Abstract
Background.
Platelets, the smallest human blood cells component, have a key role in the control of haemostasis and thrombosis but they have also been shown to be implicated in a number of different pathological states because of their involvement also in the process of inflammation end its resolution. Their peculiar anucleated morphology render the proteomics an intriguing approach to understand their biology. Given the high impact of platelet in different diseases we have started a systematic investigation of protein repertoire in controlled platelet preparation.
Material and methods.
Platelets have been extracted from blood of healthy donors (n=6) collected by venipuncture in Vacutainer. The quality of the preparation was assessed by observation and enumeration in a Bürker chamber with a conventional tissue culture microscope. To characterize human platelets proteome we analysed the pool of purified platelets combining two proteomic approaches: 2-DE separation combined with Mass Spectrometry and nanoscale ultra performances LC-MSE shotgun proteomics experiments.
Results.
The 2D gel analysis leads an average of 1900 protein spots, after the filtering of “noise” and “false positive” spots, over 500 were selected to be eligible for further analysis given their optimal spot quality value. To perform the analysis by ion accounting shotgun proteomic approach, based on nano ultra performance liquid chromatography (nUPLC) coupled to MSE processing of continuum LC-MS data, the same pool of samples was subject to liquid phase tryptic digestion and the peptide obtained used for the experiments. All the data obtained were analysed using ProteinLynx GlobalServer v2.3 (PLGS, Waters). Three analytical replicates run were acquire in high/low energy modes and associated to a human protein database returning the identification of 100 distinct genes. Comparative analysis of the Gene Ontology has been performed to evaluate the differential functional representation of the molecular repertoire investigated with these two orthogonal approaches.
Discussion.
The overall molecular function classification revealed differences between the two proteomic approaches. In particular, we found significant differences in cytoskeletal proteins (19.65% 2-DE versus 45.60 Shotgun) and receptors (0,92% 2-DE versus 6.90% Shotgun).
Keywords: platelets, proteome, mass spectrometry (MS), gene onthology
Introduction
Platelets are the smallest blood circulating cells involved in several biological processes such as haemostasis, wound repair, inflammation and pathological events causing cardiovascular diseases, including stroke and myocardial infarction1–5. Platelets arise from fragmentation of membrane-delimited cytoplasmic area of terminally differentiated megakaryocytes, located in the bone marrow1. Although translation events and protein synthesis have been recognized in platelets2, their anucleate nature renders genomic and transcriptomic techniques not very suitable for genotypic characterization, nevertheless significant analysis can be carried out on platelet protein level. In this respect, available proteomics technologies offers huge sensitivity, in terms of characterization, quantitation and identification of the proteins content of biological samples, thus representing very promising tools for the comprehensive research of platelet biology. At the beginning of the proteomics era the platelet proteome has principally been investigated by high-resolution 2-D IEF/SDS-PAGE maps as primary separation technique6–10. However, this technique is not very efficient for the separation of several kind of proteins such as, for instance, hydrophobic proteins. Hydrophobic proteins, in fact, tend to aggregate and precipitate when they reach their pI, which represents the point of their lowest solubility. As a consequence, these proteins will be underrepresented in the second dimension gel. Moreover an analysis only based on protein separation studies can be affected by protein changes or degradation due to long term storage of the analyte, that's why alternative approaches, such as peptide analysis based strategies, mainly used for quantitation studies, has been more recently shown to be a necessary complement to have a whole image of the entire expression profile of a sample11,12.
Therefore, in this study, we have employed two different but complementary approaches, namely the classic 2-DE, in association with mass analysis and nanoscale ultra-performance LC-MSE. We compared these techniques for sensibility, accuracy and data reproducibility and we consistently observed that combining these techniques resulted in a more detailed characterization of the platelet proteome.
Improving advances in proteomics technologies achieved in the last decade, leads to the possibility to find platelets proteomes available in several integrated database and the combination with transcriptomes data allowed the generation of comprehensive in silico model of platelets specific interactomes and platelets phosphorylations and kinases functional map13,14.
In our study we carried out a meta-analysis by bioinformatic investigation employing The PANTHER (Protein ANalysis THrough Evolutionary Relationships) Classification System15 combining both our experimental data with bibliographic references9 to estimate the functional distribution of the identified proteins.
Materials and methods
Platelet preparation and protein isolation
Blood samples, collected in sodium citrate-containing tubes were obtained from six healthy volunteers. Each blood sample was processed individually by adding acid citrate dextrose (ACD) (45 mM sodium citrate, 25 mM citric acid, 80 mM D-glucose) and centrifuged for 15 min at 150 × g at room temperature to obtain the platelet rich plasma (PRP). The upper third of the PRP was centrifuged at the same conditions to remove any contaminating leukocytes. Platelets were pelleted at 1000 × g and washed in ACD twice. Purity of isolated platelets was confirmed by microscopic inspection and a percentage of leukocite contamination < 0.02% and of RBC < 1% was calculated. Pellets were suspended in 200 μl sample buffer (7 M urea, 2 M thiourea, 40 mM Tris pH 7,5, CHAPS 4%, DTT 50 mM). The extracted proteins were subjected to precipitation with a solution of ice-cold acetone, ethanol and methanol at −20 °C overnight and then air-dried. Proteins were suspended in 6 M urea dissolved in 100 mM Tris pH 7,5 and protein concentration was determined by the Bradford method. Individual samples were pooled and subjected to 2-DE and nUPLC-MSE analysis.
2-DE
2-DE was performed using the IPGphor II (Amersham Biosciences) as previously described9 with some modifications. Proteins (triplicates of 100 μg for each condition) were loaded on a pH 3–10 NL IPG strips by in-gel rehydratation for 8 h at the voltage of 30V. Proteins were electrofocused at 80000 V/h at a maximum voltage of 8000V. After focusing, IPG strips were subjected to protein reduction and alkylation by two sequential immersion in the equilibration buffer containing 1% DTT for 10 min, and in the equilibration buffer containing 4% iodoacetamide for 10 min. Then, IPG strips were loaded at the top of 8–16% polyacrylamide linear gradient gels for the separation in second dimension. SDS-PAGE was carried out at a constant current of 40 mA per gel. Gels were stained by a silver staining protocol compatible with MS18. Image analysis was carried out with the DELTA 2D (DECODON-Germany) software. Statistical analysis of differential protein expression was performed by the Student's T-test. Changes were considered significant at p = 0,05.
Protein excision and tryptic digestion
Protein spots were excised manually and transferred to eppendorf tubes (0,2 mL). Protein-containing gel pieces were destained with 200 μL of 30 mM K3Fe(CN)6 and 100 mM Na2S2O3 and then washed with sequential incubation in 100 μL of 0,1 M ammonium bicarbonate and dried with 100 μL of 100% ACN. Gel immobilised proteins were reduced with 10 mM DTT, alkylated with 55 mM iodoacetamide and subsequently reswollen with 10 ng/μL trypsin in 50 mM ammonium bicarbonate and digested overnight at 37 °C. Peptides were purified and concentrated by solid phase extraction (SPE) in ZipTip C18 pipette tips (Millipore) and spotted directly onto a MALDI target upon elution with 2 μL of CHCA matrix (5 mg/mL in 50% ACN, 0,1% TFA).
Protein identification by MS
MALDI-MS and MALDI-MS/MS were performed on an UltraFlex III MALDI-TOF/TOF mass spectrometer (Bruker-Daltonik, Bremen, Germany). Data were acquired in positive reflectron mode. Two hundred shots per spectrum were accumulated. All acquisition were performed in a mass range of 700–3500 Thomson (m/z) with voltages of 25 and 21.7 kV for the first and second ion extraction stages, 9 kV for the lens, 26.3 and 13.8 kV for reflector 1 and 2 respectively. Quadratic external calibration of TOF was performed on monoisotopic mass of bradykinin (clip 1–7) [M+H]+, angiotensin II [M+H]+, angiotensin I [M+H]+, substance P [M+H]+, bombesin [M+H]+, ACTH (clip 1–17) [M+H]+, ACTH (clip 18–39) [M+H]+, somatostatin [M+H]+. MS and MS/MS data were analyzed by the Bruker FlexAnalysis 3.0 software. Peptide mass fingerprint obtained from MS analysis were used for protein identification in the Swiss-Prot database using the peptide search routine MASCOT (http://www.matrixscience.com). All peptide mass values were considered mono-isotopic and mass tolerance was set at 50 ppm. MASCOT scores greater than 56 were considered significant (p = 0,05). For MS/MS analysis, peaks were searched against the Swiss-Prot database, using the same setting of MS analysis, with a fragment tolerance of 0,3 Da.
Protein identification by nUPLC-MSE
Samples (25 μg of total protein for each pool) were diluted in 25 μL 6 M urea in 100 mM Tris pH 7,5. Proteins were reduced in the presence of 10 mM DTT for 1 h at 37 °C and subsequently alkylated with 20 mM iodoacetamide for 1 h at RT in the dark. Modified proteins were digested with 1 μL of 0,5 μg/μL trypsin solution at 37 °C overnight. The reaction was stopped by adding 1 μL of TFA 10% (v/v). A 2 μL aliquot of the digested peptides was loaded, three times for each pool, on the nano-ACQUITY UPLC™ chromatographic system. Peptides were trapped on a 5 μm Symmetry C18 column (180 μm × 620 mm) and washed for 10 min at 5 μL/min with mobile phase A (0.1% FA). Peptides were then eluted and separated using a 200 min RP gradient at 300 nL/min (3–40% ACN over 120 min) on a 1.7 mm BEH 130 C18 NanoEase™ (75 μm × 625 cm) nanoscale LC column. The column temperature was set at 50 °C. Lock mass ([Glu1]-fibrinopeptide B, 250 fmol/mL) was constantly infused by the NanoAcquity auxiliary pump at a constant flow rate of 250 nL/min. The Q-Tof Premier™ mass spectrometer was programmed to switch between low (4 eV) and high (15–40 eV) energies in the collision cell, with a scan time of 1.5 s per function over a mass range of 50–1990 Th. LC-MSE data were processed with ProteinLynx GlobalServer v2.3 (Waters) and searched in the associated human protein database (UniProtKB/SwissProt Protein Knowledge Base, Release 56.0, July 2008.)
Bioinformatic pathway analysis
Proteins identified by 2DE and LC-MSE were combined with bibliographic references9 in a unique dataset and uploaded into PANTHER (Protein ANalysis THrough Evolutionary Relationships) Classification System available online on the free web site http://www.pantherdb.org/, PANTHER Pathway version 2.5 released on line on January 06, 2009. This software allows to predict function using published scientific experimental evidence and evolutionary relationships. Proteins are classified by expert biologists into families and subfamilies of shared function, which are then categorized by molecular function and biological process ontology terms and detailed biochemical interactions are included in canonical pathways and can be viewed interactively15–17.
Results and discussion
Mapping platelets proteome
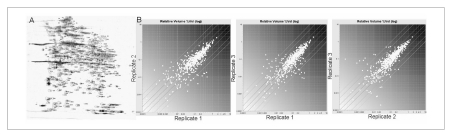
The initial analysis of platelet proteome was carried out on silver stained 2-DE maps of platelets proteins pools, solubilised under reducing condition and in the presence of urea, thiourea and CHAPS. These experimental conditions allowed effective separation and representation of platelet proteins by 2-DE over a broad range of pH gradient for the IEF steps. The 2D gels of platelet proteins were characterized also by the presence of contaminant plasma proteins, such as albumin, present in large amount, haemoglobin and some members of the apolipoprotein family. From imaging analysis by DECODON Delta 2D software, we obtained more than 1,900 distinct spots, which were compared with each other. We search for spots with a significantly high spot volume (>0.5 %Vol) and we obtained more than 500 spots matching these criteria (Figure 1A).
Figure 1.
2D_E analysis of platelets proteome. (A) Representative 2-DE map of human platelets. Spots with a volume higher than 0.5 % Vol. are enclosed in boundaries. (B) Binary comparison among the log ratios of relative spots volumes detected in the three replicate gels.
Regardless biological and gel-to-gel variation, the majority of these protein spots were found to be reproducible across the three gels. Binary comparison of the log ratios of the relative spot volumes gave the degree of variation among the three replicates. Deviation from the main 45-degree diagonal line reflects fold changes of spot volumes (Figure 1B).
The protein spot separation pattern of the three replicates is consistent with previously published work8,9 done under the same conditions of pI and MW range. In order to confirm these correspondence and the related purity of our preparation, some protein spots were selected as reference points, excised from the gel, trypsin-digested and subjected to MS analysis. Comparative analysis of experimental identification versus reference proteins identified by O'Neill et al.9 was successful, even though some of these proteins, in particular the big ones (e.g. talin), appeared in different gel position respect to the theoretical molecular weight and pI, probably because of post translational modification or sample degradation.
In order to obtain a better characterization of platelet proteome, thus overcoming the limitations of 2-DE, we decided to complement the analysis with the recently-developed nano LC-MSE.
Platelet shotgun proteomics investigation
Total protein extracts were digested and analyzed in triplicate run injections. All extracted peptides were subjected to an alternate scanning acquisition method, designed to obtain high resolution and accurate mass information for each detected precursor and any associated fragments. The acquisition mode was configured to alternate between two collision energy conditions. Low energy allows detection of eluting precursor ion peptides, while high energy allows detection of associated product ions with no precursor ion selection prior to CID. Data were collected during the entire LC-MSE experiment as pairs of chromatographic profiles and their associated ion mass measurements for all the detected peptides19.
We identified a total of 114 proteins (Table I) and 102543 EMRT (Exact Mass Retention Time clusters). Quality control measures were performed on the replicates to determine the analytical reproducibility of the analysis (Figure 2). The final results from the clustering algorithm contain all mass spectrometric and chromatographic characteristics for each peptide component.
Table I.
Proteins identified by LC-MSe
| SwissProt Acc.N° | Protein name | MW (Da) | pI | PLGS score | Matching peptides | Coverage (%) |
|---|---|---|---|---|---|---|
| A5A3E0 | ANKRD26 like family C member 1B | 121366 | 5.8 | 1303.003 | 37 | 41.3 |
| A6NKZ8 | Putative tubulin beta chain-like protein | 41748 | 4.6 | 300.3834 | 6 | 26.1 |
| A6NNZ2 | Tubulin beta-8 chain B | 49540 | 4.6 | 306.393 | 5 | 17.8 |
| O00151 | PDZ and LIM domain protein 1 | 36049 | 6.6 | 443.2623 | 17 | 76.9 |
| O00299 | Chloride intracellular channel protein 1 | 26905 | 4.9 | 336.7437 | 12 | 68.9 |
| O14950 | Myosin regulatory light chain 12B | 19766 | 4.5 | 469.5623 | 6 | 51.7 |
| O43707 | Alpha-actinin-4 | 104788 | 5.1 | 1246.604 | 32 | 48.1 |
| O94888 | UBX domain-containing protein 7 | 54828 | 4.9 | 270.1815 | 11 | 38.9 |
| O95810 | Serum deprivation-response protein | 47144 | 5.0 | 494.3606 | 14 | 49.9 |
| P00338 | L-lactate dehydrogenase A chain | 36665 | 8.4 | 309.2319 | 12 | 39.8 |
| P00488 | Coagulation factor XIII A chain | 83214 | 5.7 | 794.0464 | 31 | 54.5 |
| P00918 | Carbonic anhydrase 2 | 29227 | 7.0 | 182.8219 | 5 | 34.6 |
| P02042 | Hemoglobin subunit delta | 16045 | 8.2 | 526.4562 | 8 | 82.3 |
| P02100 | Hemoglobin subunit epsilon | 16192 | 9.2 | 224.1096 | 3 | 12.9 |
| P02671 | Fibrinogen alpha chain | 94914 | 5.6 | 975.3052 | 28 | 39.8 |
| P02675 | Fibrinogen beta chain | 55892 | 8.3 | 814.1464 | 33 | 78.4 |
| P02679 | Fibrinogen gamma chain | 51478 | 5.2 | 935.0375 | 24 | 59.4 |
| P02768 | Serum albumin | 69321 | 5.9 | 1230.0095 | 32 | 60.4 |
| P02775 | Platelet basic protein | 13885 | 9.1 | 640.334 | 7 | 38.3 |
| P02776 | Platelet factor 4 | 10837 | 8.8 | 264.0247 | 4 | 35.6 |
| P04075 | Fructose-bisphosphate aldolase A | 39395 | 8.1 | 506.852 | 14 | 58.2 |
| P04350 | Tubulin beta-4 chain | 49553 | 4.6 | 471.5167 | 11 | 48.4 |
| P04406 | Glyceraldehyde-3-phosphate dehydrogenase | 36030 | 8.7 | 929.9182 | 18 | 67.8 |
| P06396 | Gelsolin | 85644 | 5.8 | 912.8763 | 26 | 47.1 |
| P06733 | Alpha-enolase | 47139 | 7.2 | 539.9177 | 19 | 60.8 |
| P06753 | Tropomyosin alpha-3 chain | 32798 | 4.5 | 326.7619 | 19 | 53.5 |
| P07195 | L-lactate dehydrogenase B chain | 36615 | 5.6 | 271.8883 | 5 | 18.3 |
| P07437 | Tubulin beta chain | 49638 | 4.6 | 633.4846 | 11 | 47.1 |
| P07737 | Profilin-1 | 15044 | 8.5 | 637.4518 | 10 | 80.0 |
| P07951 | Tropomyosin beta chain | 32830 | 4.5 | 328.07 | 7 | 27.5 |
| P07996 | Thrombospondin-1 | 129299 | 4.5 | 2156.4783 | 46 | 49.1 |
| P08107 | Heat shock 70 kDa protein 1A/1B | 70009 | 5.3 | 459.2476 | 14 | 41.2 |
| P08514 | Integrin alpha-IIb | 113319 | 5.0 | 535.3934 | 25 | 29.9 |
| P08567 | Pleckstrin | 40071 | 8.3 | 934.5118 | 21 | 58.6 |
| P09417 | Dihydropteridine reductase | 25773 | 7.2 | 142.1144 | 9 | 50.8 |
| P09486 | SPARC | 34609 | 4.5 | 190.9292 | 9 | 51.8 |
| P09493 | Tropomyosin alpha-1 chain | 32688 | 4.5 | 297.0772 | 6 | 21.8 |
| P09972 | Fructose-bisphosphate aldolase C | 39431 | 6.4 | 367.2415 | 10 | 31.9 |
| P10720 | Platelet factor 4 variant | 11545 | 9.5 | 309.8934 | 3 | 27.9 |
| P11021 | 78 kDa glucose-regulated protein | 72288 | 4.9 | 360.8539 | 18 | 34.4 |
| P11142 | Heat shock cognate 71 kDa protein | 70854 | 5.2 | 520.1844 | 19 | 37.3 |
| P12814 | Alpha-actinin-1 | 102992 | 5.1 | 1519.6946 | 41 | 62.9 |
| P13929 | Beta-enolase | 46957 | 7.7 | 124.1352 | 6 | 20.7 |
| P14618 | Pyruvate kinase isozymes M1/M2 | 57900 | 7.8 | 611.7149 | 21 | 62.9 |
| P14649 | Myosin light chain 6B | 22749 | 5.4 | 264.5406 | 7 | 47.1 |
| P18206 | Vinculin | 123721 | 5.3 | 2245.8167 | 62 | 64.0 |
| P19105 | Myosin regulatory light chain 12A | 19781 | 4.5 | 469.5623 | 10 | 80.1 |
| P23528 | Cofilin-1 | 18490 | 8.2 | 582.6157 | 9 | 56.0 |
| P24071 | Immunoglobulin alpha Fc receptor | 32244 | 6.5 | 158.8546 | 5 | 35.2 |
| P24844 | Myosin regulatory light polypeptide 9 | 19814 | 4.6 | 372.3448 | 9 | 70.3 |
| P28065 | Proteasome subunit beta type-9 | 23249 | 4.7 | 216.371 | 9 | 60.3 |
| P30041 | Peroxiredoxin-6 | 25019 | 6.0 | 164.9216 | 4 | 31.7 |
| P31146 | Coronin-1A | 50993 | 6.2 | 307.7916 | 10 | 35.1 |
| P34931 | Heat shock 70 kDa protein 1-like | 70331 | 5.6 | 378.9034 | 22 | 41.0 |
| P35579 | Myosin-9 | 226390 | 5.3 | 4097.9404 | 114 | 57.3 |
| P35580 | Myosin-10 | 228856 | 5.3 | 1592.002 | 67 | 43.5 |
| P35749 | Myosin-11 | 227197 | 5.2 | 1598.0902 | 73 | 39.4 |
| P37802 | Transgelin-2 | 22377 | 8.4 | 1125.636 | 15 | 86.4 |
| P50552 | Vasodilator-stimulated phosphoprotein | 39805 | 9.3 | 397.6586 | 13 | 34.2 |
| P54652 | Heat shock-related 70 kDa protein 2 | 69977 | 5.4 | 377.9034 | 21 | 39.1 |
| P60174 | Triosephosphate isomerase | 26652 | 6.5 | 294.145 | 7 | 47.4 |
| P60660 | Myosin light polypeptide 6 | 16919 | 4.4 | 588.86 | 11 | 61.6 |
| P60709 | Actin, cytoplasmic 1 | 41709 | 5.1 | 2612.1707 | 27 | 76.8 |
| P62736 | Actin, aortic smooth muscle | 41981 | 5.1 | 1535.4171 | 15 | 48.3 |
| P62937 | Peptidyl-prolyl cis-trans isomerase A | 18000 | 7.9 | 245.5191 | 8 | 57.6 |
| P63104 | 14-3-3 protein zeta/delta | 27727 | 4.5 | 574.0399 | 14 | 51.8 |
| P63261 | Actin, cytoplasmic 2 | 41765 | 5.2 | 2617.9204 | 22 | 73.3 |
| P63267 | Actin, gamma-enteric smooth muscle | 41849 | 5.2 | 1502.7444 | 15 | 45.2 |
| P67936 | Tropomyosin alpha-4 chain | 28504 | 4.5 | 581.3867 | 18 | 57.7 |
| P68032 | Actin, alpha cardiac muscle 1 | 41991 | 5.1 | 1570.227 | 15 | 48.3 |
| P68133 | Actin, alpha skeletal muscle | 42023 | 5.1 | 1523.284 | 18 | 54.9 |
| P68363 | Tubulin alpha-1B chain | 50119 | 4.8 | 845.3544 | 12 | 40.1 |
| P68366 | Tubulin alpha-4A chain | 49892 | 4.8 | 964.94 | 16 | 55.1 |
| P68371 | Tubulin beta-2C chain | 49799 | 4.6 | 536.0964 | 10 | 44.9 |
| P68871 | Hemoglobin subunit beta | 15988 | 6.9 | 1363.5991 | 13 | 93.9 |
| P69891 | Hemoglobin subunit gamma-1 | 16130 | 6.8 | 211.2016 | 8 | 58.5 |
| P69892 | Hemoglobin subunit gamma-2 | 16116 | 6.8 | 207.7864 | 4 | 32.7 |
| P69905 | Hemoglobin subunit alpha | 15247 | 9.2 | 625.6528 | 8 | 89.4 |
| Q01518 | Adenylyl cyclase-associated protein 1 | 51822 | 8.1 | 426.7084 | 21 | 52.8 |
| Q05209 | Tyrosine-protein phosphatase non-receptor type 12 | 88065 | 5.3 | 504.4341 | 20 | 37.7 |
| Q13418 | Integrin-linked protein kinase | 51385 | 8.0 | 406.3417 | 13 | 44.9 |
| Q13509 | Tubulin beta-3 chain | 50400 | 4.6 | 427.0488 | 9 | 32.9 |
| Q13748 | Tubulin alpha-3C/D chain | 49927 | 4.8 | 566.2676 | 9 | 36.0 |
| Q13885 | Tubulin beta-2A chain | 49874 | 4.6 | 521.9 | 15 | 47.9 |
| Q14112 | Nidogen-2 | 151299 | 4.9 | 757.1977 | 28 | 31.8 |
| Q14185 | Dedicator of cytokinesis protein 1 | 215207 | 7.3 | 991.6309 | 39 | 26.1 |
| Q15404 | Ras suppressor protein 1 | 31520 | 9.1 | 262.5601 | 10 | 46.9 |
| Q15942 | Zyxin | 61238 | 6.2 | 517.1403 | 16 | 51.2 |
| Q3ZCM7 | Tubulin beta-8 chain | 49743 | 4.6 | 331.9643 | 9 | 32.7 |
| Q562R1 | Beta-actin-like protein 2 | 41975 | 5.3 | 793.0007 | 20 | 63.8 |
| Q58FF3 | Putative endoplasmin-like protein | 45829 | 5.0 | 295.9952 | 16 | 53.4 |
| Q6NUK1 | Calcium-binding mitochondrial carrier protein SCaMC-1 | 53320 | 5.9 | 291.726 | 16 | 40.3 |
| Q6PEY2 | Tubulin alpha-3E chain | 49884 | 4.8 | 388.5545 | 7 | 31.1 |
| Q6Q0C0 | E3 ubiquitin-protein ligase TRAF7 | 74560 | 6.7 | 432.0255 | 21 | 44.3 |
| Q6S8J3 | ANKRD26 like family C member 1A | 121285 | 5.8 | 1470.8132 | 18 | 23.4 |
| Q71U36 | Tubulin alpha-1A chain | 50103 | 4.8 | 576.6373 | 9 | 31.3 |
| Q7Z406 | Myosin-14 | 227861 | 5.6 | 1663.2179 | 62 | 39.0 |
| Q80930 | Regulatory protein E2 | 45528 | 9.2 | 336.3838 | 17 | 52.8 |
| Q86UX7 | Fermitin family homolog 3 | 75905 | 6.5 | 717.6672 | 35 | 67.2 |
| Q8IZ40 | REST corepressor 2 | 57976 | 9.3 | 243.9844 | 12 | 35.4 |
| Q8NGU1 | Putative olfactory receptor 9A1 | 29526 | 7.4 | 168.0808 | 2 | 9.1 |
| Q99867 | Putative tubulin beta-4q chain | 48403 | 4.9 | 366.3576 | 11 | 35.5 |
| Q9BQE3 | Tubulin alpha-1C chain | 49863 | 4.8 | 629.9761 | 16 | 51.2 |
| Q9BUF5 | Tubulin beta-6 chain | 49825 | 4.6 | 337.3611 | 8 | 34.1 |
| Q9BV86 | Methyltransferase-like protein 11A | 25370 | 5.2 | 152.797 | 7 | 37.2 |
| Q9BVA1 | Tubulin beta-2B chain | 49920 | 4.6 | 538.4824 | 9 | 36.6 |
| Q9BYX7 | Beta-actin-like protein 3 | 41988 | 5.9 | 587.8795 | 7 | 34.4 |
| Q9GZV4 | Eukaryotic translation initiation factor 5A-2 | 16782 | 5.2 | 101.176 | 5 | 73.9 |
| Q9H299 | SH3 domain-binding glutamic acid-rich-like protein 3 | 10431 | 4.6 | 159.5535 | 5 | 69.9 |
| Q9H4B7 | Tubulin beta-1 chain | 50294 | 4.9 | 1078.2789 | 23 | 79.8 |
| Q9HBI1 | Beta-parvin | 41688 | 6.3 | 227.9087 | 9 | 39.8 |
| Q9NY65 | Tubulin alpha-8 chain | 50061 | 4.8 | 451.669 | 11 | 41.4 |
| Q9UBW5 | Bridging integrator 2 | 61836 | 4.9 | 463.2477 | 18 | 56.3 |
| Q9UI15 | Transgelin-3 | 22458 | 7.2 | 165.844 | 6 | 43.7 |
| Q9Y281 | Cofilin-2 | 18724 | 8.2 | 362.7851 | 6 | 47.6 |
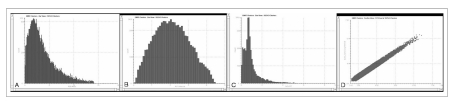
Figure 2.
Analytical reproducibility of replicate LC-MSE experiments. (A) Relative standard deviation from all the EMRT components within ± 5 ppm of the mean mass measurement. (B) Average coefficient of variation of the measured signal intensity of the clusters. (C) Average retention time coefficient of variation was centered at 0.4%. (D) Binary comparison of the log intensity measurement obtained from the matched EMRT clusters for two replicate injections.
These were subjected to statistical calculations after the clustering process. The clustering algorithm utilized the analytical reproducibility of the mass measurement and the reproducibility of the chromatographic retention time of each peptide.
The mass precision of the extracted peptide components was within ± 5 ppm (approximately 2 ppm) of the mean mass measurement (Figure 2A). The variability of intensity among the replicate injections for these EMRT components showed an average coefficient within 1.6 and 2.3% (Figure 2B). The reproducibility of retention times for most of these clusters showed a RSD centered at 0.4% (Figure 2C). Variations in intensity were evaluated by conducting binary comparisons of the intensities of all matched peptide components from two replicate injections (Figure 2D). Under ideal conditions, the binary comparison would rely on a perfect 45-degree diagonal (ln(ratio)=0) intersecting through 0. The scatter plot showed a minimal degree of deviation of the peptide components intensity values throughout the detected range, confirming that this methods is highly reproducible. We compared datasets of identified proteins to those obtained with 2-DE experiments, in order to cluster them according to the Gene Ontology hierarchy, based on molecular functions and biological processes categories.
Gene ontology
Following 2-DE and nanoscale reversed phase LC-MSE, all the identified proteins were analyzed for their involvement in known biological processes and their molecular function, respectively. Protein identification datasets derived both from our experimental work and from bibliographic reference9 have been merged and loaded on PANTHER software.
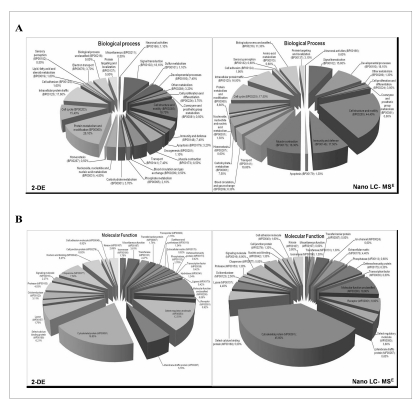
Proteins were grouped by category and compared for their ontology class. We observed differences in the functional distribution of the identified proteins between the two methods used. Using Shotgun approach, we found a major representation of proteins involved in cell structure and motility (44.40%) respect to 2-DE (19.70%). On the contrary, using 2-DE we observed a predominance of proteins involved in cell metabolism (26.10%), respect to Shotgun analysis (8.80%). Notably, when we combined these two different but complementary proteomic tools, we were able to detect additional classes of proteins with different biological functions (Figure 3A).
Figure 3.
Gene ontology distribution terms obtained from platelets proteome analysis. The proteins identified by 2-DE and LC-MSE analysis were clustered based on their (A) biological process and (B) molecular functions
Moreover, the molecular function classification revealed differences between the two proteomic approaches. In particular, we found significant differences in cytoskeletal proteins (19.65% 2-DE versus 45.60% Shotgun) and receptors (0,92% 2-DE versus 6.90% Shotgun), as expected due to the limited resolution of membrane hydrophobic proteins in the IEF first dimension. We found also differences in protein of immunity response (0.42% 2-DE versus 6.30 Shotgun) as well as in other classes of proteins (Figure 3B).
In conclusion, present results indicate that molecular profiling performed with two orthogonal proteomics analysis methodologies, enhances the possibility to obtain deeper information on the biology and pathophysiology of the systems under investigation. This becomes particularly relevant with platelets, in light of their multiple physiological functions and their involvement in a variety of human diseases.
References
- 1.Brown AS, Erusalimsky JD, Martin JF. In: Platelets and their Factors. von Bruchhausen F, Walter U, editors. Springer-Verlag; Berlin, Germany: 1997. pp. 3–19. [Google Scholar]
- 2.Denis MM, Tolley ND, Bunting M, et al. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122(3):379–91. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macaulay IC, Carr P, Gusnanto A, et al. Platelet genomics and proteomics in human health and disease. J Clin Invest. 2005;115(12):3370–7. doi: 10.1172/JCI26885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8:1223–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 5.Savage B, Cattaneo M, Ruggeri ZM. Mechanisms of platelet aggregation. Curr Opin Hematol. 2001;8:270–276. doi: 10.1097/00062752-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Gravel P, Sanchez JC, Walzer C, et al. Human blood platelet protein map established by two-dimensional polyacrylamide gel electrophoresis. Electrophoresis. 1995;16:1152–1159. doi: 10.1002/elps.11501601191. [DOI] [PubMed] [Google Scholar]
- 7.Marcus K, Immler D, Sternberger J, Meyer HE. Identification of platelet proteins separated by two-dimensional gel electrophoresis and analyzed by matrix assisted laser desorption/ionization-time of flight-mass spectrometry and detection of tyrosine-phosphorylated proteins. Electrophoresis. 2000;21:2622–2636. doi: 10.1002/1522-2683(20000701)21:13<2622::AID-ELPS2622>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Marcus K, Moebius J, Meyer HE. Differential analysis of phosphorylated proteins in resting and thrombin-stimulated human platelets. Anal Bioanal Chem. 2003;376:973–993. doi: 10.1007/s00216-003-2021-z. [DOI] [PubMed] [Google Scholar]
- 9.O’Neill EE, Brock CJ, von Kriegsheim AF, et al. Towards complete analysis of the platelet proteome. Proteomics. 2002;2:288–305. doi: 10.1002/1615-9861(200203)2:3<288::aid-prot288>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Garcia A, Prabhakar S, Brock CJ, et al. Extensive analysis of the human platelet proteome by two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2004;4:656–668. doi: 10.1002/pmic.200300665. [DOI] [PubMed] [Google Scholar]
- 11.Kubota K, Kosaka T, Ichikawa K. Combination of two-dimensional electrophoresis and shotgun peptide sequencing in comparative proteomics. Journal of Chromatography B. 2005;815:3–9. doi: 10.1016/j.jchromb.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 12.Thon JN, Schubert P, Duguay M, et al. Comprehensive proteomic analysis of protein changes during platelet storage requires complementary proteomic approaches. Transfusion. 2008;48(3):425–35. doi: 10.1111/j.1537-2995.2007.01546.x. [DOI] [PubMed] [Google Scholar]
- 13.Dittrich M, Birschmann I, Mietner S, et al. Platelet protein interactions: map, signaling components, and phosphorylation groundstate. Arterioscler Thromb Vasc Biol. 2008;28(7):1326–31. doi: 10.1161/ATVBAHA.107.161000. [DOI] [PubMed] [Google Scholar]
- 14.Senzel L, Gnatenko DV, Bahou WF. The platelet proteome. Curr Opin Hematol. 2009;16(5):329–33. doi: 10.1097/MOH.0b013e32832e9dc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas PD, Campbell MJ, Kejariwal A, et al. PANTHER: a library of protein families and subfamilies indexed by function. Genome Res. 2003;13:2129–2141. doi: 10.1101/gr.772403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mi H, Guo N, Kejariwal A, Thomas PD. PANTHER version 6: protein sequence and function evolution data with expanded representation of biological pathways. Nucl Acids Res. 2007;35:D247–D252. doi: 10.1093/nar/gkl869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas PD, Kejariwal A, Guo N, et al. Applications for protein sequence-function evolution data: mRNA/protein expression analysis and coding SNP scoring tools. Nucl Acids Res. 2006;34:W645–W650. doi: 10.1093/nar/gkl229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shevchenko A, Wilm M, Vorm O, et al. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 19.Silva JC, Denny R, Dorschel CA, et al. Quantitative proteomic analysis by accurate mass retention time pairs. Anal Chem. 2005;77:2187–2200. doi: 10.1021/ac048455k. [DOI] [PubMed] [Google Scholar]