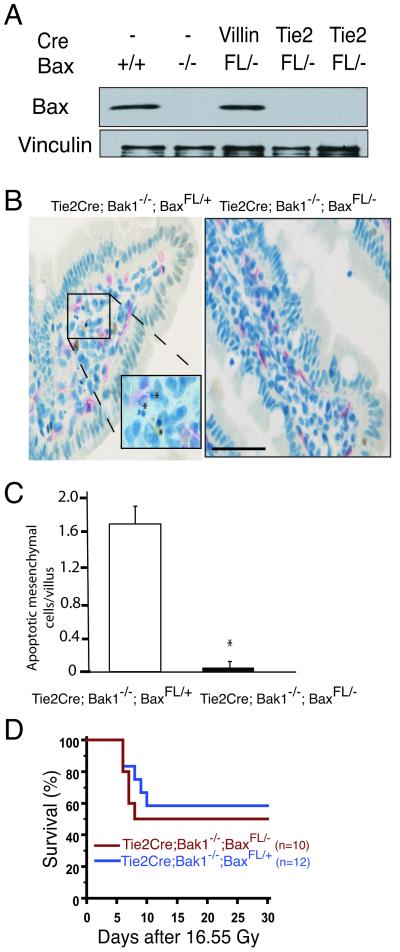
To determine whether Bax was deleted from endothelial cells in Tie2Cre; Bak1−/−; BaxFL/− mice, endothelial cells were isolated from lungs by fluorescent activated cell sorting (FACS). PECAM+, SCA-1+ cells were collected (24) and analyzed by Western blot for BAX (Fig. 2A). In these cells from Tie2Cre; Bak1−/−; BaxFL/− mice, BAX was not detected. Within the villi, apoptosis of mesenchymal cells, which includes endothelial cells and lymphocytes, occurs 4 hours after doses of radiation that cause the GI syndrome (10, 26). Therefore, we harvested the small intestine of mice 4 hours after 16.95 Gy SBI and assessed mesenchymal cell apoptosis within the villi by TUNEL staining. Deleting the intrinsic pathway of apoptosis in Tie2Cre; Bak1−/−; BaxFL/− mice significantly reduced mesenchymal cell apoptosis compared to Tie2Cre; Bak−/−; BaxFL/+ mice that retained a wild-type Bax allele (Fig. 2 B and C), but did not improve survival from the GI syndrome after SBI (Fig. 2D and fig. S4). These results suggest that mesenchymal cell apoptosis within the intervillus region of the intestine does not initiate the GI syndrome.
Fig. 2.
Deletion of the intrinsic pathway of apoptosis from endothelial cells does not protect mice from subtotal-body gamma irradiation (SBI). A. Western blot for BAX and Vinculin from lung endothelial cells isolated from mice that contained no Cre (−), VillinCre (Villin), or Tie2Cre (Tie2) and the indicated Bax genotype. B. Mesenchymal cell (endothelial cell and lymphocyte) apoptosis is decreased in Tie2Cre; Bak1−/−; BaxFL/− mice. TUNEL (brown) and MECA-32 (pink) immunohistochemistry identify apoptotic mesenchymal cells within the villi of Tie2Cre; Bak1−/−; BaxFL/+ mice. Scale bar, 100 μm. C. Quantification of mesenchymal cell apoptosis in the villi of the distal small intestine. The mean number of mesenchymal cells undergoing apoptosis in a total of at least 87 villi from 3 mice is shown; *p < 0.01 by two-tailed t test. Error bars indicate SEM. D. Kaplan-Meier survival analysis of Tie2Cre; Bak1−/−; BaxFL/+ mice and Tie2Cre; Bak1−/−; BaxFL/− litter mates after 16.55 Gy SBI. By log-rank comparison, p =NS.
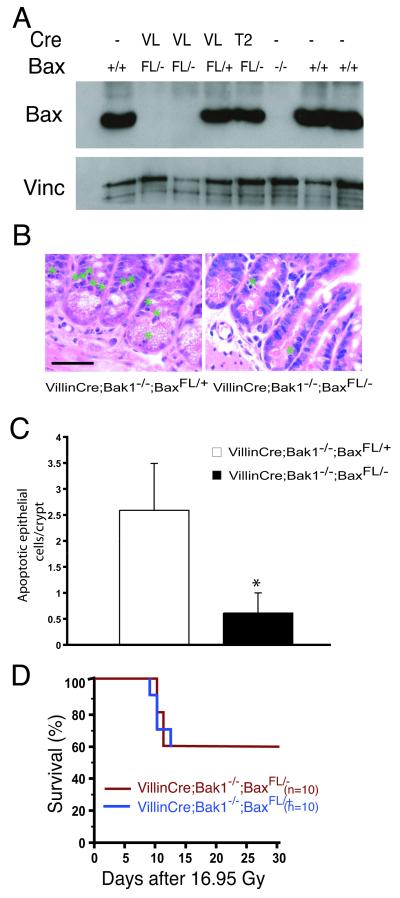
We also evaluated the role of the intrinsic pathway of apoptosis in mediating the GI syndrome in GI epithelial cells. To determine whether BAX was deleted in the GI epithelium of VillinCre; Bak1−/−; BaxFL/− mice, we isolated epithelial cells from the small intestine and analyzed them for BAX expression by Western blot (24). In VillinCre; Bak1−/−; BaxFL/− mice, BAX was deleted from the GI epithelium (Fig. 3A). Although deleting the intrinsic pathway of apoptosis in GI epithelium reduced apoptosis in the crypt epithelium 5-fold after irradiation (Fig. 3 B and C), this did not improve survival after SBI (Fig. 3D and fig. S5). Taken together, these results suggest that survival from the GI syndrome is not determined by apoptosis of either endothelial cells or GI epithelial cells.
Fig. 3.
Deletion of the intrinsic pathway of apoptosis from GI epithelial cells does not protect mice from subtotal-body gamma irradiation (SBI). A. Western blot for BAX and Vinculin from GI epithelial cells isolated from mice without Cre (−), with VillinCre (VL), or Tie2Cre (T2), and the indicated Bax genotype. B. Hematoxylin and eosin stained sections of distal small intestine 4 hours after 16.95 Gy SBI demonstrates suppression of apoptosis within the crypt epithelium in VillinCre; Bak1−/−; BaxFL/− mice. A green asterisk labels cells with characteristic apoptotic morphology. Scale bar, 100 μm. C. Quantification of GI epithelial cell apoptosis in the crypts of the distal small intestine. The mean number of apoptotic cells in a total of at least 100 crypts in 3 mice is shown. Error bars indicate SEM. *p < 0.01, by two-tailed t test. Similar results were observed in the proximal small intestine. D. Kaplan-Meier survival analysis of VillinCre; Bak1−/−; BaxFL/+ and VillinCre; Bak1−/−; BaxFL/− mice after 16.95 Gy SBI. By log-rank comparison, p = NS.
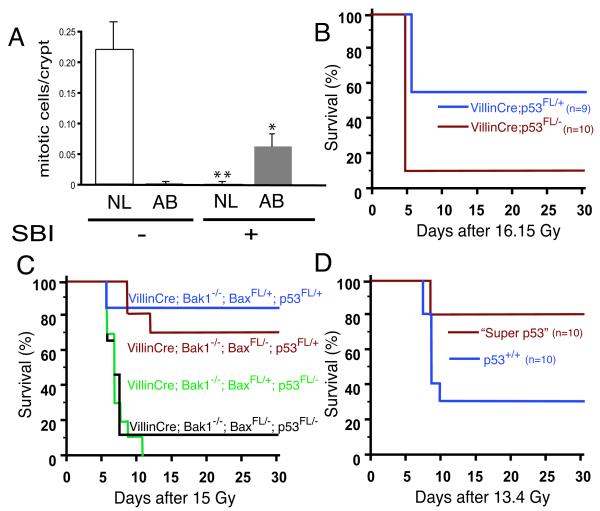
At radiation doses that can cause the GI syndrome, mitotic death occurs within the small intestine (16). Although the molecular mechanism of this type of cell death is not completely understood, it may involve progression through an aberrant mitosis with damaged DNA (27). Therefore, we looked for evidence of aberrant mitosis in the crypts of the small intestine after irradiation. In unirradiated mice, almost all cells undergoing mitosis showed chromosomes that aligned symmetrically (Fig 4A and fig. S6). 24 hours after 13.4 Gy SBI, most of the mitotic cells in the crypts were aberrant because chromosomes aligned in an asymmetrical fashion with lagging chromosomes or in micronuclei (Fig 4A and fig. S6). Because p53 is required in the small intestine for optimal cell cycle arrest after radiation to prevent inappropriate entry into mitosis (18), we generated mice in which p53 was deleted in the GI epithelium (VillinCre; p53FL/−) to investigate whether cell cycle checkpoints in GI epithelial cells regulate the GI syndrome. Indeed, the VillinCre; p53FL/− mice were more sensitive to SBI than VillinCre; p53FL/+ littermates (Fig. 4B), even though we did not observe a difference in surviving crypts at 90 hours after SBI (fig. S7). These results point to cells within the GI crypt epithelium as the primary cellular target of radiation mediating the GI syndrome. Moreover, as reported by others (14), we also found mice lacking the cyclin-dependent kinase inhibitor p21 to be sensitive to the GI syndrome (fig. S8). To investigate whether deletion of the intrinsic pathway of apoptosis could suppress sensitivity of the GI syndrome that results from loss of p53, we generated mice (VillinCre; p53FL/−; Bak1−/−; BaxFL/−) in which p53 and the intrinsic pathway of apoptosis were deleted from GI epithelial cells (24). Despite deletion of Bak1 and Bax, mice remained sensitive to the GI syndrome when p53 was deleted in GI epithelial cells (Fig 4C).
Fig. 4.
Regulation of the GI syndrome by p53 in epithelial cells is independent of apoptosis. A. Quantification of normal (NL) and aberrant (AB) mitoses in GI crypt epithelial cells of the proximal and distal small intestine from wild-type mice without (−) or 24 hours after (+) 13.4 Gy SBI with ionizing radiation. The mean number of mitoses per crypt in a total of at least 115 crypts from 6 mice is shown. Error bars indicate SEM. *p < 0.04 for aberrant mitoses, two-sided t test and **p < 0.005 for normal mitoses, two-sided t test. B. Kaplan-Meier survival analysis of VillinCre; p53FL/+ and VillinCre; p53FL/− mice after 16.15 Gy SBI with gamma irradiation. p = 0.0015 by log-rank test. C. Kaplan-Meier survival analysis of at least 9 mice of each of the indicated genotype after 15 Gy SBI with gamma irradiation. p = 0.0001 between p53FL/− and p53FL/+ mice and p = NS between BaxFL/− and BaxFL/+ mice by log-rank test. D. Kaplan-Meier survival analysis of “super p53” tgb mice and C57/BL6 p53+/+ littermate controls after 13.4 Gy SBI with ionizing radiation. p = 0.02 by log-rank test.
These results are consistent with a model where progression through p53-regulated cell cycle checkpoints after radiation leads to reproductive or mitotic death of GI epithelial cells independent of apoptosis to cause the GI syndrome (15, 16, 18). To test this model in a gain-of-function experiment, we studied “super p53” tgb mice, which contain two extra copies of the wild-type p53 gene (28). Following irradiation, “super p53” mice exhibit an enhanced DNA damage response with elevated levels of p53-dependent apoptosis and increased induction of the cyclin-dependent kinase inhibitor, p21 (28). Consistent with a model where p53-dependent cell cycle arrest regulates the GI syndrome, we observed that p53 tgb mice are protected from the GI syndrome after SBI (Fig 4D).
We also generated mice in which p53 was deleted from endothelial cells. These Tie2Cre; p53FL/− mice were not more sensitive to the acute GI syndrome after SBI (fig. S9). Surprisingly, all of these mice died from delayed radiation effects within 2 months (fig. S9). Therefore, p53 may not only protect the GI epithelium from acute injury (14), but p53 may also protect endothelial cells from late effects from radiation.
These results have potential implications for efforts to develop medical countermeasures against radiation. Drugs that block the intrinsic pathway of apoptosis may be effective for the hematopoietic syndrome, but not the GI syndrome. Indeed, the p53 inhibitor, pifithrin-α, rescues mice from the lethal effects of the hematopoietic syndrome, but does not protect mice from the GI syndrome (14). Because complete inhibition of p53 may promote the GI syndrome (Fig. 4B), drugs for the acute radiation syndrome that block apoptosis should not compromise p53 function (29) .
This study may also have important implications for cancer therapy. Inhibitors of p53 function have been proposed as a way to limit the acute toxicity of radiation therapy and increase tumor response without increasing the risk of radiation-induced cancers (30-32). For many patients, acute toxicity is not dose limiting in the clinic. Instead, the risk of late effects from radiation therapy, which can be secondary to vascular injury (33), frequently limits the radiation dose that can safely be delivered to treat a cancer. Our finding that deleting p53 from endothelial cells sensitizes mice to late effects of radiation (fig. S9) raises the possibility of increased late effects when p53 inhibitors are combined with radiation therapy.
Supplementary Material
Acknowledgments
34. We thank M. Serrano for the “super p53” mice; A.Berns for the p53FL mice; P. Crawford and J. Gordon for advice; E. Travis for providing a model jig; J. Down, C. Pope, and K. Doppke for performing dosimetry measurements; and K. Held for critically reading the manuscript. This work is funded by the Howard Hughes Medical Institute (TJ), partially by Cancer Center Support (core) grant P30-CA14051 from the NCI (TJ), NCI grant KO8 CA 114176 (DGK), pilot project from the Dana Farber Cancer Center for Medical Countermeasures Against Radiation U19-AI06775 (DGK), RC1-AI078521 (DGK), the American Society of Clinical Oncology Young Investigator Award (DGK), and NCI grant P01 CA80124 (RKJ). TJ is the David H. Koch Professor of Biology and a Daniel K. Ludwig Scholar.
References and Notes
- 1.Mettler FA, Voelz GL. N. Engl. J. Med. 2002;346:1554. doi: 10.1056/NEJMra000365. [DOI] [PubMed] [Google Scholar]
- 2.van Bekkum DW, Schotman E. Int. J. Radiat. Biol. 1974;25:361. doi: 10.1080/09553007414550431. [DOI] [PubMed] [Google Scholar]
- 3.Terry NHA, Travis EL. Int. J. Radiat. Onc. Biol. Phys. 1989;17:569. doi: 10.1016/0360-3016(89)90108-9. [DOI] [PubMed] [Google Scholar]
- 4.Mason KA, Withers HR, McBride WH, Davis CA, Smathers JB. Radiation Res. 1989;117:480. [PubMed] [Google Scholar]
- 5.Brown JM. Int. J. Radiat. Onc. Bio. Phys. 2008;70:799. [Google Scholar]
- 6.Merritt AJ, et al. Cancer Res. 1994;54:614. [PubMed] [Google Scholar]
- 7.Potten CS. Nature. 1977;269:518. doi: 10.1038/269518a0. [DOI] [PubMed] [Google Scholar]
- 8.Jeffers JR, et al. Cancer Cell. 2003;4:321. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 9.Qiu W, et al. Cell Stem Cell. 2008;2:576. doi: 10.1016/j.stem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paris F, et al. Science. 2001;293:293. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 11.Santana P, et al. Cell. 1996;86:189. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Barros M, et al. Science. 2003;300:1155. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 13.Rotolo J, et al. Int. J. Radiat. Onc. Bio. Phys. 2008;70:804. doi: 10.1016/j.ijrobp.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 14.Komarova EA, et al. Oncogene. 2004;23:3265. doi: 10.1038/sj.onc.1207494. [DOI] [PubMed] [Google Scholar]
- 15.Merritt AJ, Allen TD, Potten CS, Hickman JA. Oncogene. 1997;14:2759. doi: 10.1038/sj.onc.1201126. [DOI] [PubMed] [Google Scholar]
- 16.Hendry JH, Potten CS. Int. J. Radiat. Biol. 1982;42:621. doi: 10.1080/09553008214551601. [DOI] [PubMed] [Google Scholar]
- 17.Lesher J, Lesher S. Radiation Res. 1974;57:148. [PubMed] [Google Scholar]
- 18.Potten CS, Owen G, Roberts SA. Int. J. Radiat. Biol. 1990;57:185. doi: 10.1080/09553009014550431. [DOI] [PubMed] [Google Scholar]
- 19.Branda CS, Dymecki SM. Dev. Cell. 2004;6:7. doi: 10.1016/s1534-5807(03)00399-x. [DOI] [PubMed] [Google Scholar]
- 20.El Marjou F, et al. Genesis. 2004;39:186. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 21.Koni PA, et al. J. Exp. Med. 2001;193:741. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeuchi O, et al. Proc. Natl. Acad. Sci. U.S.A. 2005;102:11272. doi: 10.1073/pnas.0504783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindsten T, et al. Mol. Cell. 2000;6:1389. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Table describing genotypes used in this study, methods, and text are in Supporting Online Material
- 25.Salter AB, et al. Blood. 2009;113:2104. doi: 10.1182/blood-2008-06-162941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crawford PA, Gordon JI. PNAS. 2005;102:13254. doi: 10.1073/pnas.0504830102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroemer G, et al. Cell Death Differ. 2005;12:1463. doi: 10.1038/sj.cdd.4401724. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Cao I, et al. EMBO J. 2002;21:6225. doi: 10.1093/emboj/cdf595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burdelya LG, et al. Science. 2008;320:226. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komarov PG, et al. Science. 1999;285:1733. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- 31.Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI. Nature. 2006;443:214. doi: 10.1038/nature05077. [DOI] [PubMed] [Google Scholar]
- 32.Burdelya LG, et al. Cancer Res. 2006;66:9356. doi: 10.1158/0008-5472.CAN-06-1223. [DOI] [PubMed] [Google Scholar]
- 33.Coderre JA, et al. Radiation Res. 2006;166:495. doi: 10.1667/RR3597.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.