Introduction
Storage of erythrocytes for transfusion purposes is accompanied by a number of morphological and biochemical changes, the storage lesions. Some of these lesions, such as the almost complete, fast disappearance of 2,3-diphoshosphoglycerate and the relatively small, slow decrease in intracellular ATP, may be reversible. Other changes, such as the loss of haemoglobin, and of phospholipid and protein by vesiculation, are not1. Especially the irreversible lesions are likely to affect erythrocyte survival and function after transfusion. Elucidation of the underlying mechanisms may not only result in prolonged storage times, but also - and probably more importantly - in a higher quality of the erythrocyte transfusion product. One of the first requirements for a higher quality is prolonged survival time in vivo or, more specifically, a higher fraction of erythrocytes that survive the first 24 hours after transfusion. The data of recent studies, that used minor blood group antigens and compared the survival characteristics of various products in one and the same recipient, indicate that the percentage erythrocytes that is removed within a few hours after transfusion may be as high as 30%2. This fraction does not only represent nonfunctional erythrocytes, but also may be a major factor in the interaction between the transfused erythrocyte and the patient's immune system. The resulting pathological reactions may cause long-term transfusion side effects such as the development of anti-erythrocyte antibodies, especially in transfusion-dependent patients, and in patients with a chronic inflammation3–6.
Since the pivotal studies showing that physiological removal of old erythrocytes is initiated by their specific recognition by the immune system7, it has become obvious to apply the knowledge of causes and effects of the aging process in vivo to the study of the changes that erythrocytes undergo during storage, an aging process in vitro. In fact, in one of the first attempts to mimick the physiological aging process, storage in blood bank-like conditions was the treatment that yielded the most complete set of biologically relevant aging parameters then available8. These parameters were concentrated on structural and functional changes in band 3, the anion exchanger and major protein of the erythrocyte membrane: increased breakdown as deduced from immunoblot patterns and decreased anion transport capacity as deduced from sulfate exchange characteristics8. Since then, our knowledge on composition and insight in the organisation of the erythrocyte membrane and the role of band 3 therein has vastly expanded. However, our knowledge on the molecular changes that band 3 undergoes during aging in vivo, the effects of these changes on binding of physiological, naturally occurring autoantibodies, and on the induction of pathological autoantibodies, has lagged behind. This hampers a critical evaluation of claims on the occurrence of erythrocyte aging during storage8–10. Here we will review the data that are presently available on changes in band 3 structure and function during storage in blood bank conditions, focusing on their relevance for the generation of immunological removal signals as biomarkers of old and/or damaged erythrocytes.
Changes in band 3 during storage
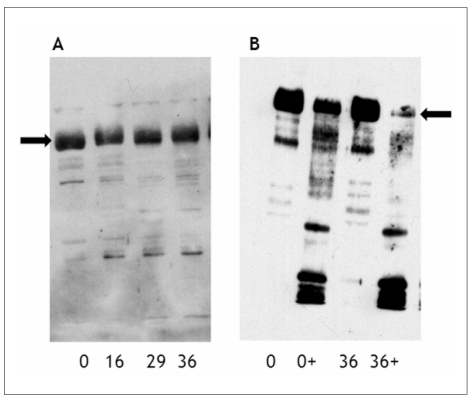
Various immunoblot data indicate progressive alterations in band 3 structure during storage8, 11. These data have been interpreted as indicating storage-associated increases in breakdown and/or aggregation of band 3 molecules (Figure 1A). The results obtained with such immunoblot analyses depend not only on the use of denaturing conditions and on appropriate gels for isolation and separation of high-molecular-weight complexes, but also on the specificity of the antibodies. This has been amply demonstrated, for example in the case of antibodies against regions of band 3 that carry Diego antigens12. In the latter study, the virtual absence of staining of intact band 3, together with the absence of storage-associated changes using anti-Diego and anti-Wright antibodies that were highly reactive in binding to intact erythrocytes, illustrate once more the problem in translating immunoblot data of integral membrane proteins to structural information. This is corroborated by the data from an extensive, semi-quantitative proteomic analysis of the distribution of band 3 peptides over the molecular weight range from 15 - 300 kDa, that showed only a small increase in aggregation and no significant changes in degradation of band 3 with storage13. The relationship between the band 3-containing aggregates and the oligomeric state of band 3 in the membrane is unknown. However, a recent spectroscopic study specifically attempting to chart changes in the formation of oligomers in intact erythrocytes during storage using eosin-5-maleimide, also showed a minor increase in large oligomers, be it after prolonged storage time14. It is unclear whether the band 3-containing complexes of 150–250 kDa consist of intact band 3 in a complex with other proteins that has survived denaturation, or of aggregates of band 3 degradation products. Mapping of the peptides detected by mass spectrometry in the various molecular weight fractions showed coverage of the complete band 3 sequence (unpublished data), which makes the former possibility more likely. One of the proteins complexed with band 3 may very well be haemoglobin, that is found in the same high molecular weight fractions13, and that is strongly associated with erythrocyte membranes after storage1.
Figure 1.
Immunoblot analysis of membrane proteins from erythrocytes of various storage times with anti-band 3 antibodies.
Immunoblot analysis of erythrocytes stored for the indicated periods (0, 16, 29, 36 days) was performed with antibodies against the membrane domain of band 3. +, membranes isolated after incubation with papain. A, increased breakdown and aggregation during storage; B, increased susceptibility to proteolytic breakdown with storage. The arrows indicate the position of intact band 3.
Additional information was obtained by immunoblot analyses of membrane fractions obtained after treatment of intact erythrocytes with proteolytic enzymes, following procedures that are commonly used in blood group serology to make some blood group antigens more accessible12. This approach has yielded data that indicate an increased susceptibility of band 3 to proteolytic degradation with storage time (Figure 1B), associated with increased exposure of epitopes that are involved in recognition of erythrocytes aged in vivo by physiological autoantibodies15. This increase is not only correlated with an increase in the capacity to bind IgG upon incubation with autologous plasma8 (Figure 2), but also with an increase in erythrocyte-bound IgG, probably as the result of the binding of IgG present in the transfusion unit13, 16, 17. The specificity of this IgG remains to be established, although the IgG fraction eluted from stored erythrocytes has been used to identify the extracellular loops of band 3 containing residues 547–553 and 824–829 as crucial in the recognition of old erythrocytes by physiological autoantibodies7. This suggests that, during storage, epitopes originate on band 3 that are identical or at least immunologically related to the epitopes that bestow senescent cell antigen activity upon erythrocytes that have become old in vivo.
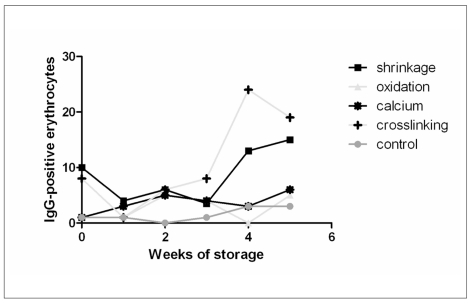
Figure 2.
The effect of treatment of erythrocytes during storage on the capacity to bind autologous IgG.
Erythrocytes of various storage periods were subjected to incubation with hyperosmotic medium inducing cell shrinkage, with hydroperoxide inducing oxidation, with ionomycin inducing an increase in intracellular calcium concentration, and with SITS inducing crosslinking of band 3 molecules. After these treatments the cells were incubated with autologous plasma and IgG binding was determined by flowcytometry using FITC-labeled anti-human IgG (Cappel). Hyperosmotic shock and SITS induce an increase in IgG binding starting at three weeks of storage.
The relationship between storage-associated alterations in band 3 structure and IgG binding is confirmed by the finding that incubation of stored erythrocytes with SITS, a high affinity ligand and crosslinker of band 318, induced a storage time-associated increase in the binding of autologous IgG (Figure 2). An identical, but (much) smaller effect was observed with the structurally related band 3 ligands and anion transport inhibitors DIDS and DNDS, that have a much lower or no crosslinking activity, respectively (data not shown). This suggests that the storage-associated increase in autologous IgG binding capacity is caused specifically by a storage-related increase in the susceptibility of band 3 to crosslinking. Treatment of freshly isolated erythrocytes with SITS also increases the binding of antibodies against senescent cell antigen-involved regions of band 319 from 1.3 to 3.0 percent of the erythrocytes. In addition, the most dense erythrocytes, obtained by discontinuous Percoll separation, are much more sensitive to SITS-induced binding of autologous IgG than the least dense erythrocytes15. Together, these data suggest that there is a relationship between the alterations in band 3 structure and organisation that occur during storage, and those alterations that occur during aging in vivo. An increase in IgG-binding capacity also was observed upon artifical shrinkage, but not by increased intracellular calcium concentrations or by oxidation in vitro (Figure 2). The latter results show that, although oxidation and increasing the intracellular calcium concentration may cause the same type of damage as accumulating during storage, this damage does not cause immunologically identical effects. Preliminary data suggest that the sensitivity to these stressors may vary with donor and/or transfusion unit (Bosman et al., in preparation).
Consequences of storage-associated changes in band 3
Thus, storage-induced changes in band 3 structure are associated with binding of autologous IgG in the transfusion unit, and are likely to induce binding of senescent cell-specific, autologous IgG after transfusion to at least a fraction of the erythrocytes. Alternatively, the central role of band 3 in the organisation of the erythrocyte membrane implicates that changes in band 3 structure may lead to loss of recognition of self by the loss of CD47 activity, or the generation of antigenic changes in Rhesus proteins20, 21. In view of the preliminary data indicating an increased susceptibility of band 3 on stored erythrocytes to various treatments in vitro (Figures 1 and 2), we postulate that antigenic changes in band 3 structure will happen after transfusion as well. These changes could very well be the result of the stress that erythrocytes undergo during their passage through the body, such as shrinkage by hyperosmotic shock, oxidation by the constant binding and release of oxygen, and increased calcium concentrations resulting from loss of membrane integrity resulting from deformation-induced mechanical stress22. This may contribute to the fast removal of a considerable fraction of the transfused erythrocytes2. Exposure of senescent cell antigen-related epitopes on band 3 that originate during storage or after transfusion may induce the formation of neoantigens and new antibodies, and eventually the development of autoimmune haemolytic anaemia. Indeed, transfusions are a risk factor for developing autoantibodies23, mostly directed against targets on the Rhesus proteins and the senescent cell antigen-associated regions of band 324, 25.
The central role of band 3 in the maintenance of erythrocyte shape is the cause of a causal link between alterations in band 3 structure, erythrocyte morphology, and cell deformability. Many mutations in band 3 are associated with an abnormal cell shape as well as with haematological problems26. During storage, morphological alterations are associated with a decrease in deformability27, and with immunochemical indications for altered membrane and band 3 organisation10, 17. A loss of binding between band 3 and the cytoskeleton may be responsible for the storage-related increase in band 3 oligomerization and/or aggregation13, 14, as well as for an increased susceptibility to crosslinking (Figure 2). The decrease in band 3-mediated linkage between lipid bilayer and the cytoskeleton by itself could be enough to cause release of vesicles28. Thus, changes in band 3 structure are not only responsible for recognition by natural autoantibodies but also for vesiculation. Vesicle release in vivo is considered an integral part of the physiological aging process, enabling the removal of damaged membrane patches from otherwise functional erythrocytes19. This theory is supported by the observations that, during storage of erythrocytes in transfusion units: 1, vesicle formation increases strongly with storage time29; 2, vesicles are enriched in modified haemoglobin species, including the erythrocyte aging marker HbA1c15; 3, vesicles contain much more aggregated as well as degraded band 3 than erythrocytes of the same storage time13; 4, vesicles are enriched in strong removal signals such as phosphatidylserine, IgG and complement proteins13,15, 17.
Band 3 is involved in mediating deoxyhaemoglobin-regulated erythrocyte metabolism by phosphorylation-dependent binding of key enzymes of the glycolysis30. Thus, band 3 alterations during storage affect the binding between cytoskeleton and lipid bilayer, as well as signaling of ATP, and 2,3-DPG production. The former will affect ion homeostasis and cell deformability, and the latter oxygen binding and release by haemoglobin. Both effects will hamper the functional quality of the erythrocytes after transfusion. Other processes may become equally affected by changes in band 3 structure during storage. The close association between glucose transporter and band 3 for example, may explain the correlation between alterations in band 3 structure and glucose transport characteristics observed earlier20,31. This may also be true for the, as yet mostly unknown, functions of the other components of the band 3 complex.
It has been proposed that in pathological conditions such as thalassemia, sickle cell disease, malaria and G6PD deficiency, the first trigger for recognition by naturally occurring anti-band 3 autoantibodies is denatured haemoglobin-induced oxidation of band 3. This would then be followed by tyrosine phosphorylation of the cytoplasmic domain of band 3, thereby potentiating clustering and subsequent neoantigen exposure32. It remains to be established if this series of events also occurs during aging in vivo, but all the observations summarized above suggest that this mechanism may be operative during storage. This would classify erythrocyte storage as a pathological condition. In our opinion, future research should focus on the prevention of the emergence of these pathological erythrocytes in the erythrocyte transfusion unit, and on their removal from the transfusion unit before transfusion. Elucidation of the molecular mechanisms of storage-associated changes in band 3 is likely to be the most efficient way towards this goal. The knowledge summarized here may provide one set of tools in this endeavour, such as erythrocyte fraction-specific antibodies and functionally relevant markers for erythrocyte damage. Another set of tools consists of the rapidly developing methodology in the field of proteomics, especially with respect to identifying and measuring posttranslational modifications. We foresee that the first inventories of the application of proteomics tools to the research of blood components such as collected in this issue, will contribute to the growth of our knowledge on erythrocyte pathology, in vivo as well as in vitro.
References
- 1.Bosman GJCGM, Werre JM, Willekens FLA, Novotný VMJ. Erythrocyte aging in vivo and in vitro: structural aspects and implications for transfusion. Transfusion Medicine. 2008;18:1–13. doi: 10.1111/j.1365-3148.2008.00892.x. [DOI] [PubMed] [Google Scholar]
- 2.Luten M, Roerdinkholder-Stoelwinder B, Schaap NPM, et al. Survival of red blood cells after transfusion: a comparison between red cell concentrates of different storage periods. Transfusion. 2008;48:1478–85. doi: 10.1111/j.1537-2995.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 3.Perrotta PL, Snyder EL. Non-infectious complications of transfusion therapy. Blood Rev. 2001;15:69–83. doi: 10.1054/blre.2001.0151. [DOI] [PubMed] [Google Scholar]
- 4.Tinmouth A, Chin-Yee I. The clinical consequences of the red cell storage lesion. Transfus Med Rev. 2001;15:91–107. doi: 10.1053/tmrv.2001.22613. [DOI] [PubMed] [Google Scholar]
- 5.Hendrickson JE, Chadwick TE, Roback JD, et al. Inflammation enhances consumption and presentation of transfused RBC antigens by dendritic cells. Blood. 2007;110:2736–43. doi: 10.1182/blood-2007-03-083105. [DOI] [PubMed] [Google Scholar]
- 6.Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risk of allogeneic blood transfusion and the available strategy for their prevention. Blood. 2009;113:3406–17. doi: 10.1182/blood-2008-10-167643. [DOI] [PubMed] [Google Scholar]
- 7.Kay MMB. Immunoregulation of cellular life span. Ann. NY Acad. Sci. 2005;1057:85–111. doi: 10.1196/annals.1356.005. [DOI] [PubMed] [Google Scholar]
- 8.Bosman GJCGM, Kay MMB. Erythrocyte aging: a comparison of model systems for simulating cellular aging in vitro. Blood Cells. 1988;14:19–35. [PubMed] [Google Scholar]
- 9.Bratosin D, Tcacenco L, Sidoroff M, et al. Active caspases-8 and -3 in circulating human erythrocytes purified on immobilized annexin-V: a cytometric demonstration. Cytometry. 2009;75:236–44. doi: 10.1002/cyto.a.20693. [DOI] [PubMed] [Google Scholar]
- 10.Antonelou MHA, Kriebardis AG, Stamoulis KE, et al. Red blood cell aging markers during storage in citrate-phosphate-dextrose-saline-adenine-glucose-mannitol. Transfusion. 2010;50:376–89. doi: 10.1111/j.1537-2995.2009.02449.x. [DOI] [PubMed] [Google Scholar]
- 11.Messana I, Ferroni L, Misiti F, et al. Blood bank conditions and RBCs: the progressive loss of metabolic modulation. Transfusion. 2000;40:353–60. doi: 10.1046/j.1537-2995.2000.40030353.x. [DOI] [PubMed] [Google Scholar]
- 12.Bosman GJCGM, Klaarenbeek JM, Luten M, Bos HJ. Storage-related changes in erythrocyte band 3: not a case for the Diego blood group antigens. Cell Mol Biol. 2005;51:195–200. [PubMed] [Google Scholar]
- 13.Bosman GJCGM, Lasonder E, Luten M, et al. The proteome of red cell membranes and vesicles during storage in blood bank conditions. Transfusion. 2008;48:827–35. doi: 10.1111/j.1537-2995.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 14.Karon BS, Hoyer JD, Stubbs JR, Thomas DD. Changes in band 3 oligomeric state precede cell membrane phospholipid loss during blood bank storage of red blood cells. Transfusion. 2009;49:1435–42. doi: 10.1111/j.1537-2995.2009.02133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosman GJCGM, Lasonder E, Groenen-Döpp YAM, et al. Comparative proteomics of erythrocyte aging in vivo and in vitro. J Proteomics. 2010;73:396–402. doi: 10.1016/j.jprot.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Luten M, Roerdinkholder-Stoelwinder B, Bos HJ, Bosman GJCGM. Survival of the fittest? Survival of stored red blood cells after transfusion. Cell Mol Biol. 2004;50:197–203. [PubMed] [Google Scholar]
- 17.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. Storage-dependent remodeling of the red blood cell membrane is associated with increased immunoglobulin G binding, lipid raft rearrangement, and caspase activation. Transfusion. 2007;47:1212–20. doi: 10.1111/j.1537-2995.2007.01254.x. [DOI] [PubMed] [Google Scholar]
- 18.Jennings ML, Passow H. Anion transport across the erythrocyte membrane, in situ proteolysis of band 3 protein, and cross-linking of proteolytic fragments by 4,4′-diisothiocyanodihydrostilbene-2,2′-disulfonate. Biochim. Biophys Acta. 1979;554:498–519. doi: 10.1016/0005-2736(79)90387-0. [DOI] [PubMed] [Google Scholar]
- 19.Willekens FLA, Werre JM, Groenen-Döpp YAM, et al. Erythrocyte vesiculation: a self-protective mechanism? Brit J Haematol. 2008;141:549–56. doi: 10.1111/j.1365-2141.2008.07055.x. [DOI] [PubMed] [Google Scholar]
- 20.Mohandas N, Gallagher PG. Red cell membrane: past, present, and future. Blood. 2008;112:3939–48. doi: 10.1182/blood-2008-07-161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruce LJ, Ghosh S, King MJ, et al. Absence of CD47 in protein 4.2-deficient hereditary spherocytosis in man: an interaction between the Rh complex and the band 3 complex. Blood. 2002;100:1878–85. doi: 10.1182/blood-2002-03-0706. [DOI] [PubMed] [Google Scholar]
- 22.Lang F, Lang KS, Lang PA, et al. Mechanisms and significance of eryptosis. Antiox Redox Signal. 2006;8:1183–92. doi: 10.1089/ars.2006.8.1183. [DOI] [PubMed] [Google Scholar]
- 23.Young PP, Uzieblo A, Trulock E, et al. Autoantibody formation after alloimmunization: are blood transfusions a risk factor for autoimmune hemolytic anemia? Transfusion. 2004;44:67–72. doi: 10.1046/j.0041-1132.2003.00589.x. [DOI] [PubMed] [Google Scholar]
- 24.Iwamoto S, Kamesaki T, Oyamada T, et al. Reactivity of autoantibodies of autoimmune hemolytic anemia with recombinant Rhesus blood group antigens or anion transporter band 3. Am J Hematol. 2001;68:106–14. doi: 10.1002/ajh.1161. [DOI] [PubMed] [Google Scholar]
- 25.Janvier D, Lam Y, Galicier L, Bierling P. A new cold autoagglutinin specificity: the third external loop of band 3. Transfusion. 2010;50:47–52. doi: 10.1111/j.1537-2995.2009.02383.x. [DOI] [PubMed] [Google Scholar]
- 26.Ann X, Mohandas N. Disorders of red cell membrane. Brit J Haematol. 2008;141:367–75. doi: 10.1111/j.1365-2141.2008.07091.x. [DOI] [PubMed] [Google Scholar]
- 27.Relevy H, Koshkaryev A, Manny N, et al. Blood banking-induced alteration of red blood cell flow properties. Transfusion. 2008;48:136–46. doi: 10.1111/j.1537-2995.2007.01491.x. [DOI] [PubMed] [Google Scholar]
- 28.Gov N, Cluitmans J, Sens P, Bosman GJCGM. Cytoskeletal control of red blood cell shape: theory and practice of vesicle formation. Adv. Planar Lipid Bilayers Liposomes. 2009;10:95–119. [Google Scholar]
- 29.Greenwalt TJ. The how and why of exocytic vesicles. Transfusion. 2006;46:143–52. doi: 10.1111/j.1537-2995.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 30.Lewis IA, Campanella ME, Markley JL, Low PS. Role of band 3 in regulating metabolic flux of red blood cells. Proc Natl Acad Sci USA. 2009;106:18515–20. doi: 10.1073/pnas.0905999106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosman GJCGM, Kay MMB. Alterations of band 3 transport protein by cellular aging and disease: erythrocyte band 3 and glucose transporter share a functional relationship. Biochem Cell Biol. 1990;68:1419–27. doi: 10.1139/o90-205. [DOI] [PubMed] [Google Scholar]
- 32.Pantaleo A, Giribaldi G, Mannu F, et al. Naturally occurring anti-band 3 antibodies and red blood cell removal under physiological and pathological conditions. Autoimm Rev. 2008;7:457–62. doi: 10.1016/j.autrev.2008.03.017. [DOI] [PubMed] [Google Scholar]