Abstract
Purpose of review
Obesity is established as an important contributor of increased diabetes mellitus, hypertension, and cardiovascular disease, all of which can promote chronic kidney disease (CKD). Recently, there is a growing appreciation that even in the absence of these risks, obesity itself significantly increases CKD and accelerates its progression.
Recent findings
Experimental and clinical studies reveal that adipose tissue, especially visceral fat, elaborates bioactive substances that contribute to the pathophysiologic renal hemodynamic and structural changes leading to obesity-related nephropathy. Adipocytes contain all the components of the renin-angiotensin-aldosterone system, plasminogen activator inhibitor, as well as adipocyte-specific metabolites such as free fatty acids, leptin, and adiponectin which affect renal function and structure. In addition, fat is infiltrated by macrophages that can alter their phenotype and foster a pro-inflammatory milieu which advances pathophysiologic changes in the kidney associated with obesity.
Summary
Obesity is an independent risk factor for development and progression of renal damage. While the current therapies aimed at slowing progressive renal damage include reduction in weight and rely on inhibition of the renin-angiotensin system, the approach will likely be supplemented by interventions aimed at obesity-specific targets including adipocyte-driven cytokines and inflammatory factors.
Keywords: Obesity, kidney, CKD
Introduction
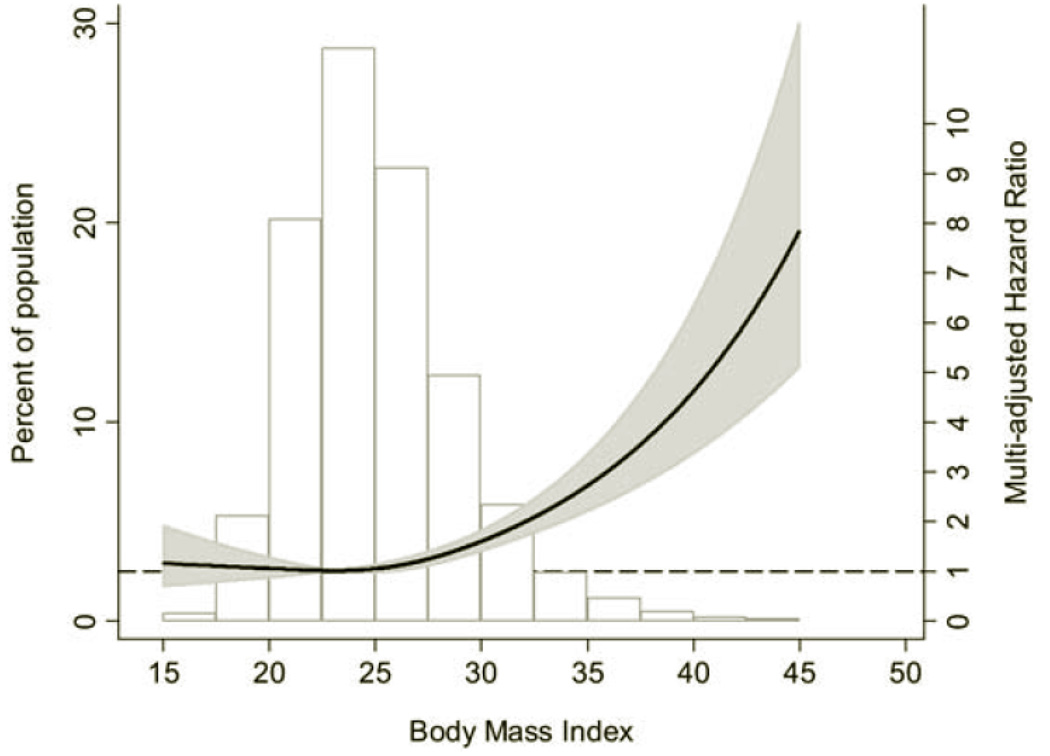
Obesity is now a worldwide epidemic, with overweight, obesity, and extreme obesity all increasing. The number of patients with CKD and end stage renal disease (ESRD) has also risen. Potentially linking these two epidemiologic observations is that many obesity-induced derangements are themselves nephrotoxic. Thus, diabetes mellitus is a common cause of renal dysfunction and ESRD and occurs with greater frequency in the obese [1] Likewise, adiposity contributes to hypertension, hyperlipidemia and cardiovascular disease, all of which promote renal disease. [2] Recently, there is increasing support that obesity per se can initiate and accelerate progression of kidney disease. [3] [4] [5]Among 75,000 Norwegians followed over 21 years, increased BMI dramatically correlated with initiation of renal replacement or death with CKD (Figure 1). Interestingly, prehypertension only adversely affected outcome in those with obesity. [5]
Figure 1.
Left axis and bar graph: Distribution of BMI in the study population of 74,986 adults in the HUNT I Study in Norway. Right axis: Hazard ratio for treated ESRD or CKD-related death by BMI, multi-adjusted for age, gender, smoking status, physical activity, and socioeconomic status. (Adapted from[5]).
Clinical Characteristics
In 1974, Weisinger et al. first reported an association between obesity and nephrotic syndrome that remitted with weight loss and returned with weight gain.[6] The renal histology was that of idiopathic focal segmental glomerulosclerosis (FSGS). The term obesity-related glomerulopathy (ORG) is now used to describe this secondary form of FSGS. Although proteinuria has been the clinical hallmark of obesity-related renal disease, ORG is now observed at lower thresholds of proteinuria. Thus, Kambham et al. documented a ten fold increase in ORG between 1986–1990 and 1996–2000. This study importantly noted that ORG was less likely than idiopathic FSGS to present with nephrotic-range proteinuria or edema, and was frequently associated with little to no hypoalbuminemia. [7] Among a Chinese cohort with ORG, half had proteinuria <1 gm/day, and a third <400 mg/day.[8] Even among extremely obese patients with normal renal function prior to bariatric surgery, majority had glomerular lesions, including glomerulomegaly, podocyte hypertrophy, increased mesangial matrix and mesangial cell proliferation.[9] Despite these structural changes, only 4% had macroalbuminuria, and 41% had only microalbuminuria. Thus, 96% of this group had no dipstick definable proteinuria. Such observations illustrate a shift in the concept of ORG as a nephropathy that does not hinge on the manifestation of proteinuria for diagnosis.
Glomerulomegaly is the primary histopathologic feature which distinguishes ORG from primary FSGS as well as obese patients with other renal diseases. [8] [10] [11] [12] [13] Increased glomerular size may be a manifestation of processes that promote cell proliferation and matrix synthesis (see below). Additionally, the link of glomerulomegaly and sclerosis may reflect the limited capacity of mature podocytes to divide. Indeed, glomerulomegaly with ORG was accompanied by a 45% reduction in podocyte density.[14] Thickening of the glomerular basement membrane (GBM) which has previously been considered an early manifestation of hyperglycemia and diabetic nephropathy, may be an additional pathologic finding associated with obesity. Obesity promotes hyperinsulinemia which may transition to hyperglycemia and type II diabetes. In obese patients with IgAN, GBM was ~25% thicker despite similar HgbA1c to the non obese.[12] Thicker GBM was also seen in biopsies from patients with benign nephrosclerosis related to essential hypertension and patients with ORG, with no data on glucose, though triglycerides and cholesterol were higher in the obese.[15] Another series found thicker GBM in obese patients, as well as direct correlation with HgbA1c in the normal range. [16] GBM thickness also correlated directly with circulating levels of cholesterol and triglycerides. Thus, glycemic and lipid abnormalities of obesity may contribute to GBM thickening which may not achieve the level seen in overt diabetes.
Obesity dramatically alters renal hemodynamics. A recent study found that glomerular filtration rate (GFR) was higher obese adults than in normal weight controls.[17, 18] The renal plasma flow (RPF) was also elevated, though not to the same degree. As a result, the filtration fraction (FF) increased, a hemodynamic adjustment that paralleled the degree of BMI and adipose mass. Even renal allografts adjust their function to the body habitus of the recipient.[19] Molecular sieving experiments in obese individuals suggest that afferent arteriolar vasodilatation, together with efferent arteriolar vasoconstriction, contribute to increased FF.[17, 20] In experimental animals, even mild adiposity enhances the antinatriuretic response.[21] By lowering tubular NaCl relative to GFR, obesity-dependent mechanisms disrupt the tubuloglomerular feedback (TGF) response, preventing suppression of GFR.[22] Given the high rate of hypertension in obesity, TGF inadequacy may allow transmission of systemic BP to the glomerulus contributing not only to increased GFR but to deleterious structural consequences.[23] Importantly, obesity-induced GFR increase is not fixed, with studies documenting improvement in hyperfiltration after gastroplasty.[24] [25]
Pathophysiology
Obesity induces several pathophysiologic disturbances that contribute to renal injury.
Renin-angiotensin-aldosterone system (RAAS)
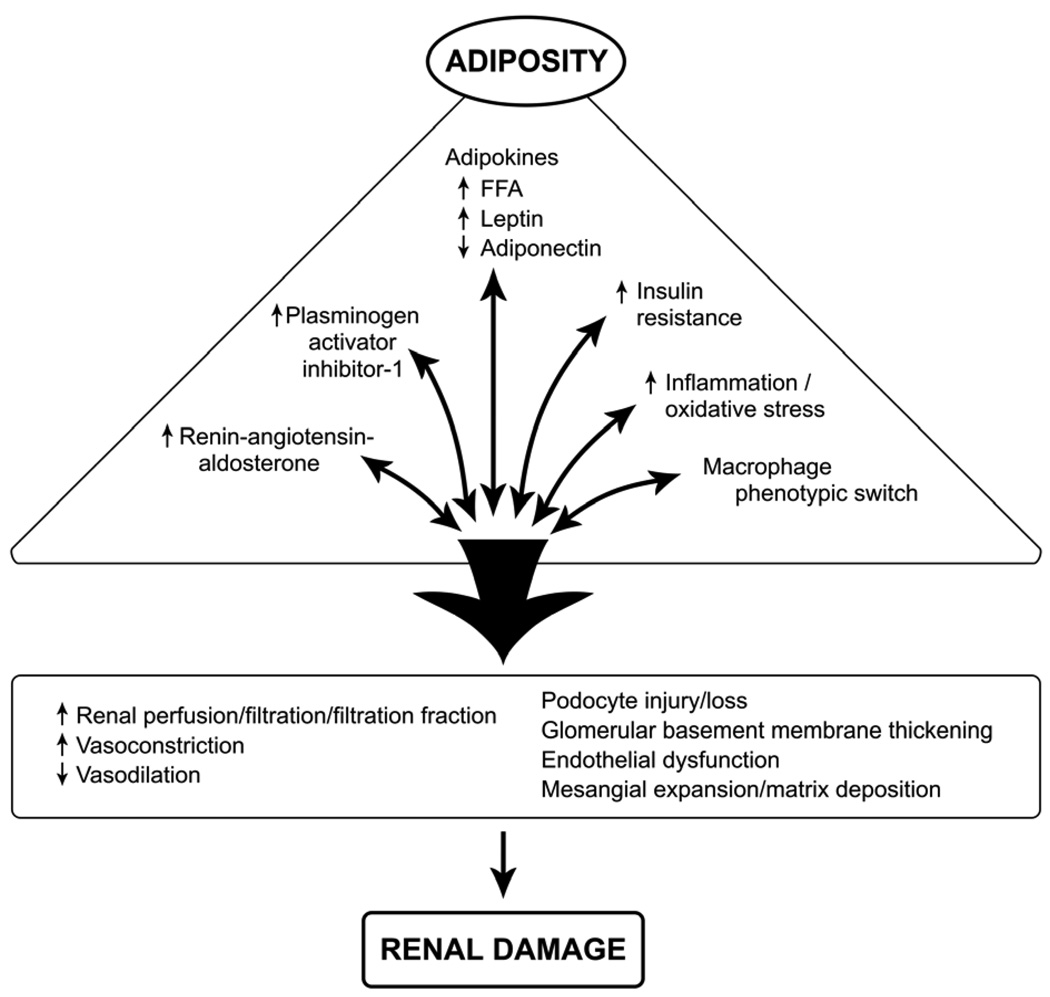
The RAAS is a major regulator of vasomotor tone and cellular proliferation that affect renal function and structure. Adipocytes and adipose-infiltrating macrophages comprise an important source of RAAS (Figure 2). Indeed, visceral fat expression of angiotensinogen (Aog) approximates that of the liver, classically considered the chief source of Aog.[26] Circulating levels of Aog increase with increasing BMI.[27] Relevant to obesity and CKD, infusion of angiotensin II (AngII) in obese mice resulted in a dramatic increase in adipocyte-derived and circulating, but not liver, Aog.[28] The AngII type 1 receptor (AT1), primarily responsible for post-glomerular vasoconstriction, is elevated in the renal cortex of obese Zucker rats.[29] Renal AT1 is also upregulated in transgenic mice overexpressing Aog exclusively in adipocytes (aP2-Agt).[30] Overall, adipose-derived increase in circulating RAAS ligands together with adipose-driven increase in renal AT1 provide a powerful combination for increasing efferent arteriolar vasoconstriction, glomerular pressure, FF, as well as cellular proliferation that culminate in renal damage. As with other proteinuric glomerulopathies, inhibition of RAAS has been used to treat ORG. Notably, although escape from the antiproteinuric benefits of angiotensin converting enzyme inhibition has been observed, it coincided with weight gain, further underscoring the prominent role of adipose tissue RAS.[31] Fasting decreases Aog and can reduce AngII production and AT1 density.[26] Such mechanisms may have contributed to decreased proteinuria observed in an obese teenager soon after bariatric surgery with negative caloric balance but minimal weight loss.[32]
Figure 2.
Mechanisms of obesity related renal disease. Adipose secretes a large number of mediators with impact on renal function and structure, culminating in renal damage.
Less easily conceptualized is the role of the AngII type 2 receptor (AT2). Obese Zucker rats treated with an AT2 receptor antagonist showed dramatic increase in blood pressure and renal cortical renin.[33] Similarly, when AT2 null mutation was introduced into the aP2-Agt strain of mice overexpressing Aog in adipocytes, exacerbation of hypertension, higher renal renin, and higher circulating AngI were observed.[34] AT2 KO/ aP2-Agt mice showed significant amelioration of elevated adipocyte levels of several angiogenic/inflammatory cytokines than aP2-Agt mice with intact AT2, including TNF-α, IL-6, IL-1β, and vascular endothelial growth factor (VEGF). These data thus suggest a role for AT2 in mediating the considerable adipose inflammatory response associated with increased Aog.[34]
Aldosterone blockade lessens renal injury. These benefits are independent of antihypertensive effects and instead, may relate to blocking aldosterone effects on plasminogen activator inhibitor (PAI-1) and transforming growth factor-β (TGF-β̣, reactive oxygen intermediates, inflammatory mediators, and podocyte function.[35] [36] [37] Adipose tissue is capable of AngII-independent aldosterone production and at least one oxidized derivative of linoleic acid is able to stimulate aldosterone production.[38] Further, complement-C1q TNF-related protein 1 (CTRP1), which in part mediates AngII stimulation of aldosterone, is also prominently expressed by adipose tissue where it may mediate AngII-independent aldosterone production.[39] Treatment with eplerenone in a mouse model of metabolic syndrome increases podocyte nephrin, reduces proteinuria and normalizes urinary markers of oxidative stress.[35] In this connection, the transgenic Ren2 rat shows podocyte foot process effacement which is normalized by treatment with spironolactone accompanied by a reduction in albuminuria as well as attenuating NADPH oxidase activity.[40] Overall, elevated aldosterone which prevails in obesity may be injurious to glomeruli through indirect effects to increase GFR as well as through direct podocyte effects.
Plasminogen activator inhibitor-1 (PAI-1)
PAI-1, as the primary physiological inhibitor of plasminogen activators, inhibits fibrinolysis and proteolysis and has a key role in obesity and insulin resistance. [41, 42] [43] [44] Obesity induces PAI-1 in adipose tissue and glomerular cells where it is an independent risk factor for renal damage through its effects to decrease protease-dependent matrix degradation and cellular migration.[45] In a podocyte injury-associated glomerulosclerosis model, renoprotection conferred by PPAR-γ agonist is achieved, in part, through decreased PAI-1.[46] Interestingly, preliminary studies suggest PAI-1 also modulates podocyte injury. Thus, renal ablation in PAI-1 deficient mice caused less proteinuria, glomerular sclerosis, podocyte damage/loss. These in vivo findings were paralleled by decreased angiotensin-induced apoptosis in cultured PAI-1 deficient podocytes compared with PAI-1 intact cells. These results are of interest because of the highly differentiated nature of podocytes, which once lost, are not replenished and thought to promote intraglomerular injuries that lead to glomerular sclerosis. (Unpublished data.)
Melanocortin
The central nervous melanocortin system plays a pivotal role in regulating body weight and energy homeostasis.[47] Melanocortin 4 receptor (MC4-R) has been identified as the cause of rare forms of monogenic obesity and heterozygous mutations in the MC4-R gene account for about 6% of early onset or severe adult obesity.[48] Novel non-selective melanocortin receptor agonists improve obesity, hyperinsulinemia and fatty liver disease in obese C57BL/6 mice.[49] Recently, the effects of melanocortin-4 receptor in obesity-associated renal injury were studied in MC4R−/− mice.[50] Although MC4R−/− mice exhibited many characteristic of the metabolic syndrome, including increased weight, hyperinsulinemia, and hyperleptinemia, they were not hypertensive. Although treatment with L-NAME caused a similar increase in systemic blood pressure in both MC4R−/− and age-matched wild type mice, the MC4R−/− developed more renal injury including greater elevation in urine albumin, renal TGF-β content and renal macrophage infiltration. These results emphasize that hypertension is an important risk factor for obesity related kidney injury in MC4R−/− mice.
Metabolic/adipose factors
Obesity causes lipid disturbances that may directly contribute to renal damage. Young C57BL/6 mice fed a HFD became heavier, developed hyperglycemia, hyperinsulinemia, elevated triglycerides and cholesterol and lower circulating adiponectin. They became proteinuric and had morphological abnormalities including, glomerulomegaly, expanded mesangial matrix, GBM thickening and podocyte effacement.[51] A dramatic increase in mesangial area was also observed in young obese Zucker rats fed a HFD, an abnormality which normalized by treatment with rosuvastatin.[52] Lipid moieties can directly injure renal parenchymal cells. Human mesangial cells exposed to LDL, oxidized LDL, and glycated LDL at concentrations approximating those in circulation dramatically increased synthesis of mesangial matrix components, fibronectin and laminin.[53] The lipid moieties also promoted mesangial production of macrophage migration inhibitory factor, and increased expression/release of inflammatory activators, CD40 and IL-6.[53] Treatment of hyperlipidemic mice with anti-IL-6 monoclonal antibody ameliorated lipid-induced renal toxicity, including glomerular lipid accumulation, mesangial cell proliferation and matrix accumulation, resulting in normalization of proteinuria.[54] Lipids also directly damage podocytes. [14] Oxidized LDL causes redistribution and loss of nephrin as well as podocyte apoptosis by decreasing phosphorylation of Akt, a prominent pathway for cell survival.[55, 56] Additional podocyte metabolic pathways may be altered by lipids. Thus, podocytes cultured with the saturated fatty acid, palmitate, increased ceramide production resulting in blockade of insulin-stimulated glucose uptake.[57] Fatty acid-induced insulin resistance in podocytes appears to represent a novel nexus where lipid abnormalities and altered glucose metabolism may interact directly to foster nephropathy.
Sterol regulatory element binding protein-1 (SREBP-1) appears to play a critical role in the renal lipid accumulation, subsequent inflammatory/fibrotic response, and resultant injury.[58] [59] Thus, renal effects of a HFD were not seen in SREBP-1c −/− mutant mice, while SREBP-1a transgenic mice had increased glomerular lipid accumulation, markers of glomerulosclerosis as well as increased albuminuria. Ameliorating effects were recently observed for farnesoid X receptor.[60] Lending credence to these data for human disease are observations that glomerular expression of SREBP-1 is up-regulated two fold in glomeruli from patients with obesity related glomerulopathy.[61]
Adipose tissue produces a number of bioactive substances. Leptin was originally identified as a murine obesity gene product abundantly produced by adipose tissue and regulates the hypothalamic-pituitary axis involved in food intake, energy expenditure and intracellular lipid homeostasis. Circulating levels of leptin parallel fat stores and absence of leptin or mutation in the leptin receptor gene causes massive hyperphagia in animals and humans. Despite severe obesity, these mutations are not accompanied by renal dysfunction. Contrasting adipose-originating cytokines which are elevated, adiponectin levels are depressed in obesity. Low adiponectin levels have been associated with inflammation, atherosclerosis, insulin resistance, and augmentation of blood pressure.[62] Experimental and clinical hypoadiponectinemia is associated with endothelial cell dysfunction, impaired endothelium-dependent vasodilation, disinhibition of leukocyte-endothelium adhesion, and activation of RAAS. Adiponectin also supports normal function of the podocyte[63] and hypoadiponectinemia may impair the pivotal role of podocytes in maintaining an intact glomerular sieving barrier and promote intraglomerular injuries that lead to glomerular sclerosis. Thus, adiponectin null mutant mice have an exaggerated response to renal injury including glomerulomegaly, glomerular collagen deposition, podocyte foot process effacement, increased TGF-β, and albuminuria.[64] Adiponectin treatment normalizes podocyte effacement and albuminuria. At least in part, adiponectin’s benefit may be through reduction in oxidant stress.[63] [65] Conversely, adiponectin deficiency leads to augmentation of NADPH oxidase and increase in urinary reactive oxygen species. It is of interest that obese African-Americans show a strong negative correlation between plasma adiponectin levels and albuminuria.[63] Importantly, adiponectin level can increase even with modest weight loss. Only 1 month after bariatric surgery, obese patients had a significant increase in adiponectin.[66]
Adiposity-driven proinflammatory cytokines
Fat distribution, specifically visceral adiposity, is a key determinant of renal dysfunction, even in normal weight individuals.[67] The role of the visceral fat relates not only to secretion of bioactive substances, but also to promote a low grade chronic inflammatory state. Visceral fat is infiltrated by macrophages which constitute an important source of pro-inflammatory mediators. Macrophages also have a reciprocal relationship with adipocytes. For example, fatty acids released by adipocytes stimulate TNF-α release by macrophages which, in turn, can stimulate production of IL-6 by fat cells further amplifying the inflammatory response in adipose tissue as well as the kidney.[68] TNF-α is a key mediator of progressive renal fibrosis. Gene expression profiles in glomeruli obtained from renal biopsy samples of patients with ORG showed increased TNF-α and its receptors, suggesting TNF-α’s role in development of ORG.[61] Interleukin-6 is secreted by adipose tissue and circulating levels increase with obesity, with as much as 30% derived from adipose tissue.[69] Glomeruli from patients with ORG show increased expression of IL-6 signal transducer, pointing to the possibility of IL-6 pathogenicity in glomeruli.[61] Many of the bioactive substances produced by macrophages also inhibit preadipocyte differentiation, further expanding a population of large, dysfunctional, insulin-resistant adipocytes that fuel the vicious cycle between obesity and renal injury.
Adiposity-driven macrophage infiltration and phenotypic switch
Obesity-related macrophage infiltration of adipose tissue is believed to be key in inflammation and insulin resistance.[70] [71] Importantly, depending on the local microenvironment and stage of tissue injury, macrophages display heterogeneity in functions.[72] [73] [74] Thus, M1 or “classically activated” macrophages are induced by classical immune pathways and function to enhance proinflammatory cytokine production (IL-1β, TNF-α, IL-6). By contrast, M2 or “alternatively activated” macrophages function in the resolution of inflammation and tissue repair through synthesis of anti-inflammatory cytokines IL-10 and IL-1 decoy receptor and possess high endocytic clearance capacities.[75] [74] [73] Obesity induces macrophage phenotypic switch in adipose tissue,[76] shifting from M2 phenotype predominating in lean rodents to a robust increase in proinflammatory M1 macrophage population in obese animals.[77] [78]
Experimental approaches to inhibit proinflammatory macrophages have been successful in reducing kidney injury.[79] [80] [81] The possibility that phenotypic alteration of macrophages modulate obesity-associated CKD has recently been evaluated. Using AT1a receptor knockout mice (AT1aKO) and a high-fat diet-induced obesity model, we recently found that HFD feeding augmented renal injury, including mesangial expansion and tubular vacuolization in AT1aKO (submitted for publication). There was significantly greater macrophage infiltration in visceral adipose tissue and kidney of obese AT1aKO. Kidney M1 macrophage activation was markedly induced while kidney M2 activation was reduced by half in obese AT1aKO. Further, M1, but not M2, activation in peritoneal macrophages was enhanced in obese AT1aKO. These data reveal a new role of macrophage AT1 receptor in mediating macrophage polarization and suggest that AT1a deficiency reduces the population of potentially beneficial M2 macrophages and promotes obesity-related renal damage.
In conclusion, new evidence indicates that in addition to promoting diabetes, hypertension, and cardiovascular disease; obesity per se, causes pathophysiologic disturbances that adversely affect kidney function and structure. These abnormalities are remediable through weight reduction and inhibition of RAS, an approach that will likely be supplanted with interventions that directly target adipocyte-associated cytokines and inflammatory factors.
Acknowledgments
This work has been supported by NIH DK044757 (VK, LM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest.
References
- 1.US RDS. USRDS 2009 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; National Institutes of Health NIoDaDa. 2009
- 2.Rheaume C, Arsenault BJ, Belanger S, et al. Low cardiorespiratory fitness levels and elevated blood pressure: what is the contribution of visceral adiposity? Hypertension. 2009;54:91–97. doi: 10.1161/HYPERTENSIONAHA.109.131656. [DOI] [PubMed] [Google Scholar]
- 3.Goncalves Torres MR, Cardoso LG, de Abreu VG, et al. Temporal relation between body mass index and renal function in individuals with hypertension and excess body weight. Nutrition. 2009;25:914–919. doi: 10.1016/j.nut.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Nomura I, Kato J, Kitamura K. Association between body mass index and chronic kidney disease: a population-based, cross-sectional study of a Japanese community. Vasc Health Risk Manag. 2009;5:315–320. doi: 10.2147/vhrm.s5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Munkhaugen J, Lydersen S, Wideroe TE, Hallan S. Prehypertension, obesity, and risk of kidney disease: 20-year follow-up of the HUNT I study in Norway. Am J Kidney Dis. 2009;54:638–646. doi: 10.1053/j.ajkd.2009.03.023. ** Among 75,000 adults followed over 21 years, increased BMI dramatically correlated with initiation of renal replacement or death with CKD. Prehypertension only adversely affected outcome in those with obesity.
- 6.Weisinger JR, Kempson RL, Eldridge FL, Swenson RS. The nephrotic syndrome: a complication of massive obesity. Ann Intern Med. 1974;81:440–447. doi: 10.7326/0003-4819-81-4-440. [DOI] [PubMed] [Google Scholar]
- 7.Kambham N, Markowitz GS, Valeri AM, et al. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59:1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 8. Chen HM, Li SJ, Chen HP, et al. Obesity-related glomerulopathy in China: a case series of 90 patients. Am J Kidney Dis. 2008;52:58–65. doi: 10.1053/j.ajkd.2008.02.303. * The paper documents a dramatic increase in frequency of biopsy-proven obesity-related glomerulopathy in China. Importantly, the authors show that more than half of these patients have surprisingly low levels of proteinuria.
- 9. Serra A, Romero R, Lopez D, et al. Renal injury in the extremely obese patients with normal renal function. Kidney Int. 2008;73:947–955. doi: 10.1038/sj.ki.5002796. ** Renal biopsies were analyzed from obese patients with normal renal function presenting for bariatric surgery. 77% had some glomerular lesion including glomerulomegaly, FSGS, global sclerosis, podocyte hypertrophy, etc, despite the fact that 41% of the group had merely microalbuminuria and only 4% had macroalbuminuria.
- 10.Kambham N, Markowitz GS, Valeri AM, et al. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59:1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 11.Danilewicz M, Wagrowska-Danielwicz M. Morphometric and immunohistochemical insight into focal segmental glomerulosclerosis in obese and non-obese patients. Nefrologia. 2009;29:35–41. doi: 10.3265/Nefrologia.2009.29.1.35.1.en.full.pdf. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka M, Yamada S, Iwasaki Y, et al. Impact of obesity on IgA nephropathy: comparative ultrastructural study between obese and non-obese patients. Nephron Clin Pract. 2009;112:c71–c78. doi: 10.1159/000213084. [DOI] [PubMed] [Google Scholar]
- 13.Rea DJ, Heimbach JK, Grande JP, et al. Glomerular volume and renal histology in obese and non-obese living kidney donors. Kidney Int. 2006;70:1636–1641. doi: 10.1038/sj.ki.5001799. [DOI] [PubMed] [Google Scholar]
- 14.Chen HM, Liu ZH, Zeng CH, et al. Podocyte lesions in patients with obesity-related glomerulopathy. Am J Kidney Dis. 2006;48:772–779. doi: 10.1053/j.ajkd.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 15.Kato S, Nazneen A, Nakashima Y, et al. Pathological influence of obesity on renal structural changes in chronic kidney disease. Clin Exp Nephrol. 2009;13:332–340. doi: 10.1007/s10157-009-0169-3. [DOI] [PubMed] [Google Scholar]
- 16.Goumenos DS, Kawar B, El Nahas M, et al. Early histological changes in the kidney of people with morbid obesity. Nephrol Dial Transplant. 2009;24:3732–3738. doi: 10.1093/ndt/gfp329. [DOI] [PubMed] [Google Scholar]
- 17.Chagnac A, Herman M, Zingerman B, et al. Obesity-induced glomerular hyperfiltration: its involvement in the pathogenesis of tubular sodium reabsorption. Nephrol Dial Transplant. 2008;23:3946–3952. doi: 10.1093/ndt/gfn379. [DOI] [PubMed] [Google Scholar]
- 18.Bosma RJ, van der Heide JJ, Oosterop EJ, et al. Body mass index is associated with altered renal hemodynamics in non-obese healthy subjects. Kidney Int. 2004;65:259–265. doi: 10.1111/j.1523-1755.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- 19.Bosma RJ, Kwakernaak AJ, van der Heide JJ, et al. Body mass index and glomerular hyperfiltration in renal transplant recipients: cross-sectional analysis and long-term impact. Am J Transplant. 2007;7:645–652. doi: 10.1111/j.1600-6143.2006.01672.x. [DOI] [PubMed] [Google Scholar]
- 20.Rook M, Bosma RJ, van Son WJ, et al. Nephrectomy elicits impact of age and BMI on renal hemodynamics: lower postdonation reserve capacity in older or overweight kidney donors. Am J Transplant. 2008;8:2077–2085. doi: 10.1111/j.1600-6143.2008.02355.x. [DOI] [PubMed] [Google Scholar]
- 21.Michaels S, Eppel GA, Burke SL, et al. Altered responsiveness of the kidney to activation of the renal nerves in fat-fed rabbits. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1889–R1896. doi: 10.1152/ajpregu.90931.2008. [DOI] [PubMed] [Google Scholar]
- 22. Hashimoto S, Yamada K, Kawata T, et al. Abnormal autoregulation and tubuloglomerular feedback in prediabetic and diabetic OLETF rats. Am J Physiol Renal Physiol. 2009;296:F598–F604. doi: 10.1152/ajprenal.00074.2008. * OLETF rat is a model of Type II diabetes. The paper finds renal autoregulatory abnormalities before onset of diabetes. Importantly, the paper shows a degraded tubuloglomerular feedback response in these obese rats.
- 23.Griffin KA, Kramer H, Bidani AK. Adverse renal consequences of obesity. Am J Physiol Renal Physiol. 2008;294:F685–F696. doi: 10.1152/ajprenal.00324.2007. [DOI] [PubMed] [Google Scholar]
- 24.Chagnac A, Weinstein T, Herman M, et al. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol. 2003;14:1480–1486. doi: 10.1097/01.asn.0000068462.38661.89. [DOI] [PubMed] [Google Scholar]
- 25.Navarro-Diaz M, Serra A, Romero R, et al. Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. J Am Soc Nephrol. 2006;17:S213–S217. doi: 10.1681/ASN.2006080917. [DOI] [PubMed] [Google Scholar]
- 26.Frederich RC, Jr, Kahn BB, Peach MJ, Flier JS. Tissue-specific nutritional regulation of angiotensinogen in adipose tissue. Hypertension. 1992;19:339–344. doi: 10.1161/01.hyp.19.4.339. [DOI] [PubMed] [Google Scholar]
- 27.Schorr U, Blaschke K, Turan S, et al. Relationship between angiotensinogen, leptin and blood pressure levels in young normotensive men. J Hypertens. 1998;16:1475–1480. doi: 10.1097/00004872-199816100-00011. [DOI] [PubMed] [Google Scholar]
- 28.Lu H, Boustany-Kari CM, Daugherty A, Cassis LA. Angiotensin II increases adipose angiotensinogen expression. Am J Physiol Endocrinol Metab. 2007;292:E1280–E1287. doi: 10.1152/ajpendo.00277.2006. [DOI] [PubMed] [Google Scholar]
- 29.Xu ZG, Lanting L, Vaziri ND, et al. Upregulation of angiotensin II type 1 receptor, inflammatory mediators, and enzymes of arachidonate metabolism in obese Zucker rat kidney: reversal by angiotensin II type 1 receptor blockade. Circulation. 2005;111:1962–1969. doi: 10.1161/01.CIR.0000161831.07637.63. [DOI] [PubMed] [Google Scholar]
- 30.Kim S, Soltani-Bejnood M, Quignard-Boulange A, et al. The adipose Renin-Angiotensin system modulates systemic markers of insulin sensitivity and activates the intrarenal Renin-Angiotensin system. J Biomed Biotechnol. 2006;2006:27012. doi: 10.1155/JBB/2006/27012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Praga M, Hernandez E, Morales E, et al. Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2001;16:1790–1798. doi: 10.1093/ndt/16.9.1790. [DOI] [PubMed] [Google Scholar]
- 32.Fowler SM, Kon V, Ma L, et al. Obesity-related focal and segmental glomerulosclerosis: normalization of proteinuria in an adolescent after bariatric surgery. Pediatr Nephrol. 2009;24:851–855. doi: 10.1007/s00467-008-1024-6. [DOI] [PubMed] [Google Scholar]
- 33.Siddiqui AH, Ali Q, Hussain T. Protective role of angiotensin II subtype 2 receptor in blood pressure increase in obese Zucker rats. Hypertension. 2009;53:256–261. doi: 10.1161/HYPERTENSIONAHA.108.126086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yvan-Charvet L, Massiera F, Lamande N, et al. Deficiency of angiotensin type 2 receptor rescues obesity but not hypertension induced by overexpression of angiotensinogen in adipose tissue. Endocrinology. 2009;150:1421–1428. doi: 10.1210/en.2008-1120. * The study underscores the importance of the angiotensin II type 2 receptor (AT2) in obesity. Using transgenic mice with a deletion of AT2, an overexpression of angiotensinogen, or both compound mutants, the study shows the necessary role of the AT2 for the onset of obesity, renin production as well as mediating adipose inflammatory response.
- 35. Nagase M, Fujita T. Aldosterone and glomerular podocyte injury. Clin Exp Nephrol. 2008;12:233–242. doi: 10.1007/s10157-008-0034-9. * Podocytes express mineralocorticoid receptor in vivo and in vitro. Aldosterone blockade with eplerenone, as well as antioxidant tempol, ameliorated aldosterone-induced podocyte injury and proteinuria. Aldosterone has direct deleterious podocyte effects which are modulatable by aldosterone blockers.
- 36.de Paula RB, da Silva AA, Hall JE. Aldosterone antagonism attenuates obesity-induced hypertension and glomerular hyperfiltration. Hypertension. 2004;43:41–47. doi: 10.1161/01.HYP.0000105624.68174.00. [DOI] [PubMed] [Google Scholar]
- 37.Bomback AS, Klemmer PJ. Renal injury in extreme obesity: the important role of aldosterone. Kidney Int. 2008;74:1216. doi: 10.1038/ki.2008.429. author reply 1216-7. [DOI] [PubMed] [Google Scholar]
- 38.Goodfriend TL, Ball DL, Egan BM, et al. Epoxy-keto derivative of linoleic acid stimulates aldosterone secretion. Hypertension. 2004;43:358–363. doi: 10.1161/01.HYP.0000113294.06704.64. [DOI] [PubMed] [Google Scholar]
- 39. Jeon JH, Kim KY, Kim JH, et al. A novel adipokine CTRP1 stimulates aldosterone production. FASEB J. 2008;22:1502–1511. doi: 10.1096/fj.07-9412com. ** Complement-C1q TNF-related protein 1(CTRP1) is highly espressed in adipose tissue and stimulates aldosterone production in a dose dependent manner in human adrenal cortical cell line H295R.
- 40. Whaley-Connell A, Habibi J, Wei Y, et al. Mineralocorticoid receptor antagonism attenuates glomerular filtration barrier remodeling in the transgenic Ren2 rat. Am J Physiol Renal Physiol. 2009;296:F1013–F1022. doi: 10.1152/ajprenal.90646.2008. * Using transgenic (mRen2)27 (Ren2) rats with increased tissue RAAS activity and elevated serum aldosterone levels the study shows the mechanisms by which mineralocorticoid receptor antagonism attenuates glomerular/podocyte remodeling and albuminuria includes reductions in redox-mediated impairment of insulin metabolic signaling.
- 41.Ma LJ, Fogo AB. PAI-1 and kidney fibrosis. Front Biosci. 2009;14:2028–2041. doi: 10.2741/3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loskutoff DJ, Quigley JP. PAI-1, fibrosis, and the elusive provisional fibrin matrix. J Clin Invest. 2000;106:1441–1443. doi: 10.1172/JCI11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma LJ, Mao SL, Taylor KL, et al. Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes. 2004;53:336–346. doi: 10.2337/diabetes.53.2.336. [DOI] [PubMed] [Google Scholar]
- 44.Liang X, Kanjanabuch T, Mao SL, et al. Plasminogen activator inhibitor-1 modulates adipocyte differentiation. Am J Physiol Endocrinol Metab. 2006;290:E103–E113. doi: 10.1152/ajpendo.00605.2004. [DOI] [PubMed] [Google Scholar]
- 45.Eddy AA, Fogo AB. Plasminogen activator inhibitor-1 in chronic kidney disease: evidence and mechanisms of action. J Am Soc Nephrol. 2006;17:2999–3012. doi: 10.1681/ASN.2006050503. [DOI] [PubMed] [Google Scholar]
- 46.Yang HC, Ma LJ, Ma J, Fogo AB. Peroxisome proliferator-activated receptor-gamma agonist is protective in podocyte injury-associated sclerosis. Kidney Int. 2006;69:1756–1764. doi: 10.1038/sj.ki.5000336. [DOI] [PubMed] [Google Scholar]
- 47.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 48.Farooqi IS, Keogh JM, Yeo GS, et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 49.Kumar KG, Sutton GM, Dong JZ, et al. Analysis of the therapeutic functions of novel melanocortin receptor agonists in MC3R- and MC4R-deficient C57BL/6J mice. Peptides. 2009;30:1892–1900. doi: 10.1016/j.peptides.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.do Carmo JM, Tallam LS, Roberts JV, et al. Impact of obesity on renal structure and function in the presence and absence of hypertension: evidence from melanocortin-4 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2009;297:R803–R812. doi: 10.1152/ajpregu.00187.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deji N, Kume S, Araki S, et al. Structural and functional changes in the kidneys of high-fat diet-induced obese mice. Am J Physiol Renal Physiol. 2009;296:F118–F126. doi: 10.1152/ajprenal.00110.2008. [DOI] [PubMed] [Google Scholar]
- 52.Reisin E, Ebenezer PJ, Liao J, et al. Effect of the HMG-CoA reductase inhibitor rosuvastatin on early chronic kidney injury in obese zucker rats fed with an atherogenic diet. Am J Med Sci. 2009;338:301–309. doi: 10.1097/MAJ.0b013e3181b27195. [DOI] [PubMed] [Google Scholar]
- 53.Santini E, Lupi R, Baldi S, et al. Effects of different LDL particles on inflammatory molecules in human mesangial cells. Diabetologia. 2008;51:2117–2125. doi: 10.1007/s00125-008-1127-4. [DOI] [PubMed] [Google Scholar]
- 54.Tomiyama-Hanayama M, Rakugi H, Kohara M, et al. Effect of interleukin-6 receptor blockage on renal injury in apolipoprotein E-deficient mice. Am J Physiol Renal Physiol. 2009;297:F679–F684. doi: 10.1152/ajprenal.90680.2008. [DOI] [PubMed] [Google Scholar]
- 55. Bussolati B, Deregibus MC, Fonsato V, et al. Statins prevent oxidized LDL-induced injury of glomerular podocytes by activating the phosphatidylinositol 3-kinase/AKT-signaling pathway. J Am Soc Nephrol. 2005;16:1936–1947. doi: 10.1681/ASN.2004080629. ** IL-6 blockade prevented proteinuria and ameliorated glomerular lipid deposits as well as mesangial proliferation and matrix accumulation in high fat diet-induced renal injury. This study underscores the importance of adipose-derived and dietary lipid-derived inflammatory mediators in obesity-related renal disease.
- 56.Cormack-Aboud FC, Brinkkoetter PT, Pippin JW, et al. Rosuvastatin protects against podocyte apoptosis in vitro. Nephrol Dial Transplant. 2009;24:404–412. doi: 10.1093/ndt/gfn528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lennon R, Pons D, Sabin MA, et al. Saturated fatty acids induce insulin resistance in human podocytes: implications for diabetic nephropathy. Nephrol Dial Transplant. 2009;24:3288–3296. doi: 10.1093/ndt/gfp302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sun L, Halaihel N, Zhang W, et al. Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in diabetes mellitus. J Biol Chem. 2002;277:18919–18927. doi: 10.1074/jbc.M110650200. ** Cultured human podocytes exposed to the saturated fatty acid palmitate show decreased insulin-stimulated glucose uptake. This study points to the podocyte as a nexus of lipid and glucose metabolism and glomerular disease. Beyond diabetic nephropathy per se, this study has implications for obesity related renal disease.
- 59.Jiang T, Wang Z, Proctor G, et al. Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem. 2005;280:32317–32325. doi: 10.1074/jbc.M500801200. [DOI] [PubMed] [Google Scholar]
- 60.Wang XX, Jiang T, Shen Y, et al. The farnesoid X receptor modulates renal lipid metabolism and diet-induced renal inflammation, fibrosis, and proteinuria. Am J Physiol Renal Physiol. 2009;297:F1587–F1596. doi: 10.1152/ajprenal.00404.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wu Y, Liu Z, Xiang Z, et al. Obesity-related glomerulopathy: insights from gene expression profiles of the glomeruli derived from renal biopsy samples. Endocrinology. 2006;147:44–50. doi: 10.1210/en.2005-0641. * In a rodent model of high fat diet induced proteinuria and glomerular disease, Farnesoid X receptor activation ameliorated triglyceride accumulation, podocyte loss, mesangial expansion, proteinuria as well as inflammatory and oxidative stress.
- 62.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 63.Sharma K, Ramachandrarao S, Qiu G, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118:1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ohashi K, Iwatani H, Kihara S, et al. Exacerbation of albuminuria and renal fibrosis in subtotal renal ablation model of adiponectin-knockout mice. Arterioscler Thromb Vasc Biol. 2007;27:1910–1917. doi: 10.1161/ATVBAHA.107.147645. ** Demonstration of an important communication between adipocytes and podocytes through adiponectin. Decreased adiponectin correlated with increased urinary microalbumin in obese African Americans. Adiponectin null mutant mice had foot process fusion, oxidant stress, and albuminuria which increased with age or diabetes and was ameliorated by treatment with adiponectin.
- 65.Cammisotto PG, Bendayan M. Adiponectin stimulates phosphorylation of AMP-activated protein kinase alpha in renal glomeruli. J Mol Histol. 2008;39:579–584. doi: 10.1007/s10735-008-9198-6. [DOI] [PubMed] [Google Scholar]
- 66.Linscheid P, Christ-Crain M, Stoeckli R, et al. Increase in high molecular weight adiponectin by bariatric surgery-induced weight loss. Diabetes Obes Metab. 2008;10:1266–1270. doi: 10.1111/j.1463-1326.2008.00899.x. [DOI] [PubMed] [Google Scholar]
- 67.Mathieu P, Poirier P, Pibarot P, et al. Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. Hypertension. 2009;53:577–584. doi: 10.1161/HYPERTENSIONAHA.108.110320. [DOI] [PubMed] [Google Scholar]
- 68.Lee DL, Leite R, Fleming C, et al. Hypertensive response to acute stress is attenuated in interleukin-6 knockout mice. Hypertension. 2004;44:259–263. doi: 10.1161/01.HYP.0000139913.56461.fb. [DOI] [PubMed] [Google Scholar]
- 69.Zoccali C, Mallamaci F. Obesity, diabetes, adiponectin and the kidney: a podocyte affair. Nephrol Dial Transplant. 2008;23:3767–3770. doi: 10.1093/ndt/gfn517. [DOI] [PubMed] [Google Scholar]
- 70.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 73.Kluth DC. Pro-resolution properties of macrophages in renal injury. Kidney Int. 2007;72:234–236. doi: 10.1038/sj.ki.5002332. [DOI] [PubMed] [Google Scholar]
- 74.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 76.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 78. Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–3246. doi: 10.2337/db08-0872. ** This study, by combination of immunofluorescence and flow cytometry looking at an array of M1 and M2 markers, showed macrophage phenotypic switch induced by obesity is due to recruitment of different macrophage subtypes.
- 79.Duffield JS, Tipping PG, Kipari T, et al. Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am J Pathol. 2005;167:1207–1219. doi: 10.1016/S0002-9440(10)61209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lim AK, Ma FY, Nikolic-Paterson DJ, et al. Antibody blockade of c-fms suppresses the progression of inflammation and injury in early diabetic nephropathy in obese db/db mice. Diabetologia. 2009;52:1669–1679. doi: 10.1007/s00125-009-1399-3. [DOI] [PubMed] [Google Scholar]
- 81. Wang Y, Wang YP, Zheng G, et al. Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int. 2007;72:290–299. doi: 10.1038/sj.ki.5002275. * Adoptive transfer of classically activated M1 or alternatively activated M2 macrophages into mice with adriamycin nephropathy found that M1 macrophages promote more severe histological and functional injury, whereas M2 macrophage-induced transfused mice had reduced histological and functional injury. The study reiterates that macrophages are effectors of immune injury but can also provide protection against immune injury.