Abstract
Background.
Deficiency or dysfunction of coagulation factor VIII (FVIII) is the underlying cause of haemophilia A. Haemophilic patients are at present treated with plasma-derived FVIII (pdFVIII) or recombinant FVIII (rFVIII) in order to correct their clotting deficiency. pdFVIII concentrates are exclusively produced from human plasma upon pooling from multiple donors. It is not know whether the presence of excess of other plasma proteins, in addition to von Willebrand factor, could stimulate untoward immune responses in the recipient. Thus, information regarding the presence of contaminants in commercial products is of concern.
Materials and Methods.
Two commercially available pdFVIII concentrates were characterized through SDS-PAGE and mass spectrometry Emoclot® and Beriate®.
Results.
The components of two pdFVIII products considered in this study were well identified by mass spectrometry analysis, in both cases we found abundant components coming from blood plasma, and some other contaminants. Only in Beriate® we also found truncated form of pdFVIII.
Conclusion.
The two pdFVIII examined showed the presence of vWF, Fibrinogen in excess, and other substances that could be considered as contaminants or impurities.
Keywords: contaminants, mass spectrometry, SDS-PAGE, haemophilia A, plasma derived FVIII
Introduction
Factor VIII (FVIII) is a plasma glycoprotein that functions as a cofactor for the serine protease factor IXa in the proteolytic activation of factor X to Xa. Although FVIII is synthesized as a single chain, it circulates in plasma as a heterodimer. The chains can be dissociated by EDTA, indicating that they are kept together by metal ions1.
The non-covalent heterodimer contains a heavy chain (HCh), with a molecular weight between 90 to 200 kDa (A1-A2-B domains), and a light chain (LCh), with a molecular weight of 80 kDa (A3-C1-C2 domains).
FVIII plays an important role in the intrinsic pathways of the blood coagulation cascade. It circulates as an inactive pro-cofactor in complex with von Willebrand factor (vWF). Thrombin is the principal physiological activator of FVIII. This protease cleaves the protein at sites in both the HCh and the LCh. Activated FVIII (FVIIIa) is a heterotrimer of 50, 43 and 73 kDa subunits, which are required for procoagulant activity2,3.
FVIII occurs in human plasma in very low concentrations of about 100–200 ng/mL, corresponding to one unit of FVIII per mL4. Insufficient levels or the total absence of this protein in the bloodstream lead to coagulation disorders, widely known as haemophilia A2,3. This is a severe bleeding disorder, which results from a defect or a deficiency in FVIII. Haemophilia A is an X-linked disease caused by defects in the expression or function of the plasma glycoprotein FVIII5–7. Maintenance of normal levels of FVIII in the circulation is dependent on its formation of a complex with vWF. FVIII and vWF are assembled in a tight non covalent complex which is essential for its stability in the plasma. Patients with severe von Willebrand disease, where vWF is deficient or where the binding between FVIII and vWF is impaired, have a secondary deficiency of FVIII2,3,8. When FVIII is bound to vWF, a stable association of its HCh and LCh is maintained8–9. von Willebrand factor also protects FVIII from cleavage by activated FX and from protein C-catalyzed degradation2,10,11.
The clinical manifestations of haemophilia A are categorized with respect to the severity of FVIII deficiency. FVIII activity levels of 5–25% are associated with a mild disease state, 1–5% as moderate, and less than 1% as severe12.
Severe haemophiliacs may have spontaneous haemorrhages, requiring therapy two to four times monthly13.
Haemophilic patients are at present treated with plasma-derived FVIII (pdFVIII) or recombinant FVIII (rFVIII), to correct their clotting defciency14,15. Bleeding episodes are managed by administration of FVIII-rich plasma cryoprecipitate or commercial preparations of lyophilised FVIII concentrates1, which are prepared either from human plasma or by recombinant technology. In the case of plasma prepared FVIII, viral safety is an essential requirement and the purification process should inactivate both enveloped and non-enveloped viruses. Solvent-detergent and heat treatment are frequently used to inactivate both viruses. According to literature data, these treatments could result in undesired effect such as: (a) increase of the antigenic properties of the injected material that may induce formation of inhibitory antibodies (Abs) in patients with severe haemophilia A9–11; (b) increase of high molecular weight (HMW) aggregates which may interfere with the complete dissolution of the lyophilised FVIII concentrates, when properly reconstituted. Therefore the European Pharmacopoeia is modifying the FVIII monograph, allowing the use of a filter, provided in the package, to eliminate small flakes or particles16. Regardless of the grade of purity, all FVIII concentrates and especially plasma derived concentrates could present formation of HMW aggregates, having variable solubility, even if the formation mechanism and aggregates composition can be different. Nowadays it is not known if virus inactivation processes may induce protein alterations as well, as pdFVIII may contain impurities or contaminants.
In this study, by using monodimensional gel electrophoresis (SDS-PAGE) and Mass Spectrometry, we revealed in Beriate® the presence of galectin 3 binding protein which coeluted with truncated form of FVIII, inter-alfa globulin and, in Emoclot® alfa 1 microglobulin, IGHM that could be considered as contaminants.
Materials and method
Commercially-available pdFVIII concentrates were used in this study. Materials consisted of Beriate® 1000 UI/10mL (Aventis Behring) batch 81865011E and Emoclot® 1000 UI/10mL batch KA0805 (Kedrion), both products were reconstituted according to the manufacturers’ instructions.
Sample preparation
Protein concentration was estimated by the Bradford method. 40 μg of samples were precipitated with 80% acetone for 2h at −20 °C.
The sample was centrifuged at 14 000 rpm for 12 min, the supernatant was removed and the pellet was air dried. The pellets were suspended in 100 μL of sample buffer and were incubated for 20 min in 100 mM DTT, 12% sucrose, 0.6% urea, 50 mM Tris-HCl pH 6.8, 0.01% bromophenol blue at room temperature.
SDS-PAGE
SDS-PAGE was performed according to Laemmli’s17 procedure under reducing conditions. Reagents for electrophoretic analyses were obtained from Bio-Rad. The experiment was performed on a 12%, 11%, 9% and 7.5% acrylamide gel, in a chamber with the following dimension 12x16x18 cm. The second electrophoresis gel was performed at 7.5% using a chamber with the following dimension 29x32x17 cm. A Protean II Bio-Rad (Hercules, CA, USA) electrophoresis system (1803160 mm, 0.75 mm thick) was used for the first dimension. Gels were fixed and stained for 30 min in a 5:1:4 (v/v) methanol-glacial acetic acid-water mixture. Protein bands were stained with Coomassie Brilliant Blue R-250.
In-gel-digestion
Bands from the monodimensional gel were carefully excised and subjected to in-gel trypsin digestion according to Shevchenko et al.18 with minor modifications.
The gel pieces were swollen in a digestion buffer containing 50 mM NH4HCO3 and 12.5 ng/μL trypsin (modified porcine trypsin, sequencing grade, Promega, Madison, WI) in an ice bath. After 30 min, the supernatant was removed and discarded, 20 μL of 50 mM NH4HCO3 were added to the gel pieces, and digestion was allowed to proceed at 37 °C overnight. The supernatant containing tryptic peptides was dried by vacuum centrifugation.
RP-nanoHPLC mass spectrometry
Prior to mass spectrometric analysis, the peptide mixtures were re-dissolved in 10 μL of 5% FA (formic acid). Peptide Sequencing was performed by Nano-RP-HPLC-ESI-MS/MS. Peptide mixtures were separated using a nano flow-HPLC system (Ultimate; Switchos; Famos; LC Packings, Amsterdam, The Netherlands). A sample volume of 10 μL was loaded through the autosampler onto a homemade 2 cm fused silica precolumn (75 μm i.d.; 375 μm o.d.; Reprosil C18-AQ, 3 μm, Ammerbuch- Entringen, Germany) at a flow rate of 2 μL/min. Sequential elution of peptides was accomplished using a flow rate of 200 nL/min and a linear gradient from Solution A (2% acetonitrile; 0.1% formic acid) to 50% of Solution B (98% acetonitrile; 0.1%formic acid) in 40 min over the precolumn in-line with a homemade 10–15 cm resolving column (75 μm i.d.; 375 μmo.d.; Reprosil C18-AQ, 3 μm, Ammerbuch-Entringen). Peptides were eluted directly into a high capacity ion trap (model HCTplus Bruker-Daltonik, Germany). Capillary voltage was 1.5–2 kV and a dry gas flow rate of 10 L/min was used with a temperature of 200 °C. The scan range used was from 300 to 1800 m/z. Protein identification was performed by searching in the National Center for Biotechnology Information nonredundant (NCBInr) database using the MASCOT program (http://www.matrixscience.com). The following parameters were adopted for database searches: complete carbamidomethylation of cysteines, partial oxidation of methionines, peptide mass tolerance (1.2 Da, fragment mass tolerance (0.9 Da and missed cleavages 2. For positive identification, the score of the result of [−10 × Log(P)] had to be over the significance threshold level (P< 0.05).
Results
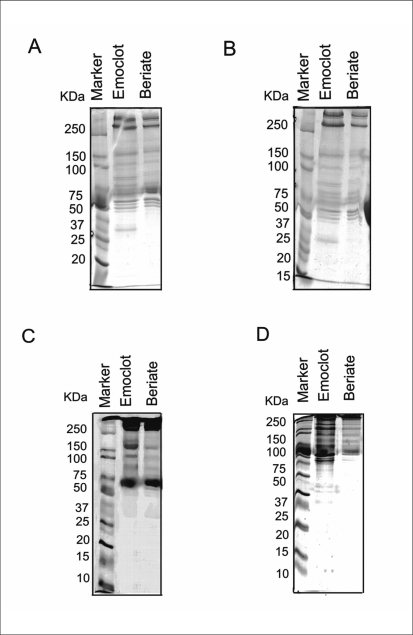
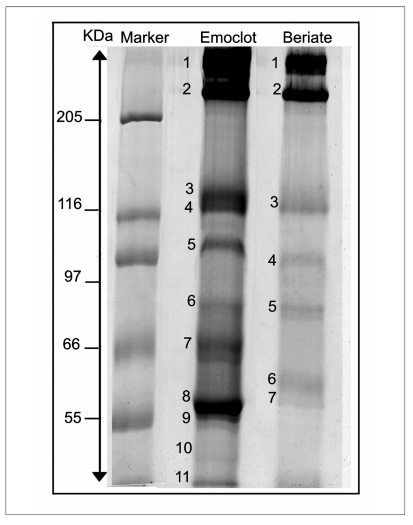
Two commercially-available preparations of plasma FVIII concentrates Beriate® and Emoclot ® were examined to determine eventual impurities and/or contaminants. To this regard, SDS-PAGE was used for protein separation, and mass spectrometry for protein identification. Figure 1 compares the mini gel electrophoretic separation pattern of pdFVIII from two different manufacturers: Emoclot® and Beriate®. We use different concentrations of acrylamide, ranging from 7.5% to 12 % (see Figure 1 A–D) and mini gel to set up the best condition. As shown in fig. 1, best results were obtained by using acrylamide at 7,5%. Figure 2 shows a new large gel-electorophoresis SDS PAGE performing at 7.5% acrylamide. We apply mass spectrometry to identify proteins, by cutting bands from e SDS-PAGE of Beriate® and Emoclot® (Figure 2). This avoided any further manipulation of samples.
Figure 1.
Mini gel SDS PAGE of two pdVIII preparations: Emoclot ® 1000 UI/10 mL batch KA0805 Kedrion and Beriate® P1000 batch 81865011E CLS Behring.
Samples are reduced according to Laemmli’s17 method and are analysed at different concentration of acrilamide a) 7,5% b) 9% c) 11% d) 12%.
Figure 2.
SDS-PAGE (7.5%) of reduced samples (pdFVIII) according to Laemmli’s17 method. Protein are detected by Coomassie Brilliant Blue.
Lane 1 HMW standard, Lane 2: 40 ug of pdFVIII Emoclot ® 1000 UI/10 mL batch KA0805 Kedrion, Lane 3: 40 ug of pdFVIII Beriate® P1000 batch 81865011E CLS Behring.
Table I reports the identification spots numbered as in Figure 2.
Table I.
List of protein identifications
| Spot number | Protein name | ID | Mascot score % | p/M r | Number mathced peptides | NCBI Accession number |
|---|---|---|---|---|---|---|
| BERIATE | ||||||
| 1 BE | von Willebrand factor prepropeptide | 5.44 | 1756 | 237661 | 53 | gi|340361 |
| 2 BE | inter-alpha (globulin) inhibitor H2 [Homo sapiens] | 6.59 | 849 | 99813 | 29 | gi|119585664 |
| 2 BE | alpha-1-microglobulin; bikunin [Homo sapiens] | 5.58 | 251 | 17215 | 5 | gi|579676 |
| 3 BE | inter-alpha (globulin) inhibitor H2 [Homo sapiens] | 6.56 | 816 | 105606 | 20 | gi|55958063 |
| 4 BE | Chain A, Crystal Structure Analysis Of Coagulation Factor VIII | 5.74 | 186 | 85365 | 4 | gi|183448129 |
| 4 BE | galectin 3 binding protein [Homo sapiens] | 5.13 | 285 | 66202 | 5 | gi|5031863 |
| 5 BE | Chain B, Crystal Structure Of Human Fibrinogen | 8.31 | 819 | 55545 | 22 | gi|237823915 |
| 6 BE | kininogen 1 isoform 2 [Homo sapiens] | 6.29 | 147 | 48936 | 3 | gi|4504893 |
| 7 BE | fibrinogen gamma chain [Homo sapiens] | 5.61 | 293 | 50077 | 6 | gi|182439 |
| EMOCLOT | ||||||
| 1 EMO | von Willebrand factor preproprotein [Homo sapiens] | 5.44 | 1665 | 237661 | 50 | gi|340361 |
| 2 EMO | von Willebrand factor prepropeptide | 5.44 | 703 | 237661 | 20 | gi|340361 |
| 3 EMO | alpha-1-microglobulin; bikunin [Homo sapiens] | 5.58 | 172 | 17215 | 3 | gi|579676 |
| 3 EMO | von Willebrand factor preproprotein [Homo sapiens] | 5.30 | 782 | 322429 | 19 | gi|89191868 |
| 4 EMO | coagulation factor VIII, procoagulant component (hemophilia A), isoform CRA_b [Homo sapiens] | 6.51 | 73 | 239233 | 4 | gi|171849087 |
| gi|119593052 | ||||||
| 4 EMO | alpha-1-microglobulin; bikunin [Homo sapiens] | 5.58 | 154 | 17215 | 3 | gi|579676 |
| 5 EMO | kininogen 1 isoform 2 [Homo sapiens] | 6.29 | 486 | 48936 | 16 | gi|4504893 |
| 6 EMO | IGHM protein [Homo sapiens] | 5.82 | 292 | 66032 | 7 | gi|12804853 |
| 6 EMO | alpha-fibrinogen precursor | 8.26 | 250 | 70223 | 7 | gi|182424 |
| 6 EMO | B domain-deleted coagulation factor VIII [synthetic construct] | 6.36 | 600 | 168754 | 16 | gi|157863686 |
| 7 EMO | alpha-fibrinogen precursor | 8.26 | 1166 | 70223 | 39 | gi|182424 |
| 8 EMO | beta-fibrinogen precursor | 8.31 | 209 | 55545 | 6 | gi|182430 |
| 9 EMO | fibrinogen, beta chain preproprotein [Homo sapiens] | 1311 | 56577 | 53 | gi|70906435 | |
| 10 EMO | fibrinogen gamma chain [Homo sapiens] | 5.61 | 480 | 50077 | 12 | gi|182439 |
| 11 EMO | fibrinogen gamma chain [Homo sapiens] | 5.61 | 657 | 50077 | 17 | gi|182439 |
| 11 EMO | Chain B, Structure Of Complement C3b: Insights Into Complement Activation And Regulation | 5.18 | 325 | 104912 | 8 | gi|118137965 |
The most abundant proteins are in the spot 1 and 2 for both Emoclot ® and Beriate®. They contain vWF as a predominant protein with 49 recognized peptides covering the 17% of sequence. The percentage excess of vWF over pdFVIII is more than 90% in pdFVIII preparations. In both cases we found a co-eluted fibronectin 1 protein which is one of the most abundant components in the blood plasma. Besides the presence of fibronectin, both samples showed a significant amount of inter-alpha inhibitor (IaI), pre-alpha inhibitor (PaI) which represented the major contaminant, together to fibrinogen and Kininogen. Interestingly Emoclot® contained human IgM heavy chain (spot 6) and in the same band we found FVIII, which is very weak comparing to fibrinogen and vWF. In Beriate®, it is interesting to underline that we detected the presence galectin 3 binding protein (G3BP), which co-eluted with FVIII. In this case we are in presence of truncated form of FVIII revealed by mass spectrometry as ‘Chain A, Crystal Structure Analysis Of Coagulation FVIII’ with accession number: gi|183448129.
Discussion
From our analysis, it came out that both Beriate® and Emoclot® contained a large amount of vWF, as well as of other plasma proteins such us fibrinogen, fibronectin, and IgM heavy chain. This is not surprising, since the production of pdFVIII concentrates requires large plasma volumes and includes several consecutive concentration and purification steps using cryoprecipitation techniques, and chromatographic steps, which reduce but not eliminate all components of plasma. Several in vitro studies have indicated that the presence of excess of these plasma proteins, in addition to vWF, could influence the function of cellular components of the immune system19.
In particular, in agreement with Josic,20 in Beriate® and Emoclot® we have isolated IaIp as a by-product of clotting of FVIII from human plasma. IaIp are a family of structurally-related serine protease inhibitors which are found in relatively high concentrations in human plasma. Recent studies have implicated a role for IaIp in sepsis, and have demonstrated their potential as biomarkers in sepsis and cancer, as IaIp likely exert an important role in tumour invasion and metastasis and in inflammation21.
Recently, a significant decrease of IaIp levels has been shown in plasma of adult patients and newborns with clinically-proven sepsis22,23.
Moreover, experimental animal studies have demonstrated the beneficial effects of IaIp in improving survival and halting the progression of sepsis in adult animals24,25.
These results highlight the potential of IaIp as a diagnostic and therapeutic agent22,24–28. However in this specific case, during pdFVIII purification, they could represent a protein burden to the patient and, in improved pdFVIII preparations, protein contaminations should be reduced as they may cause an immunogenic response,29 as reported also by Josic et al. 20. Hereby we showed that pdFVIII concentrates contain a significant amount of foreign proteins which, have been shown to be related to severe side reactions in the recipient 30.
In Beriate®, we found G3BP which Bolsen et al.,31 claimed to be a potential contaminant of recombinantly produced factor IX. G3BP is a secreted glycoprotein present in the extracellular matrix of many tissues. Galectins and their binding proteins have primarily been described in cell-cell and cell-matrix interactions involved in autoimmunity, inflammation and cancer biology 32,33.
In the present study we found truncated form of pdFVIII in Beriate® because of proteolytic process that could be occurred during purification procedure30. FVIII is sensitive to proteolysis34, and, therefore, it has to be stabilized during the production process and in the final formulation 34. Besides proteolysis, most serious complication is the development of inhibitor antibodies most frequently at an early stage of therapy, caused from truncated form of FVIII. These antibodies are capable of blocking FVIII procoagulant activity35,36. There have been dangerous outbreaks of inhibitors in multitransfused patients in the past, and they seem to be due to the creation of neoepitopes in the FVIII molecule during the manufacturing process 37–39.
In the present study, G3BP appears in the same spot of pdFVII due to the similar apparent molecular weight, G3BP plays a role in cell apoptosis and have relevant immunomodulatory activities given its role in autoimmunity, tumour metastasis and inflammation. This may be especially relevant in haemophiliacs as these patients are often chronically infected with hepatitis C and/or HIV and may be immuno-compromised. Elevated G3BP levels in AIDS patients seem to correlate with higher viral loads, especially in haemophiliac HIV-positive patients 40–42. Therefore, the presence of G3BP may have untoward effects on the health of these patients.
In conclusion most pdFVIII concentrates contain excess of plasma proteins which could influence the function of cellular components of the immune system19 and other contaminants, whose broad biological effects could result in unforeseen consequences in the recipients when infused for therapeutic purposes.
References
- 1.Iannetti A, Acquaviva R, Formisano S, et al. Characterization of high molecular weight aggregates in human plasma derived Factor VIII. Haema. 2006;9:535–42. [Google Scholar]
- 2.O’Brien DP, Tuddenham EGD. Bloom AL, Forbes CD, Thomas DP, editors. The structure and function of factor VIII. Haemostasis and Thrombosis. 1997. p. 333.
- 3.Kemball CG, Tuddenheim EGD. Forbes CD, Aledort L, Medhok R, editors. The molecular defect in hemophilia A. Haemophilia. p. 21.
- 4.Blum H, Beier H, Gross HJ. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–9. [Google Scholar]
- 5.Davie EW, Fujikawa K, Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991;30:10363–70. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- 6.Davie EW. Biochemical and molecular aspects of the coagulation cascade. Thrombosis and Haemostasis. 1995;74:1–6. [PubMed] [Google Scholar]
- 7.Mann KG. Biochemistry and physiology of blood coagulation. Thrombosis and Haemostasis. 1999;82:165–74. [PubMed] [Google Scholar]
- 8.Saenko EL, Scandella DJ. The acidic region of the factor VIII light chain and the C2 domain together form the high affinity binding site for von Willebrand factor. Biol Chem. 1997;272:18007–14. doi: 10.1074/jbc.272.29.18007. [DOI] [PubMed] [Google Scholar]
- 9.Wise RJ, Dorner AJ, Krone M, et al. The role of von Willebrand factor multimers and propeptide cleavage in the binding and stabilization of factor VIII. J Biol Chem. 1991;266:21948–55. [PubMed] [Google Scholar]
- 10.Koppelman SJ, Koedam JA, Wijnen M, et al. Von Willebrand factor as a regulator of intrinsic factor X activation. J Lab Clin Med. 1994;123:585–93. [PubMed] [Google Scholar]
- 11.Fay PJ, Coumans JV, Walker FJ. Von Willebrand factor mediates protection of factor VIII from activated protein C-catalyzed inactivation. J Biol Chem. 1991;266:2172–7. [PubMed] [Google Scholar]
- 12.Hoyer LW, Trabold NC. The effect of thrombin on human factor VIII. Cleavage of the factor VIII procoagulant protein during activation. J Lab Clin Med. 1997;1:50–64. [PubMed] [Google Scholar]
- 13.Levine PH, Brettler DB.Clinical Aspects and Therapy for Hemophilia A. In: Hematology: Basic Principles and Practice Hoffman R, Benz EJ, Shattil SJ, et al.19911290–1304.
- 14.Toole JJ, Pittman DD, Orr EC, et al. A large region (approximately equal to 95 kDa) of human factor VIII is dispensable for in vitro procoagulant activity. Proc Natl Acad Sci USA. 1986;83:5939–52. doi: 10.1073/pnas.83.16.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prescott R, Nakai H, Saenko E, et al. Recombinate and kogenate study groups. Blood. 1997;89:3663–71. [PubMed] [Google Scholar]
- 16.European Pharmacopoeia 5th Ed., Suppl 5.3 -Monography 0275: Human Coagulation Factor VIII.
- 17.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–6. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–8. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 19.Mannucci PM. The choice of plasma-derived clotting factor concentrates. Bailliere’s Clinical Haematology. 1996;9:273–90. doi: 10.1016/s0950-3536(96)80063-1. [DOI] [PubMed] [Google Scholar]
- 20.Josic D, Brown MK, Huang F, et al. Proteomic characterization of inter-alpha inhibitor proteins from human plasma. Proteomics. 2006;6:2874–85. doi: 10.1002/pmic.200500563. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi H, Shinohara H, Oi H, et al. Urinary trypsin inhibitor (UTI) and fragments derived from UTI by limted proteolysts efficiently inhibit tumor cell invasion. Clin Exp Metastasis. 1994;12:117–28. doi: 10.1007/BF01753978. [DOI] [PubMed] [Google Scholar]
- 22.Lim YP, Bendelja K, Opal SM, et al. Correlation between mortality and the levels of inter-alpha inhibitors in the plasma of patients with severe sepsis. J Infect Dis. 2003;188:919–26. doi: 10.1086/377642. [DOI] [PubMed] [Google Scholar]
- 23.Baek YW, Brokat S, Padbury JF, et al. Inter-alpha Inhibitor Proteins in infants and Decreased Levels in Neonatal Sepsis. J Pediatr. 2003;143:11–5. doi: 10.1016/S0022-3476(03)00190-2. [DOI] [PubMed] [Google Scholar]
- 24.Yang S, Lim YP, Zhou M, et al. Administration of human inter-alpha inhibitors maintains hemodynamic stability and improves survival during sepsis. Crit Care Med. 2002;30:617–22. doi: 10.1097/00003246-200203000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Wu R, Cui X, Lim Y-P, et al. Delayed administration of human inter-alpha inhibitor proteins reduces mortality in sepsis. Crit Care Med. 2004;32:1747–52. doi: 10.1097/01.ccm.0000132903.14121.0e. [DOI] [PubMed] [Google Scholar]
- 26.Himmelfarb M, Klopocki E, Grube S, et al. ITIH5, a novel member of the inter-alpha-trypsin inhibitor heavy chain family is downregulated in breast cancer. Cancer Lett. 2004;204:69–77. doi: 10.1016/j.canlet.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Yip C, Ying Y, et al. Mass spectrometric analysis of protein markers for ovarian cancer. Clin Chem. 2004;50:1939–42. doi: 10.1373/clinchem.2004.036871. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z, Bast RC, Yinhua Y. Three biomarkers identified from serum proteomic analysis for the detection of early stage ovarian cancer. Chan1 Cancer Research. 2004;64:5882–90. doi: 10.1158/0008-5472.CAN-04-0746. [DOI] [PubMed] [Google Scholar]
- 29.Burnouf T. Safety aspects in the manufacturing of plasma-derived coagulation factor concentrates. Biologicals. 1992;20:91–100. doi: 10.1016/s1045-1056(05)80056-9. [DOI] [PubMed] [Google Scholar]
- 30.Josic D, Buchacher A, Kannicht C, et al. Degradation products of factor VIII which can lead to increases immunogenicity. Sang. 1999;77:90–9. doi: 10.1159/000056726. [DOI] [PubMed] [Google Scholar]
- 31.Blostein M, Cuerquis J, Galipeau J. Galectin 3-binding protein is a potential contaminant of recombinantly produced factor IX. Haemophilia. 2007;13:701–6. doi: 10.1111/j.1365-2516.2007.01525.x. [DOI] [PubMed] [Google Scholar]
- 32.Barondes SH, Cooper DN, Gitt MA, et al. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807–10. [PubMed] [Google Scholar]
- 33.Perillo NL, Marcus ME, Baum LG. Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. J Mol Med. 1998;76:402–12. doi: 10.1007/s001090050232. [DOI] [PubMed] [Google Scholar]
- 34.Stadler M, Gruber G, Kannicht, et al. Characterisation of a novel high-purity, double virus inactivated von Willebrand Factor and Factor VIII concentrate (Wilate®) Biologicals. 2006;34:281–288. doi: 10.1016/j.biologicals.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Kasper CK. Concentrate safety and efficacy. Haemophilia. 2002;8:161–65. doi: 10.1046/j.1365-2516.2002.00601.x. [DOI] [PubMed] [Google Scholar]
- 36.Gomperts ED. The need for previously untreated patient population studies in understanding the development of factor VIII inhibitors. Haemophilia. 2006;12:573–78. doi: 10.1111/j.1365-2516.2006.01341.x. [DOI] [PubMed] [Google Scholar]
- 37.Josic D, Buchacher A, Kannicht C, et al. Degradation Products of Factor VIII Which Can Lead to Increased Immunogenicity. Vox Sanguinis. 1999;77:90–9. doi: 10.1159/000056726. [DOI] [PubMed] [Google Scholar]
- 38.Rosendaal FR, Nieuwenhuis HK, van den Berg HM, et al. A Sudden Increase in Factor VI11 Inhibitor Development in Multitransfused Hemophilia A Patients in The Netherlands. Blood. 1993;81:2180–86. [PubMed] [Google Scholar]
- 39.Peerlinck K, Arnout J, DiGiambattista M, et al. Factor VIII inhibitors in previouslytreated hemophilia A patients with a double virus-inactivated plasma derived factor VIII concentrate. Thromb Haemost. 1997;77:80–6. [PubMed] [Google Scholar]
- 40.Cherayil BJ, Weiner SJ, Pillai S. The Mac-2 antigen is a galactose-specific lectin that binds IgE. J Exp Med. 1989;170:1959–72. doi: 10.1084/jem.170.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diaz JA, Ramacciotti E, Wakefield TW.Do galectins play a role in venous thrombosis? A review Throm Res 2009. in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Groschel B, Braner JJ, Funk M, et al. Elevated plasma levels of 90 K (Mac-2 BP) immunostimulatory glycoprotein in HIV-1-infected children. J Clin Immunol. 2000;20:117–22. doi: 10.1023/a:1006634530672. [DOI] [PubMed] [Google Scholar]