Abstract
Context
Emerging data suggest that psychological and experiential factors are associated with risk of Alzheimer disease (AD), but the association of purpose in life with incident AD is unknown.
Objective
To test the hypothesis that greater purpose in life is associated with a reduced risk of AD.
Design
Prospective, longitudinal epidemiologic study of aging.
Setting
Senior housing facilities and residences across the greater Chicago metropolitan area.
Participants
More than 900 community-dwelling older persons without dementia from the Rush Memory and Aging Project.
Main Outcome Measures
Participants underwent baseline evaluations of purpose in life and up to 7 years of detailed annual follow-up clinical evaluations to document incident AD. In subsequent analyses, we examined the association of purpose in life with the precursor to AD, mild cognitive impairment (MCI), and the rate of change in cognitive function.
Results
During up to 7 years of follow-up (mean, 4.0 years), 155 of 951 persons (16.3%) developed AD. In a proportional hazards model adjusted for age, sex, and education, greater purpose in life was associated with a substantially reduced risk of AD (hazard ratio, 0.48; 95% confidence interval, 0.33-0.69; P< .001). Thus, a person with a high score on the purpose in life measure (score=4.2, 90th percentile) was approximately 2.4 times more likely to remain free of AD than was a person with a low score (score=3.0, 10th percentile). This association did not vary along demographic lines and persisted after the addition of terms for depressive symptoms, neuroticism, social network size, and number of chronic medical conditions. In subsequent models, purpose in life also was associated with a reduced risk of MCI (hazard ratio, 0.71; 95% confidence interval, 0.53-0.95; P=.02) and a slower rate of cognitive decline (mean [SE] global cognition estimate, 0.03 [0.01], P<.01).
Conclusion
Greater purpose in life is associated with a reduced risk of AD and MCI in community-dwelling older persons.
Alzheimer disease (AD) IS one of the most dreaded consequences of aging, and the identification of modifiable factors associated with the risk of AD is a top public health priority for the 21st century, particularly given the large and rapidly increasing aging population. Although relatively few such risk factors have been identified, emerging data suggest that a variety of potentially modifiable psychological factors (eg, conscientiousness, extraversion, and neuroticism) and experiential factors (eg, social networks) are associated with risk of AD.1-4 Purpose in life, the psychological tendency to derive meaning from life's experiences and to possess a sense of intentionality and goal directedness that guides behavior, has long been hypothesized to protect against adverse health outcomes.5-9 Indeed, purpose in life has been linked to positive outcomes, including better mental health and happiness, and it was recently reported that purpose in life is associated with longevity.9-14 However, the association of purpose in life with the risk of AD remains unknown.
In this study, we tested the hypothesis that greater purpose in life is associated with a reduced risk of incident AD using data from more than 900 participants in a large community-based epidemiologic study of aging, the Rush Memory and Aging Project.15 In subsequent analyses, we examined whether these associations persisted after adjustment for several potential confounders. Next, we examined the association of purpose in life with the development of mild cognitive impairment (MCI), the precursor to AD. Finally, we examined the association of purpose in life with the rate of change in cognitive function in older persons.
Methods
Participants
Participants are from the Rush Memory and Aging Project, a longitudinal clinicopathologic study of common chronic conditions of aging approved by the institutional review board of Rush University Medical Center, Chicago, Illinois.15 Participants are older persons recruited from approximately 40 continuous care retirement communities and senior subsidized housing facilities in and around the Chicago metropolitan area. Study participation required agreeing to detailed annual clinical evaluations and organ donation at the time of death, and all participants provided written consent. Between 1997 and 2008, more than 1200 older persons enrolled in the study. The purpose in life measure was added to the interview in 2001.
Eligibility for these analyses required a valid baseline score on the purpose in life measure, absence of dementia at the baseline evaluation, and at least 1 follow-up clinical evaluation. At the time of these analyses, 1151 persons had undergone their baseline clinical evaluation and completed the purpose in life measure. Of those, we excluded 75 with dementia at baseline and an additional 125 because they had not yet reached or had died before the first follow-up. This left 951 eligible persons; analyses are based on this group. At baseline, the mean (SD) age of participants was 80.4 (7.4) years, their mean (SD) years of education was 14.5 (3.0), and they had a mean (SD) score of 27.9 (2.2) on the Mini-Mental State Examination16; 74.9% were women, 91.8% were white, and 26.6% had MCI at baseline (see the following subsection). In the 951 persons, the mean (SD) number of annual clinical evaluations was 5 (1.57) (range, 1-8). The mean (SD) number of follow-ups was 4.0 (1.58) (range, 1-7), and the number of persons who completed 1 to 7 follow-ups was as follows: 1=76 (8.0%), 2=103 (10.8%), 3=187 (19.7%), 4=159 (16.7%), 5=230 (24.2%), 6=184 (19.3%), and 7=12 (1.3%).
Clinical Diagnosis of AD and MCI
Participants in the Memory and Aging Project undergo detailed annual clinical evaluations that include a medical history, a complete neurologic examination, and cognitive function testing, as previously described14,15; follow-up evaluations (identical to the baseline evaluation in all essential details) are conducted annually by examiners blinded to all previous data. Clinical diagnoses were performed using a 3-stage process, as previously described.15 First, neuropsychological tests were administered by trained technicians and scored by a computer, and ratings of impairment were assigned based on education-adjusted cutoff scores on 11 cognitive tests commonly used in the assessment of AD. Second, an experienced neuropsychologist blinded to subject age, sex, and race reviewed the results of the cognitive testing, including impairment ratings and data on education, sensory, and motor deficits, and rendered a clinical judgment regarding the presence of cognitive impairment. Third, diagnostic classification was performed by an experienced clinician after a review of all available data from that year's clinical evaluation, including ratings by the neuropsychologist and details of the neurologic examination. The clinician specified whether the participant met the clinical criteria for dementia and probable AD recommended by the joint working group of the National Institute of Neurologic and Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association,17 which require evidence of cognitive decline in memory and in at least 1 other domain of cognitive function. We previously reported15,18 that 90% of participants in this cohort who met the clinical criteria for AD had the diagnosis confirmed at autopsy. The diagnosis of MCI was rendered for individuals who were found to have cognitive impairment by the neuropsychologist but who, in the judgment of the examining clinician, did not meet the criteria for dementia.15,19,20 Although there are no universally accepted criteria for MCI, these criteria have been used in numerous publications on MCI in this and other cohorts.4,15,19,20 Persons who did not meet the criteria for MCI or dementia were classified as having no cognitive impairment.
Assessment of cognition
Cognitive function is assessed annually via a battery of 21 tests, as previously described.15,19,20 This battery includes the Mini-Mental State Examination, but these scores are used only to describe the cohort. Scores on 19 tests are used to create summary indices of global cognitive function and 5 specific cognitive domains: episodic memory, semantic memory, working memory, perceptual speed, and visuospatial ability. Episodic memory is assessed via 7 tests: immediate and delayed recall of story A from Logical Memory, immediate and delayed recall of the East Boston Story, Word List Memory, Word List Recall, and Word List Recognition. Semantic memory is assessed via 3 tests: a 15-item version of the Boston Naming Test, Verbal Fluency, and a 15-item reading test. Working memory is assessed via 3 tests: Digit Span Forward, Digit Span Backward, and Digit Ordering. Perceptual speed is assessed via 4 tests: Symbol Digit Modalities Test, Number Comparison, and 2 indices from a modified version of the Stroop Neuropsychological Screening Test. Visuospatial ability is assessed via 2 tests: a 15-item version of Judgment of Line Orientation and a 16-item version of Standard Progressive Matrices. One additional test, Complex Ideational Material, is used for diagnostic classification purposes only.
To compute the composite measure of global cognitive function, raw scores on each of the individual tests are converted to z scores using the baseline mean (SD) of the entire cohort, and the z scores of all 19 tests are averaged. Summary scores for the 5 cognitive domains (ie, episodic memory, semantic memory, working memory, perceptual speed, and visuospatial ability) also are derived by converting raw scores on each of the individual tests to z scores using the mean (SD) of the entire cohort and then averaging the z scores from tests in a specific domain. Psychometric information on these summary scores, including factor analytic support for the 5 domains, is contained in previous publications.19-21
Assessment of Purpose in Life
Purpose in life is a psychological construct that refers to the tendency to derive meaning from life's experiences and to possess a sense of intentionality and goal directedness that guides behavior. Purpose in life was assessed using a 10-item scale derived from Ryff's Scales of Psychological Well-Being (Table 1).7,11 Although the original scale included 20 items designed to measure purpose in life, several shortened versions (ranging from 3 to 14 questions) have been developed and evaluated psychometrically.10,13,14 For each of the 10 items, participants rated their level of agreement using a 5-point scale, as previously described.14,22 Scoring for the negatively worded items was flipped, and item scores were averaged to yield a total score for each participant, with higher scores indicating greater purpose in life. As previously reported, the Cronbach coefficient α on the scale used in this study indicated a moderate level of internal consistency.22 In previous studies,14,22 we found small to moderate correlations between purpose in life and depressive symptoms, neuroticism, and disability, and purpose in life was associated with longevity even after controlling for these covariates; these data suggest that purpose in life is a relatively distinct psychological construct that has predictive validity for adverse health outcomes. The mean (SD) score on the purpose in life measure was 3.6 (0.5) (range, 2-5).
Table 1. 10-Item Measure of Purpose in Life.
| Statement | |
|---|---|
| 1 | I feel good when I think of what I have done in the past and what I hope to do in the future. |
| 2 | I live life 1 day at a time and do not really think about the future. |
| 3 | I tend to focus on the present because the future nearly always brings me problems. |
| 4 | I have a sense of direction and purpose in life. |
| 5 | My daily activities often seem trivial and unimportant to me. |
| 6 | I used to set goals for myself, but that now seems like a waste of time. |
| 7 | I enjoy making plans for the future and working them to a reality. |
| 8 | I am an active person in carrying out the plans I set for myself. |
| 9 | Some people wander aimlessly through life, but I am not one of them. |
| 10 | I sometimes feel as if I have done all there is to do in life. |
Other Covariates
Other variables used in the analyses included age (based on date of birth), sex, and education (years of schooling completed).15 Depressive symptoms were assessed using a 10-item version of the Center for Epidemiologic Studies Depression Scale, as previously described.15 Persons were asked whether they had experienced each of 10 symptoms in the past week, and the score was the number of symptoms reported. The mean (SD) score on the Center for Epidemiologic Studies Depression Scale was 1.3 (1.8) (range, 0-9).
Neuroticism, the personality trait that refers to the tendency to experience psychological distress, was measured using the neuroticism subscale of the NEO Five-Factor Inventory to assess personality.15 Summary scores of the trait were computed, with higher scores indicating a higher level of neuroticism. The mean (SD) score was 15.1 (7.1) (range, 0-44).
Social network size was quantified using standard questions regarding the number of children, family, and friends that participants had and how often they interacted with them. Social network size was the number of these individuals seen at least once a month, as reported elsewhere.23 The mean (SD) social network size was 6.6 (5.8) (range, 0-66).
A self-report history of 7 medical conditions was recorded at baseline: stroke, cancer, diabetes mellitus, heart disease, hypertension, thyroid disease, and head injury The total number of conditions present was used as an index of chronic illness, as previously described.15 The mean (SD) number of medical conditions was 1.3 (1.1) (range, 0-6).
Data Analysis
We first examined the crude associations of purpose in life with age, sex, and education. Next, we examined the relation of baseline purpose in life with risk of AD using a proportional hazards model for discrete (tied) data24 adjusted for age, sex, and education (core model). In subsequent models, we added terms for the interactions of age, sex, and education with purpose in life and examined several potential confounders of the association of purpose in life with AD. We also conducted sensitivity analyses in which we sequentially excluded persons who developed AD in the early follow-up years (years 1-3). We then repeated the core model but excluded persons with MCI and dementia at baseline (because persons with MCI at baseline were included in the core model predicting incident AD) to further examine the association of purpose in life with risk of MCI. Finally, we used a series of mixed-effect models adjusted for age, sex, education, and level of cognition at baseline to examine the association of purpose in life with rate of decline in global cognitive function and the 5 cognitive domains (ie, episodic memory, working memory, semantic memory, perceptual speed, and visuospatial ability). Model validation was performed graphically and analytically, and there was no evidence of nonlinearity or nonproportionality. Programming was performed using a software program (SAS; SAS Institute Inc, Cary, North Carolina).25
Results
Psychometric Properties of Purpose in Life
Baseline scores on the measure of purpose in life ranged from 2 to 5 (mean [SD], 3.6 [0.5]), with higher scores indicating greater purpose in life. In unadjusted analyses, purpose in life was associated with age (r=−0.25) and education (r = 0.26) (P<.001 for both); women reported lower purpose in life compared with men (P=.02).
Purpose in Life and Risk of AD
During up to 7 years of follow-up (mean, 4.0 years), 155 of 951 persons (16.3%) developed AD; mean follow-up was similar in those who did (mean, 4.3 years) vs those who did not (mean, 4.0 years) develop AD. Those who developed AD were older and reported lower purpose in life than did those who did not (Table 2). In addition, those who developed AD reported higher baseline levels of neuroticism and fewer chronic medical conditions.
Table 2. Baseline Characteristics of Participants Who Developed AD vs Those Who Did Not.
| Characteristic | Did Not Develop AD | Developed AD | P Valuea |
|---|---|---|---|
| Age, mean (SD), y | 79.5 (7.4) | 84.7 (5.9) | <.001 |
| Female sex, % | 75.9 | 69.7 | .10 |
| Race, white, non-Hispanic, % | 91.1 | 95.5 | .07 |
| Education, mean (SD), y | 14.5 (3.0) | 14.6 (2.9) | 76 |
| Purpose in life score, mean (SD) | 3.7 (0.5) | 3.4 (0.4) | <.001 |
| Depressive symptoms score, mean (SD) | 1.3 (1.7) | 1.6 (2.0) | .35 |
| Neuroticism score, mean (SD) | 14.8 (7.0) | 16.8 (7.2) | .002 |
| Social network size, mean (SD) | 6.8 (6.0) | 5.8 (4.8) | .08 |
| No. of medical conditions, mean (SD) | 1.3 (1.1) | 1.2 (1.1) | .03 |
| MMSE score, mean (SD) | 28.3 (1.8) | 26.3 (3.2) | <.001 |
Abbreviations: AD, Alzheimer disease; MMSE, Mini-Mental State Examination.
Statistical significance is based on t tests or χ2 tests, as appropriate.
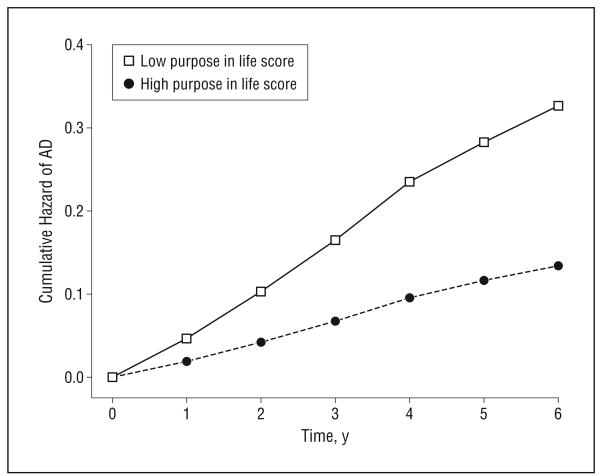
We constructed a proportional hazards model to test the hypothesized association of purpose in life with risk of AD; this core model and all subsequent models adjusted for age, sex, and education. In this analysis, greater purpose in life was associated with a substantially reduced risk of AD (hazard ratio [HR], 0.48; 95% confidence interval [CI], 0.33-0.69; P<.001) (model 1 in Table 3). Thus, a person with a high score on the purpose in life measure (score=4.2, 90th percentile) was approximately 2.4 times more likely to remain free of AD than was a person with a low score (score=3.0, 10th percentile) (Figure 1).
Table 3. Relation of Purpose in Life With AD in Sensitivity Analyses.
| Model (No. of Incident AD Cases) | Hazard Ratio (95% CI)a | P Value |
|---|---|---|
| Model 1: core model (n=155) | 0.48 (0.33-0.69) | <.001 |
| Model 2: excluding AD in year 1 (n=118) | 0.52 (0.34-0.79) | .002 |
| Model 3: excluding AD in years 1-2 (n=86) | 0.45 (0.27-0.74) | .002 |
| Model 4: excluding AD in years 1-3 (n=62) | 0.30 (0.17-0.53) | <.001 |
Abbreviations: AD, Alzheimer disease; CI, confidence interval.
Based on separate models; all models controlled for age, sex, and education.
Figure 1.
Cumulative hazard of Alzheimer disease (AD) for participants with high (90th percentile) vs low (10th percentile) purpose in life scores.
Next, because the association of purpose in life with AD may vary along demographic lines, we repeated the analysis described previously herein with additional terms for the interactions of age, sex, and education with purpose in life in separate models. No interactions were found (data not shown). In addition, because negative affect (ie, depressive symptoms), neuroticism, social networks, and chronic medical conditions may affect the association of purpose in life with AD, we repeated the core model described previously herein with additional terms to control for these important covariates. The association of purpose in life with AD persisted even after adjustment for all the covariates together in a single model (HR, 0.60; 95% CI, 0.39-0.92; P=.02).
Because it is possible that the inclusion of persons with mild, undiagnosed AD could have affected the findings reported previously herein, we repeated the core analysis 3 times after sequentially excluding persons who developed AD during each of the first 3 years of follow-up. In each case, and with as few as 62 cases of incident AD, purpose in life remained associated with the risk of AD (models 2-4 in Table 3).
Purpose in Life and Risk of MCI
It is widely recognized that AD has a long preclinical phase during which most persons transition through a stage referred to as MCI, when cognitive deficits are present but of insufficient severity to warrant a diagnosis of AD. To ensure that the present findings were not due to the inclusion of persons with early preclinical AD, we examined the relation of purpose in life with risk of MCI. In these models, persons with MCI at the baseline evaluation were excluded, leaving a final sample of 698 persons without cognitive deficits. During up to 7 years of follow-up, 285 of 698 persons (40.8%) developed MCI. Those who developed MCI were older and reported lower purpose in life and a higher number of depressive symptoms compared with those who did not develop MCI (Table 4).
Table 4. Baseline Characteristics of Participants Who Developed MCI vs Those Who Did Not.
| Characteristica | Did Not Develop MCI | Developed MCI | P Value |
|---|---|---|---|
| Age, mean (SD), y | 78.1 (7.3) | 81.6 (6.5) | <.001 |
| Female sex, % | 76.8 | 75.4 | .69 |
| Race, white, non-Hispanic, % | 89.4 | 92.6 | .14 |
| Education, mean (SD), y | 14.5 (3.0) | 14.7 (3.2) | .38 |
| Purpose in life score, mean (SD) | 3.7 (0.5) | 3.6 (0.4) | .002 |
| Depressive symptoms score, mean (SD) | 1.1 (1.7) | 1.4 (1.9) | .04 |
| Neuroticism score, mean (SD) | 14.3 (7.2) | 15.1 (6.4) | .12 |
| Social network size, mean (SD) | 7.0 (6.3) | 6.6 (6.1) | .25 |
| No. of medical conditions, mean (SD) | 1.4 (1.1) | 1.2 (1.0) | .10 |
| MMSE score, mean (SD) | 28.7 (1.4) | 28.2 (1.8) | <.001 |
Abbreviations: MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination.
Statistical significance is based on t tests, Wilcoxon rank sum tests or χ2 tests, as appropriate.
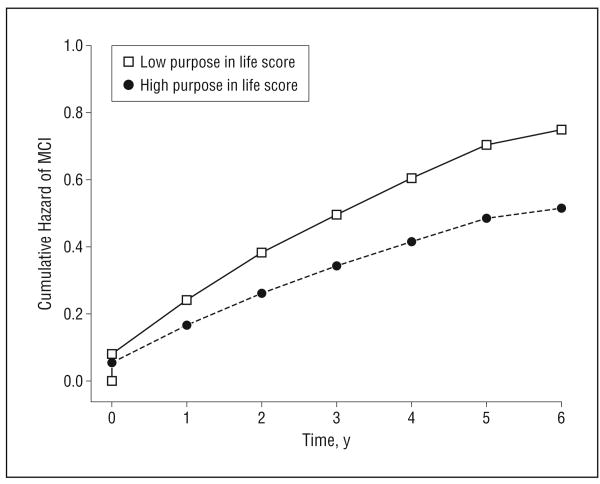
In a proportional hazards model adjusted for age, sex, and education, greater purpose in life at baseline was associated with a substantially reduced risk of MCI (HR, 0.71; 95% CI, 0.53-0.95; P=.02). Thus, a person with a high score on the purpose in life measure (score=4.2, 90th percentile) was approximately 1.5 times more likely to remain free of MCI than was a person with a low score (score=3.1, 10th percentile) (Figure 2).
Figure 2.
Cumulative hazard of mild cognitive impairment (MCI) for participants with high (90th percentile) vs low (10th percentile) purpose in life scores.
Next, because MCI does not uniformly progress to dementia or even persist,19,20 we examined the association of purpose in life with persistent MCI, defined as having MCI on 2 or more consecutive examinations (or MCI followed by dementia or death), as done in previous studies.4 Of 285 persons with incident MCI in the analyses described previously herein, 124 had MCI, dementia, or death at the next evaluation; the remaining 161 were included in the reference group. In a proportional hazards model adjusted for age, sex, and education, purpose in life was associated with a reduced risk of MCI (HR, 0.65; 95% CI, 0.43-1.00; P=.05). Although the association was stronger (ie, the point estimate was lower), the CI included 1.0 due to reduced power from the smaller sample size.
Purpose in Life and Change in Cognitive Function
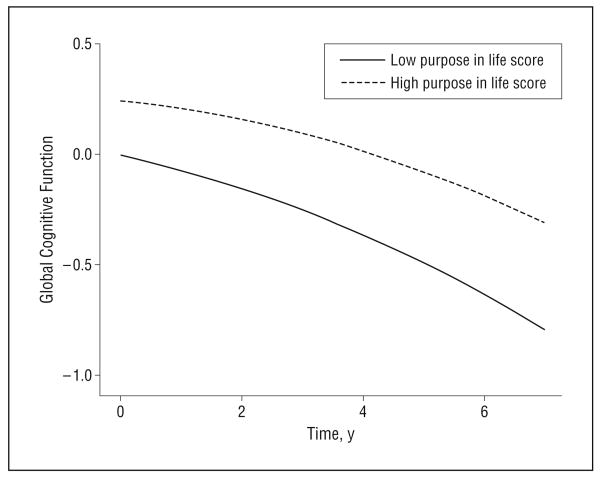
Because the principal manifestation of AD is cognitive decline and because AD develops slowly across many years, we next examined the association of purpose in life with the rate of change in cognitive function. To make use of all the cognitive data, we began with the composite measure of global cognition and constructed a series of mixed-effect models that allowed us to estimate the association of purpose in life with rate of change in cognition while controlling for baseline level of cognition. The initial analyses included all persons without dementia at the baseline evaluation. In these analyses, purpose in life was related to baseline level of global cognition (estimate for purpose in life) (Table 5, column A) and to rate of global cognitive decline (estimate for purpose in life × time) (Table 5, column A). This is illustrated in Figure 3, which shows the trajectories of cognitive decline for participants with high vs low purpose in life. Persons with greater purpose started at a higher level of cognition than did those with low purpose but declined less rapidly than did those with low purpose. Additional models examined 5 separate cognitive abilities starting with episodic memory, the hallmark of AD, and the 4 other measures, including semantic memory, working memory, perceptual speed, and visuospatial ability (Table 5). Purpose in life was related to baseline level of cognition in all 5 systems and was most strongly related to decline in semantic memory, followed by episodic memory, perceptual speed, and working memory.
Table 5. Relation of Purpose in Life With Change in Cognitive Functiona.
| Estimate, Mean (SE) | |||
|---|---|---|---|
| Cognitive Domain | Model Term | A: In Persons Without Dementia at Baseline | B: In Persons Without MCI at Baseline |
| Global cognition | Purpose in life | 0.21 (0.04)b | 0.13 (0.03)b |
| Purpose in life×time | 0.03 (0.01)c | 0.02 (0.01)d | |
| Episodic memory | Purpose in life | 0.19 (0.05)b | 0.09 (0.04)d |
| Purpose in life×time | 0.03 (0.01)d | 0.03 (0.01)d | |
| Semantic memory | Purpose in life | 0.17 (0.04)b | 0.08 (0.04) |
| Purpose in life×time | 0.04 (0.01)b | 0.03 (0.01)b | |
| Working memory | Purpose in life | 0.18 (0.05)b | 0.11 (0.06)d |
| Purpose in life×time | 0.02 (0.01)d | 0.03 (0.01)d | |
| Perceptual speed | Purpose in life | 0.24 (0.05)b | 0.18 (0.06)b |
| Purpose in life×time | 0.04 (0.01)c | 0.03 (0.05)d | |
| Visuospatial ability | Purpose in life | 0.18 (0.05)b | 0.15 (0.05)c |
| Purpose in life×time | 0.02 (0.01) | 0.01 (0.01) | |
Abbreviation: MCI, mild cognitive impairment.
Derived from mixed-effect models including terms forage, sex, education, time, time squared, and the interactions of time with age, sex, and education.
P<.001.
P<.01.
P<.05.
Figure 3.
Decline in global cognition for participants with high vs low scores on the purpose in life measure.
Finally, we repeated the mixed-effects models just described after excluding persons with MCI at baseline to further clarify the relation of purpose in life with the level and rate of change in cognitive function. In these models, findings were similar; purpose in life was associated with a reduced rate of decline in global cognition, semantic memory, episodic memory, working memory, and perceptual speed (Table 5, column B).
Comment
We examined the association of purpose in life with risk of incident AD in more than 900 community-dwelling older persons. During up to 7 years of follow-up, greater purpose in life was associated with a substantially reduced risk of AD such that a person with a high score (90th percentile) on the purpose in life measure was approximately 2.4 times more likely to remain free of AD than was a person with a low score (10th percentile). The association of purpose in life with AD persisted after adjustment for several important covariates (ie, depressive symptoms, neuroticism, social network size, and number of chronic medical conditions) and in sensitivity analyses in which we excluded persons who developed AD during the first 3 years of follow-up. Furthermore, in subsequent analyses, purpose in life was associated with a reduced risk of incident MCI, an early preclinical manifestation of AD. Finally, in models that controlled for baseline level of cognitive function, we found that purpose in life was associated with a less rapid rate of cognitive decline in persons without AD and in persons without AD or MCI. These findings suggest that the tendency to derive meaning from life's experiences and to possess a sense of intentionality and goal directedness are associated with a substantially reduced risk of AD and a less rapid rate of cognitive decline in old age.
Emerging data suggest that psychological and experiential factors are related to the risk of AD, with some (eg, neuroticism, loneliness, social isolation, and depressive symptoms) increasing risk and others (eg, conscientiousness, extraversion, and social integration) decreasing risk. Purpose in life is an indicator of human thriving that has long been hypothesized to buffer against adverse health outcomes.5-14 In recent years, purpose in life has been conceptualized as a component of psychological well-being9-11; in this context, purpose in life has been shown in cross-sectional studies to be associated with psychological health, including happiness, satisfaction, personal growth, self-acceptance, and better sleep.9-12,14 Although prospective data on the association of purpose in life with health outcomes are limited, we recently reported14 that purpose in life was associated with a reduced risk of all-cause mortality in older persons from this cohort. We are not aware of any previous study that has examined the association of purpose in life with risk of AD.
The finding that purpose in life is associated with cognitive outcomes may have important public health implications. In particular, these findings may provide a new treatment target for interventions aimed at enhancing health and well-being in older adults. Purpose in life is a potentially modifiable factor that may be increased via specific behavioral strategies that help older persons identify personally meaningful activities and engage in goal-directed behaviors. Even small behavioral modifications ultimately may translate into an increased sense of intentionality, usefulness, and relevance. Furthermore, purpose in life may be modifiable, even in persons for whom participation in more effortful activities (ie, physical activity, volunteerism, and other activities involving travel outside the home or local area) may be limited because of underlying health problems. Thus, purpose in life may offer a new treatment focus and may represent a target accessible to most of the aging population. If true, the implications could be far reaching, and efforts to increase purpose in life may help reduce the rapidly increasing burden of cognitive impairment in old age.
At present, the biological basis of the association of purpose in life with AD is unknown. Purpose in life is hypothesized to provide protective benefit in the face of illness or disease because it contributes to the optimal functioning of multiple physiologic systems.11,20,24 Although relatively few data are available on the association of purpose in life with physiologic measures, emerging evidence suggests that psychological well-being in general and purpose in life in particular are associated with important disease-related biomarkers.5,6,9 For example, purpose in life is negatively associated with immune markers, including salivary cortisol and the proinflammatory cytokines interleukin-6 receptor in women older than 75 years.26-28 In addition, purpose in life is positively correlated with high-density lipoprotein cholesterol levels and negatively with waist-hip ratios. Immune function and vascular health are related to cognitive function and the risk of dementia in old age,29 and it is possible that purpose in life is associated with a reduced risk of AD via its beneficial effect on these systems. However, future studies are needed to elucidate the biological basis of the association of purpose in life with cognition. In addition, because purpose in life is a potentially modifiable factor that all persons experience to some degree,30 future studies could examine potential strategies (eg, facilitation of participation in personally meaningful activities and goal setting) to increase purpose in life in older persons.
This study has some notable strengths, including the assessment of purpose in life in a large group of community-dwelling older persons free of dementia at baseline who underwent detailed annual structured clinical evaluations to document incident AD. Cognitive function was assessed at evenly spaced intervals using psychometrically sound composite measures for up to 8 years with high rates of follow-up participation, which enhanced our ability to reliably characterize individual paths of cognitive decline. Finally, we assessed purpose in life using a standard scale previously shown to have predictive validity for adverse health outcomes in older persons.14 One limitation of the study is the selected nature of the cohort, which may have restricted the range of scores on the purpose in life measure and may limit the generalizability of findings. In addition, there are factors that we did not measure (eg, apathy, motivation, and spirituality or religiosity) that may affect the associations of purpose in life with health outcomes. Recent data have shown that apathy, a syndrome of decreased motivation and interest, is common in persons with early cognitive impairment (ie, MCI and early AD) and is associated with functional status.31,32 Apathy and other lifestyle factors (eg, exercise and nutrition) may be related to the level of engagement in purposeful behaviors and may have implications for our understanding of the link between purpose in life and cognition. Finally, an inherent limitation of this study, as in all epidemiologic studies, is the inability to establish causality with certainty. Note that purpose in life was protective against AD even when examined carefully in a series of sensitivity analyses and after controlling for several important covariates; however, our ability to make causal inferences is limited. Future studies are needed to further clarify the role of apathy and other lifestyle factors as they relate to purpose in life and to examine the biological basis of the association of purpose in life with adverse health outcomes in old age.
Acknowledgments
Funding/Support: This work was supported by grants R01AG17917 (Drs Buchman and Bennett), R01AG24480 (Drs Buchman and Bennett), and K23AG23040 (Dr Boyle) from the National Institute on Aging; by the Illinois Department of Public Health; and by the Robert C. Borwell Endowment Fund.
Role of the Sponsors: The organizations funding this study had no role in the design or conduct of the study; in the collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr Boyle had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Additional Contributions: We thank the participants and the staff of the Rush Memory and Aging Project and the Rush Alzheimer's Disease Center. Woojeong Bang, MS, provided statistical programming.
Financial Disclosure: None reported.
References
- 1.Wang HX, Karp A, Herlitz A, Crowe M, Kåreholt I, Winblad B, Fratiglioni L. Personality and lifestyle in relation to dementia incidence. Neurology. 2009;72(3):253–259. doi: 10.1212/01.wnl.0000339485.39246.87. [DOI] [PubMed] [Google Scholar]
- 2.Fratiglioni L, Wang HX, Ericsson K, Maytan M, Winblad B. Influence of social network on occurrence of dementia: a community-based longitudinal study. Lancet. 2000;355(9212):1315–1319. doi: 10.1016/S0140-6736(00)02113-9. [DOI] [PubMed] [Google Scholar]
- 3.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3(6):343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 4.Wilson RS, Schneider JA, Arnold SE, Bienias JL, Bennett DA. Conscientiousness and the incidence of Alzheimer disease and mild cognitive impairment. Arch Gen Psychiatry. 2007;64(10):1204–1212. doi: 10.1001/archpsyc.64.10.1204. [DOI] [PubMed] [Google Scholar]
- 5.Frankl VE. Man's Search for Meaning. New York, NY: Washington Square Press, Simon and Schuster; 1963. [Google Scholar]
- 6.Ryff CD, Dienberg Love G, Urry HL, Muller D, Rosenkranz MA, Friedman EM, Davidson RJ, Singer B. Psychological well-being and ill-being: do they have distinct or mirrored biological correlates? Psychother Psychosom. 2006;75(2):85–95. doi: 10.1159/000090892. [DOI] [PubMed] [Google Scholar]
- 7.Ryff CD. Happiness is everything, or is it? explorations on the meaning of psychological well-being. J Pers Soc Psychol. 1989;57(6):1069–1081. [Google Scholar]
- 8.Stranges S, Dorn JM, Shipley MJ, Kandala NB, Trevisan M, Miller MA, Donahue RP, Hovey KM, Ferrie JE, Marmot MG, Cappuccio FP. Correlates of short and long sleep duration: a cross-cultural comparison between the United Kingdom and the United States: the Whitehall II Study and the Western New York Health Study. Am J Epidemiol. 2008;168(12):1353–1364. doi: 10.1093/aje/kwn337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryff CD, Singer BH, Dienberg Love G. Positive health: connecting well-being with biology. Philos Trans R Soc Lond B Biol Sci. 2004;359(1449):1383–1394. doi: 10.1098/rstb.2004.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samman E. Psychological and Subjective Wellbeing: A Proposal for Internationally Comparable Indicators OPHI Working Paper Series. Oxford, England: Oxford University Press; 2007. [Google Scholar]
- 11.Ryff CD, Keyes CL. The structure of psychological well-being revisited. J Pers Soc Psychol. 1995;69(4):719–727. doi: 10.1037//0022-3514.69.4.719. [DOI] [PubMed] [Google Scholar]
- 12.Wrosch C, Scheier MF, Miller GE, Schulz R, Carver CS. Adaptive self-regulation of unattainable goals: goal disengagement, goal reengagement, and subjective well-being. Pers Soc Psychol Bull. 2003;29(12):1494–1508. doi: 10.1177/0146167203256921. [DOI] [PubMed] [Google Scholar]
- 13.Abbott RA, Ploubidis GB, Huppert FA, Kuh D, Wadsworth ME, Croudace TJ. Psychometric evaluation and predictive validity of Ryff's psychological well-being items in a UK birth cohort sample of women. Health Qual Life Outcomes. 2006;4:76. doi: 10.1186/1477-7525-4-76. published online ahead of print October 4, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyle PA, Barnes LL, Buchman AS, Bennett DA. Purpose in life is associated with mortality among community-dwelling older persons. Psychosom Med. 2009;71(5):574–579. doi: 10.1097/PSY.0b013e3181a5a7c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25(4):163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 18.Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, Fox JH, Cochran EJ, Arends D, Treinkman AD, Wilson RS. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27(3):169–176. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- 19.Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Bennett DA. Motor dysfunction in mild cognitive impairment and the risk of incident Alzheimer disease. Arch Neurol. 2006;63(12):1763–1769. doi: 10.1001/archneur.63.12.1763. [DOI] [PubMed] [Google Scholar]
- 20.Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology. 2006;67(3):441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- 21.Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17(2):179–193. [PubMed] [Google Scholar]
- 22.Barnes LL, Wilson RS, Bienias JL, de Leon CF, Kim HJ, Buchman AS, Bennett DA. Correlates of life space in a volunteer cohort of older adults. Exp Aging Res. 2007;33(1):77–93. doi: 10.1080/03610730601006420. [DOI] [PubMed] [Google Scholar]
- 23.Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 2006;5(5):406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- 24.Gail MH, Lubin JH, Rubinstein LV. Likelihood calculations for matched case-control studies and survival studies with tied death times. Biometrika. 1981;68:703–707. [Google Scholar]
- 25.SAS Institute Inc. SAS 9.1.3 Help and Documentation. Cary, NC: SAS Institute Inc; 2000. [Google Scholar]
- 26.Lindfors P, Lundberg U. Is low cortisol level an indicator of positive health? Stress Health. 2002;18:153–160. [Google Scholar]
- 27.Friedman EM, Hayney M, Love GD, Singer BH, Ryff CD. Plasma interleukin-6 and soluble IL-6 receptors are associated with psychological well-being in aging women. Health Psychol. 2007;26(3):305–313. doi: 10.1037/0278-6133.26.3.305. [DOI] [PubMed] [Google Scholar]
- 28.Ryff CD, Singer B. From social structure to biology integrative science in pursuit of human health and well-being. In: Snyder CR, Lopez SJ, editors. Handbook of Positive Psychology. New York, NY: Oxford University Press; 2002. pp. 541–555. [Google Scholar]
- 29.Cechetto DF, Hachinski V, Whitehead SN. Vascular risk factors and Alzheimer's disease. Expert Rev Neurother. 2008;8(5):743–750. doi: 10.1586/14737175.8.5.743. [DOI] [PubMed] [Google Scholar]
- 30.Pinquart M. Creating and maintaining purpose in life in old age: a meta-analysis. Ageing Int. 2002;27(2):90–114. [Google Scholar]
- 31.Geda YE, Roberts RO, Knopman DS, Petersen RC, Christianson TJ, Pankratz VS, Smith GE, Boeve BF, Ivnik RJ, Tangalos EG, Rocca WA. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry. 2008;65(10):1193–1198. doi: 10.1001/archpsyc.65.10.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters KR, Rockwood K, Black SE, Hogan DB, Gauthier SG, Loy-English I, Hsiung GY, Jacova C, Kertesz A, Feldman HH. Neuropsychiatric symptom clusters and functional disability in cognitively-impaired not-demented individuals. Am J Geriatr Psychiatry. 2008;16(2):136–144. doi: 10.1097/JGP.0b013e3181462288. [DOI] [PubMed] [Google Scholar]