Blood transfusion is the key life-saving treatment in many traumatic emergencies, chronic or acute pathologies, and during or upon surgical interventions. The primary goal of blood transfusion is the fast recovery of oxygen delivery to organs, especially the brain. Furthermore, circulation volume is restored, thus maintaining the blood pressure at levels that guarantee an efficient blood flux. Whereas this secondary need can be fulfilled to some extent by blood expanders, such as colloid- and crystalloid-based solutions, up to now no approved alternative to red cells is available for oxygen supply. The main transfusion active principle, red blood cells, is derived from the voluntary action of blood donors. Transfusions can be and are considered a very safe and effective oxygen-based therapy, provided that detailed guidelines are followed1. This evaluation has been somewhat challenged in recent years2–5. Presently, clinical studies are underway to provide a more solid ground to the safety and efficacy of blood transfusions (examples available at: http://clinicaltrials.gov/ct2/show/NCT01038557 and http://clinicaltrialsfeeds.org/clinical-trials/show/NCT00991341). Indeed, transfusions are exposed to a series of limitations, listed in table I, that can lead to potential health risks. For example, in stored blood the intracellular levels of the haemoglobin allosteric effector 2–3 bisphosphoglycerate (2,3-BPG), that maintains the p50 (the oxygen pressure at 50% saturation) in vivo at 26 torr, decreases in one week from 5 mM to negligible concentrations, resulting in a drop of the p50 to about 12 torr3. The concentration of potassium ions in red cells increases from 4 mM in fresh blood to about 20 mM after three weeks of aging3. In the same time range, lactate concentration increases from about 1 to 15 mM, and solfohaemoglobin (HbSO2) from 30% to almost 100%. The intracellular pH of red cells drops from 7.4 to 6.9 in a few hours and to a value of 6.7 in three weeks3. Furthermore, free haemoglobin, i.e. haemoglobin released due to red cell haemolysis, increases from 5 μM at 3 hours after blood withdrawal to about 20 μM after three weeks3. Free haemoglobin scavenges nitric oxide (NO), the molecule that controls vessel tone, and undergoes oxidation, leading to pro-oxidative radical-based reactions6. Studies on blood units aging have also pointed to adverse effects associated to the decreased capability of red cell haemoglobin to supply nitric oxide4. However, this is a controversial issue7,8.
Table I.
Existing and emerging issues related to blood transfusions
|
There are several clinical needs that cannot be satisfied by blood transfusions, some of them listed in table II. For example, in Italy blood donations are between about 30 and 60 units per year per 1000 inhabitants, with an average of 42 units (2008 data from Italian Blood Center, National Health Institute). Consumption varies from more than 60 units per 1000 inhabitants in Sardinia to less than 30 units in Campania, with an average of 41.7 units. This leads to a delicate balance, exposed to potential crises due to pandemic events that exclude from donation significant fractions of blood donors. About 6000 Italian patients, affected by genetic-derived haemoglobinopathies, are blood transfusion-dependent and require about 200,000 units per year. As the population ages, less blood donors are available and more blood units are needed. Moreover, about 5% (140,000 units) of the total collected blood units (2,400,000) are discarded for technical and safety issues, and due to expiration. The latter counts for about 53,000 units (2005 data from Italian Blood Center). The precious haemoglobin contained in the red cells should not be wasted but “recycled”.
Table II.
Clinical needs unmet by blood transfusions
|
The development of safe alternatives to blood transfusions is a great challenge that so far has not been met by researchers. Over the years, four distinct approaches have been proposed to generate alternatives to transfusions:
cell-free haemoglobin-based oxygen carriers obtained via either chemical or genetic modifications of haemoglobin9–13;
artificial red cells based on either liposomes entrapping native or modified haemoglobin or nanocapsules adsorbing haemoglobin9–13;
enzyme-modified, polyethylene glycol-conjugated and antibody-reacted human red cells to remove or to mask cell type determinants, creating a universal red cell14–17 and red cells derived from multipotent stem cells18,19.
In the present review, only the state of the art of haemoglobin-based oxygen carriers (HBOCs) will be discussed.
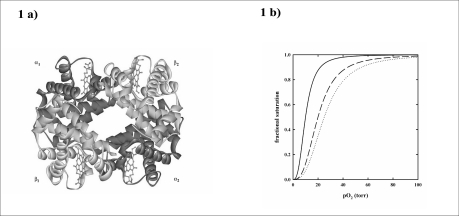
Haemoglobin is the protein contained in red blood cells that loads oxygen in the lungs and delivers it to tissues20. It is a tetramer constituted of two αβ dimers and four haems (Figure 1a). Oxygen binds to the ferrous haem with a physiological p50 of 26 ± 1 torr. Oxygen binds to haemoglobin cooperatively, i.e. the oxygen affinity increases as a function of saturation, following a sigmoidal curve (Figure 1b). The oxygen affinity is modulated by allosteric effectors such as protons, 2,3-BPG, chloride ions and carbon dioxide, that lower oxygen affinity (Figure 1b). The cooperativity and the variation of oxygen affinity caused by allosteric effectors result from a change in the equilibrium between a high affinity quaternary conformation of haemoglobin, predominantly present in the lung for optimal oxygen loading, called R, and a low affinity quaternary conformation, predominantly present in the tissues for optimal oxygen delivery, called T21. The concerted quaternary transition, explaining the cooperative oxygen binding of haemoglobin, is the basis of the Monod, Wyman and Changeux model for allosteric proteins (MWC)22. Other models that take into account the fine-tuning of oxygen affinity brought about by allosteric effectors have been recently proposed and are based on the central role of tertiary conformational changes23–25.
Figure 1.
a) Three dimensional structure of human haemoglobin tetramer (2DN2). b) Oxygen binding curves of haemoglobin in the absence (solid line) (p50 = 10 torr) and presence of 2,3-BPG (dashed line) (p50 = 20 torr) and 2,3-BPG and carbon dioxide (dotted line) (p50 = 25 torr), pH 7.2, 37 °C.
The haemoglobin tetramer dissociates into dimers depending on the oxygen fractional saturation and protein concentration. Oxyhaemoglobin free in solution at μM concentrations is significantly dissociated in dimers, whereas, inside the erythrocytes, with a haemoglobin concentration of about 5 mM, the dimers concentration is negligible. Moreover, in the absence of oxygen, the tetramer is more stable.
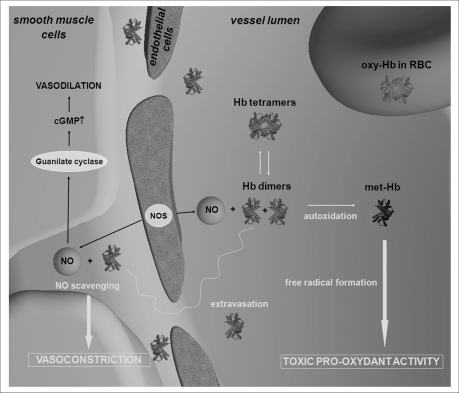
The presence of dimers free in the plasma (Figure 2) leads to several primary effects:
dimers exhibit a molecular weight of 32 KDa and therefore can, unlike tetramers, undergo glomerular filtration, thus causing nephrotoxicity.
Dimers show a higher p50, about 5 torr, thus keeping oxygen tightly bound and releasing it only at very low oxygen tensions.
The oxygen affinity of dimers is not modulated by allosteric effectors present in the plasma, such as chloride ions and carbon dioxide, thus the oxygen release is not cooperative.
The dimer haem iron is oxidized more easily than in the tetramer, leading to molecules unable to bind oxygen (Figure 2). Moreover, the formation of ferric ions triggers a cascade of reactions that generate reactive oxygen species and reactive nitrogen species, the molecular basis of oxidative damage6. The compartmentalization of haemoglobin in erythrocytes provides a protective environment that limits the oxidation to methaemoglobin and reduces the formation of potentially toxic oxidative products due to the presence of a reductase system that keeps physiological methaemoglobin levels below 1%. Red cell catalase and superoxide dismutase remove oxygen reactive species produced by haem autoxidation26. Plasma has an antioxidant activity associated to the presence of reducing agents, such as urate and ascorbate27. However, plasma antioxidant capacity is inadequate to counterbalance the oxidative cascade triggered by high levels of cell-free haemoglobin in blood.
Dimers extravasate and scavenge NO, the key controller of vessel tension produced by the endothelium cells, thus causing hypertensive effects (Figure 2). The vasodilating effect of NO is critical in the regulation of the vascular tone. NO, formed by the NO-synthase expressed in vascular endothelial cells, diffuses to smooth muscle cells where it binds to the cytoplasmic guanylate cyclase, causing vasorelaxation28. NO can react either with deoxyhaemoglobin on the haem group, forming nitrosyl-haemoglobin, or with oxyhaemoglobin, forming Cysb93 S-nitroso oxyhaemoglobin or methaemoglobin7,29. Two haemoglobin-dependent mechanisms that preserve NO vasoactivity have been proposed and are still subject of debates, the formation of S-nitrosohaemoglobin (SNO-Hb) and the reaction of deoxyhaemoglobin with nitrite to form NO7,24–30,33.
Figure 2.
Biochemical and physiological effects of the presence of haemoglobin tetramers and dimers free in the plasma.
In order to develop a haemoglobin-based oxygen carrier, it is fundamental to consider not only its oxygen transport properties, but also the influence that this product may have on NO homeostasis. Haemoglobin inside the erythrocytes has a limited interaction with NO because the erythrocyte membrane forms a diffusional barrier between NO and haemoglobin30. Moreover, the intravascular flow creates an erythrocyte-free zone of streaming plasma along the endothelium31. As result, the rate of NO scavenging by haemoglobin within the erythrocytes is decreased by more than 6000 fold32. Scavenging of NO by haemoglobin causes vasoconstriction in case haemoglobin is free in the plasma, as, for example, during haemolysis or exogenous administration of stripped haemoglobin or HBOCs. Cell-free haemoglobin is much more effective in scavenging NO with respect to haemoglobin inside red blood cells for two reasons. First, the reaction with NO is not limited by diffusion through the erythrocyte membrane or the cell-free layer adjacent to vessel walls. Second, as mentioned above (Figure 2), dimeric and, to a lesser extent, tetrameric haemoglobin extravasate through the inter-endothelial junctions and scavenge NO directly in its site of action33. Therefore, hypertension is an important side effect of most HBOCs. The increase of mean arterial blood pressure is immediate after HBOCs infusion and is often associated with reduced cardiac output and increased total peripheral resistance34. Vasoconstriction can limit tissue perfusion and oxygenation and is therefore a severe adverse effect for patients that are suffering from blood loss and haemodilution.
Although NO scavenging by HBOCs is considered the primary mechanism leading to vasoconstriction, other factors should be considered, such as oxygen pressure in the pre-capillary arterioles and solution viscosity. Winslow, Intaglietta and co-workers proposed the “autoregulation theory” suggesting that oxygen itself triggers arteriolar vasoconstriction35–37. When cell-free haemoglobin or a HBOC with low oxygen affinity are in the terminal arterioles, they may deliver excess oxygen due to an oxygen facilitated-diffusion mechanism associated to Hb molecules closer to the vessel wall than red cells. This process is further increased by the low oxygen affinity. In turn, the excess oxygen, detected by blood vessel oxygen sensors, triggers vasoconstriction and, paradoxically, decreases oxygen delivery to tissues. According to this theory, a HBOC should possess a low p50 in order to reduce pre-capillary oxygen release and target oxygen delivery to the anoxic areas, bypassing the oxygenated tissues36. Another issue associated to the hypertensive response triggered by HBOCs is the viscosity of the infused solution38. Blood exerts a shear stress on capillary walls, directly proportional to its viscosity39. If shear forces are weaker, NO production is lower, causing vasoconstriction. In animal experiments, under extreme haemodilution, elevated plasma viscosity increases perivascular NO concentration and microvascular perfusion40 whereas conventional low-viscosity plasma expanders cause microvascular impairment41. Therefore, multiple factors should be considered in order to limit the hypertensive effect of HBOCs.
Given the negative effects associated with the presence of dimers free in the plasma, the research on HBOCs has been primarily focused on the development of haemoglobin tetramers that either do not dissociate into dimers or, if they do, do not undergo ultrafiltration and extravasation because their size is artificially increased. This goal is achieved via either genetic or chemical modifications of haemoglobin. Different strategies of haemoglobin genetic manipulation have been applied, ranging from the generation of constructs that link human a chains via a glycine bridge42 to fusion proteins of two α-α or two β-β chains43. The engineered haemoglobin molecules, expressed in bacteria with yields that are of the order of a few milligrams per liter of culture, exhibit functional properties that can be different from the parent haemoglobin44. However, recombinant technology allows to modulate functional properties, for example NO scavenging, by amino acid substitutions45. An alternative genetic approach is based on mutations that generate a haemoglobin containing a single cysteine on the bovine beta chains46,47. Under oxidizing conditions a sulfide bridge is formed, linking a human α - bovine β dimer to another dimer, generating a polymer containing three-four tetramers. The resulting polymer is called haemoglobin Polytaur48.
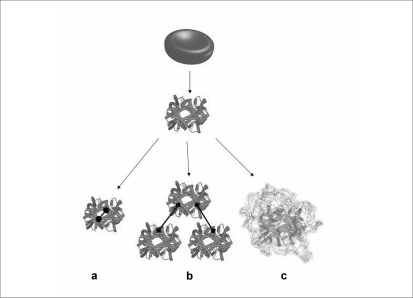
The different approaches that make use of haemoglobin chemical modifications are reported in figure 3. They are based on reagents, such as glutaraldehyde, that cross-link tetramers intra- or inter-molecularly, generating stable tetramers or polymers49,50, or on compounds that decorate the surface of haemoglobin with organic molecules, such as polyethylene glycol (PEG), thus increasing protein molecular weight and actual size51–53. Unavoidably, the chemical modifications lead to heterogeneous mixtures of products characterized by variable structural and functional properties, as shown for PEGylated haemoglobins54. As a result of the FP6 funded project “Eurobloodsubstitutes” (available at: http://www.eurobloodsubstitutes.com), a HBOC consisting of haemoglobin conjugated with PEG, under anaerobic conditions to preserve relevant functional residues from chemical modification, was obtained and called Euro-PEG-Hb55,56. This procedure and the resulting product is different from Hemospan® that is PEGylated under aerobic conditions57. Careful structural and functional investigations were carried out to compare these PEG-haemoglobins54. Results highlighted two major shortcomings: heterogeneity in the degree of PEGylation and enhanced tetramer dissociation upon PEGylation that may cause, as discussed above, vasoconstriction due to enhanced HBOC extravasation.
Figure 3.
Chemical approaches for the development of HBOCs: intra- (a) and inter- (b) molecular cross-linking and PEGylation (c).
The clinical studies carried out on the most promising HBOCs are reported in table III12. So far, no product has been approved by U.S. FDA and EMEA. A few of them, notably Hemopure®, Polyheme®, Hemospan® and Hemolink®, have received approval for compassionate use for the treatment of Jehovah’s Witnesses, sickle cell patients or red cell immune- and autoimmune-reactive patients. Hemopure® was licensed for use in South Africa but, recently, the Medicines Control Council of South Africa decided to discontinue its registration. Northfield Laboratories and Biopure, that produced the former two HBOCs, closed their activities in 2009, and the latter has been acquired by OPK Biotech. Sangart has completed a Phase III clinical trial in Europe aimed at demonstrating the validity and safety of Hemospan® as blood expander. Results should be reported to EMEA soon. Moreover, Sangart will initiate a Phase IIa study in severe trauma patients with haemorrhagic shock.
Table III.
Clinical studies on HBOCs
| Name | Company | Chemical/genetic modification | Proposed Application | Status |
|---|---|---|---|---|
| HemAssist | Baxter | α-α intra-tetramer cross-linked human Hba | Trauma, stroke | Suspended - USA 2008 |
| Hemopure | Biopure (now OPK Biotech) | Intra- and inter-tetramer cross-linked bovine Hba | Surgery, sickle cell crisis, trauma | Phase III - US FDA denied further clinical trials, 2008 |
| PolyHeme | Northfield Laboratories (closed activities) | Inter-tetramer cross-linked human Hba | Trauma | Phase III - completed with FDA approval denied, 2008 |
| Hemospan | Sangart | PEGylated human Hba | Elective orthopedic surgery as blood expander | Phase III, completed in Europe. Results under evaluation |
| Hemolink | Hemosol | Inter-tetramer cross-linked human Hba | Elective surgery | Phase II - suspended, 2004 |
| PEG-Hb | Enzon | PEGylated bovine Hba | Tumor therapy | Phase Ib - suspended in USA, 1997 |
| PHP/Hemoximer | Apex Bioscience/Curacyte | PEGylated Hba | Septic shock (NO scavenging) | Phase III – undergoing in Europe |
Hemoglobin, Hb
Particularly in relation to hypertensive effects, no difference in systemic and pulmonary arterial pressure and vascular resistance was observed for Polyheme®58. Patients treated with Hemopure®, undergoing preoperative haemodilution for elective abdominal aortic surgery, at a dose of 3 mL/Kg exhibited an increased mean arterial pressure and systemic vascular resistance, and a decrease in cardiac index, impairing oxygen delivery59. A human haemoglobin, pyridoxalated and PEGylated through the activated ester of PEG-bis reacted with S-nitrosoglutathione (SNO-PEGylated haemoglobin) was demonstrated to increase coronary blood flow and to attenuate myocardial injury of ischemic heart disease in dogs60. Hemospan® studies on swine model showed that systemic and pulmonary resistance is minimal and no short term tissue pathology61. The product did not show vasoconstriction effects and was more effective in resuscitation from acute, massive uncontrolled abdominal haemorrhage than conventional therapies62,63. Studies on rhesus monkeys after an exchange transfusion of 30% of blood volume showed no haemodynamic effects and only transient elevation of aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase was observed64. A recent investigation has correlated the molecular mass of polymerized haemoglobin with blood pressure and vasocostriction65.
Critical evaluations of the effects of HBOCs on human health have been reported6,66. These adverse effects, highlighted in table IV, may limit the applicability of currently available HBOCs, given the low therapeutic index, the ratio of benefit to risk, and cast doubts on some of the applied strategies. A meta-analysis of the clinical studies carried out with HBOCs indicated that their use is associated with a significantly increased risk of myocardial infarction and death67. This report triggered strong reactions, especially from researchers associated to companies68 (available at: http://www.sangart.com/newsroom/press_061108.htm). Equilibrated views are reported in recent reviews of the presentations and discussions that took place at a workshop organized in the USA by the National Heart, Lung and Blood Institute in 200669, at another workshop on “Safety of Haemoglobin-based Oxygen Carriers: Current Status and Future Directions”, organized by the FDA and the NIH in 200813, in a Biochimica et Biophysica Acta special issue on HBOCs70 and in a chapter of Burger’s Medicinal Chemistry book on “Oxygen delivery by allosteric effectors of haemoglobin and blood substitutes”12. At the XII International Symposium on Blood Substitutes that was held in Parma, Italy, in 2009, the common view was that more deep and extensive investigations are needed on the relationship between structure and function of HBOCs and adverse effects in animal models before continuing or designing new clinical trials. It has been reported that, in general, HBOCs exhibit a nitrite reductase activity at low oxygen tension, thus increasing the production of NO53–56. Our laboratory is carrying out a pre-clinical study on guinea pigs to test and compare two products, Hemospan® and Euro-PEG-Hb. Biochemical and haematological parameters diagnostic of liver, pancreas and heart damages are recorded. Moreover, a proteomic analysis of the same organs is carried out using bidimensional electrophoresis and MALDI-TOF spectrometry. These analyses will allow identifying damage biomarkers, thus increasing the understanding of the relationship between a specific product and the outcome of adverse effects. These data and body imaging analysis of transfused guinea pigs with single photon emission computed tomography, carried out in collaboration with Dr. Andras Eke, Semmelweis University, Budapest, Hungary, will lead to the implementation of a total body safety assessment, an essential requisite to direct the development of a safe HBOC.
Table IV.
Potential adverse effects caused by HBOCs48
|
Conclusions
The need for an alternative to blood transfusions has triggered thorough investigations. However, after almost fifty years, a safe product has not been developed yet. This is not surprising, as whole blood, with its many components, of which haemoglobin contained in red cells is just one, is involved in many distinct, essential functions and regulative mechanisms, such as oxygen and NO homeostasis, coagulation, and vessel tension. Therefore, a HBOC designed to be an alternative to just one component, coping predominantly with a single function, oxygen transport, might interfere with other functions or lead to an unbalanced system. Moreover, in order to fulfil this single function, a HBOC must be injected in a quantity of the order of 100 grams. Therefore, less than 0.1% impurity would result in a significant amount, possibly triggering unwanted reactions. This implies that HBOC homogeneity is a critical issue for this very special drug. The same or similar issues are common to other approaches aimed at replacing transfusions. For the years to come, transfusion medicine has to count on blood donors, with, however, a more clear awareness that also red cells transfusion exhibits limitations and drawbacks, and that blood alternatives are urgent.
Acknowledgments
This work was supported by a grant from Fondazione Cariparma to A. M.
References
- 1.Napolitano LM, Kurek S, Luchette FA, et al. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med. 2009;37(12):3124–57. doi: 10.1097/CCM.0b013e3181b39f1b. [DOI] [PubMed] [Google Scholar]
- 2.Bonaventura J. Clinical implications of the loss of vasoactive nitric oxide during red blood cell storage. Proc Natl Acad Sci U S A. 2007;104(49):19165–6. doi: 10.1073/pnas.0708871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett-Guerrero E, Veldman TH, Doctor A, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104(43):17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynolds JD, Ahearn GS, Angelo M, et al. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci U S A. 2007;104(43):17058–62. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zubair AC. Clinical impact of blood storage lesions. Am J Hematol. 2010;85(2):117–22. doi: 10.1002/ajh.21599. [DOI] [PubMed] [Google Scholar]
- 6.Buehler PW, Alayash AI. Toxicities of hemoglobin solutions: in search of in-vitro and in-vivo model systems. Transfusion. 2004;44(10):1516–30. doi: 10.1111/j.1537-2995.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- 7.Patel RP. Biochemical aspects of the reaction of hemoglobin and NO: implications for Hb-based blood substitutes. Free Radic Biol Med. 2000;28(10):1518–25. doi: 10.1016/s0891-5849(00)00259-8. [DOI] [PubMed] [Google Scholar]
- 8.Isbell TS, Sun CW, Wu LC, et al. SNO-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation. Nat Med. 2008;14(7):773–7. doi: 10.1038/nm1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winslow RM. Blood Substitutes. Academic Press Inc; U.S.: 2006. [Google Scholar]
- 10.Chang TMS. Artficial cells. London: World Scientific Publishing Co; 2007. [Google Scholar]
- 11.Campanini B, Bruno S, Raboni S, Mozzarelli A. Oxygen delivery via allosteric effectors of hemoglobin, blood substitutes and plasma expanders. In: Abraham D, editor. Burger’s Medicinal Chemistry. 6th edition. John Wiley and Sons, Inc; 2003. pp. 385–442. [Google Scholar]
- 12.Bruno S, Ronda L, Faggiano S, et al. Oxygen delivery via allosteric effectors of hemoglobin and blood substitutes. VII Ed. Hoboken: John Wiley &Sons, Inc.; 2010. [Google Scholar]
- 13.Silverman T, Weiskopf RB. Hemoglobin-based oxygen carriers. Anesthesiology. 2009;111:946–963. doi: 10.1097/ALN.0b013e3181ba3c2c. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein J, Siviglia G, Hurst R, et al. Group B erythrocytes enzymatically converted to group O survive normally in A, B, and O individuals. Science. 1982;215(4529):168–70. doi: 10.1126/science.6274021. [DOI] [PubMed] [Google Scholar]
- 15.Scott MD, Murad KL, Koumpouras F, et al. Chemical camouflage of antigenic determinants: stealth erythrocytes. Proc Natl Acad Sci U S A. 1997;94(14):7566–71. doi: 10.1073/pnas.94.14.7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu A, Leng L, Monahan C, et al. Characterization of recombinant alpha-galactosidase for use in seroconversion from blood group B to O of human erythrocytes. Arch Biochem Biophys. 1996;327(2):324–9. doi: 10.1006/abbi.1996.0129. [DOI] [PubMed] [Google Scholar]
- 17.Murad KL, Mahany KL, Brugnara C, et al. Structural and functional consequences of antigenic modulation of red blood cells with methoxypoly(Ethylene Glycol) Blood. 1999;93(6):2121–2127. [PubMed] [Google Scholar]
- 18.Ma F, Ebihara Y, Umeda K, et al. Generation of functional erythrocytes from human embryonic stem cell-derived definitive hematopoiesis. Proc Natl Acad Sci U S A. 2008;105(35):13087–92. doi: 10.1073/pnas.0802220105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Douay L, Lapillonne H, Turhan AG. Stem cells-a source of adult red blood cells for transfusion purposes: present and future. Crit Care Clin. 2009;25(2):383–98. doi: 10.1016/j.ccc.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Eaton WA, Henry ER, Hofrichter J, Mozzarelli A. Is cooperative oxygen binding by hemoglobin really understood? Nat Struct Biol. 1999;6(4):351–8. doi: 10.1038/7586. [DOI] [PubMed] [Google Scholar]
- 21.Bunn HF, Forget BG. Hemoglobin: Molecular, Genetic and Clinical Aspects. Philadelphia, PA: W. B. Saunders Company; 1986. [Google Scholar]
- 22.Monod J, Wyman J, Changeux J-P. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 23.Henry ER, Bettati S, Hofrichter J, Eaton W. A tertiary two-state allosteric model for hemoglobin. Biophys Chem. 2002;98:149–164. doi: 10.1016/s0301-4622(02)00091-1. [DOI] [PubMed] [Google Scholar]
- 24.Viappiani C, Bettati S, Bruno S, et al. New insights into allosteric mechanisms from trapping unstable protein conformations in silica gels. Proc Natl Acad Sci U S A. 2004;101(40):14414–9. doi: 10.1073/pnas.0405987101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yonetani T, Park SI, Tsuneshige A, et al. Global allostery model of hemoglobin. Modulation of O(2) affinity, cooperativity, and Bohr effect by heterotropic allosteric effectors. J Biol Chem. 2002;277(37):34508–20. doi: 10.1074/jbc.M203135200. [DOI] [PubMed] [Google Scholar]
- 26.Yeh LH, Alayash AI. Redox side reactions of haemoglobin and cell signalling mechanisms. J Intern Med. 2003;253(5):518–26. doi: 10.1046/j.1365-2796.2003.01152.x. [DOI] [PubMed] [Google Scholar]
- 27.Sies H. Total antioxidant capacity: appraisal of a concept. J Nutr. 2007;137:1493–1495. doi: 10.1093/jn/137.6.1493. [DOI] [PubMed] [Google Scholar]
- 28.Wallis JP. Nitric oxide and blood: a review. Transfus Med. 2005;15(1):1–11. doi: 10.1111/j.1365-3148.2005.00542.x. [DOI] [PubMed] [Google Scholar]
- 29.Cooper CE. Nitric oxide and iron proteins. Biochim Biophys Acta - Bioenerg. 1999;1411:290–309. doi: 10.1016/s0005-2728(99)00021-3. [DOI] [PubMed] [Google Scholar]
- 30.Vaughn MW, Huang KT, Kuo L, Liao JC. Erythrocytes possess an intrinsic barrier to nitric oxide consumption. J Biol Chem. 2000;275(4):2342–8. doi: 10.1074/jbc.275.4.2342. [DOI] [PubMed] [Google Scholar]
- 31.Liao JC, Hein TW, Vaughn MW, et al. Intravascular flow decreases erythrocyte consumption of nitric oxide. Proc Natl Acad Sci U S A. 1999;96(15):8757–61. doi: 10.1073/pnas.96.15.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gladwin MT, Crawford JH, Patel RP. The biochemistry of nitric oxide, nitrite, and hemoglobin: role in blood flow regulation. Free Radic Biol Med. 2004;36(6):707–17. doi: 10.1016/j.freeradbiomed.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 33.Olson JS, Foley EW, Rogge C, et al. NO scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic Biol Med. 2004;36(6):685–97. doi: 10.1016/j.freeradbiomed.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 34.Hess JR, MacDonald VW, Brinkley WW. Systemic and pulmonary hypertension after resuscitation with cell-free hemoglobin. J Appl Physiol. 1993;74(4):1769–78. doi: 10.1152/jappl.1993.74.4.1769. [DOI] [PubMed] [Google Scholar]
- 35.Winslow RM. Current status of blood substitute research: towards a new paradigm. J Intern Med. 2003;253(5):508–17. doi: 10.1046/j.1365-2796.2003.01150.x. [DOI] [PubMed] [Google Scholar]
- 36.Tsai AG, Vandegriff KD, Intaglietta M, Winslow RM. Targeted O2 delivery by low-P50 hemoglobin: a new basis for O2 therapeutics. Am J Physiol Heart Circ Physiol. 2003;285(4):H1411–9. doi: 10.1152/ajpheart.00307.2003. [DOI] [PubMed] [Google Scholar]
- 37.Tsai AG, Cabrales P, Manjula BN, et al. Dissociation of local nitric oxide concentration and vasoconstriction in the presence of cell-free hemoglobin oxygen carriers. Blood. 2006;108(10):3603–10. doi: 10.1182/blood-2006-02-005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaucher-Di Stasio C, Paternotte E, Prin-Mathieu C, et al. The importance of the effect of shear stress on endothelial cells in determining the performance of hemoglobin based oxygen carriers. Biomaterials. 2009;30(4):445–51. doi: 10.1016/j.biomaterials.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 39.Melkumyants AM, Balashov SA, Khayutin VM. Endothelium dependent control of arterial diameter by blood viscosity. Cardiovasc Res. 1989;23(9):741–7. doi: 10.1093/cvr/23.9.741. [DOI] [PubMed] [Google Scholar]
- 40.Tsai AG, Acero C, Nance PR, et al. Elevated plasma viscosity in extreme hemodilution increases perivascular nitric oxide concentration and microvascular perfusion. Am J Physiol Heart Circ Physiol. 2005;288(4):H1730–9. doi: 10.1152/ajpheart.00998.2004. [DOI] [PubMed] [Google Scholar]
- 41.Salazar Vazquez BY, Wettstein R, Cabrales P, et al. Microvascular experimental evidence on the relative significance of restoring oxygen carrying capacity vs. blood viscosity in shock resuscitation. Biochim Biophys Acta. 2008;1784(10):1421–7. doi: 10.1016/j.bbapap.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Looker D, Abbott-Brown D, Cozart P, et al. A human recombinant haemoglobin designed for use as a blood substitute. Nature. 1992;356(6366):258–60. doi: 10.1038/356258a0. [DOI] [PubMed] [Google Scholar]
- 43.Panetta G, Arcovito A, Morea V, et al. Hb(alphaalpha,betabeta): a novel fusion construct for a dimeric, four-domain hemoglobin. Biochim Biophys Acta. 2008;1784(10):1462–70. doi: 10.1016/j.bbapap.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Graves PE, Henderson DP, Horstman MJ, et al. Enhancing stability and expression of recombinant human hemoglobin in E. coli: Progress in the development of a recombinant HBOC source. Biochim Biophys Acta. 2008;1784(10):1471–9. doi: 10.1016/j.bbapap.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Doherty DH, Doyle MP, Curry SR, et al. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat Biotechnol. 1998;16(7):672–6. doi: 10.1038/nbt0798-672. [DOI] [PubMed] [Google Scholar]
- 46.Fronticelli C, Arosio D, Bobofchak KM, Vasquez GB. Molecular engineering of a polymer of tetrameric hemoglobins. Proteins. 2001;44(3):212–22. doi: 10.1002/prot.1086. [DOI] [PubMed] [Google Scholar]
- 47.Fronticelli C, Koehler RC. Design of recombinant hemoglobins for use in transfusion fluids. Crit Care Clin. 2009;25:357–371. doi: 10.1016/j.ccc.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 48.Bobofchak KM, Mito T, Texel SJ, et al. A recombinant polymeric hemoglobin with conformational, functional, and physiological characteristics of an in vivo O2 transporter. Am J Physiol Heart Circ Physiol. 2003;285(2):H549–61. doi: 10.1152/ajpheart.00037.2003. [DOI] [PubMed] [Google Scholar]
- 49.Chang TM. Modified hemoglobin-based blood substitutes: crosslinked, recombinant and encapsulated hemoglobin. Vox Sang. 1998;74(Suppl 2):233–41. doi: 10.1111/j.1423-0410.1998.tb05425.x. [DOI] [PubMed] [Google Scholar]
- 50.Keipert P, Minkowitz J, Chang TM. Cross-linked stroma-free polyhemoglobin as a potential blood substitute. Int J Artif Organs. 1982;5(6):383–5. [PubMed] [Google Scholar]
- 51.Vandegriff KD, McCarthy M, Rohlfs RJ, Winslow RM. Colloid osmotic properties of modified hemoglobins: chemically cross-linked versus polyethylene glycol surface-conjugated. Biophys Chem. 1997;69:23–30. doi: 10.1016/s0301-4622(97)00079-3. [DOI] [PubMed] [Google Scholar]
- 52.Manjula BN, Tsai AG, Intaglietta M, et al. Conjugation of multiple copies of polyethylene glycol to hemoglobin facilitated through thiolation: Influence on hemoglobin structure and function. Protein J. 2005;24(3):133–146. doi: 10.1007/s10930-005-7837-2. [DOI] [PubMed] [Google Scholar]
- 53.Li D, Hu T, Manjula BN, Acharya SA. Extension arm facilitated PEGylation of alphaalpha-hemoglobin with modifications targeted exclusively to amino groups: functional and structural advantages of free Cys-93(beta) in the PEG-Hb adduct. Bioconjug Chem. 2009;20(11):2062–70. doi: 10.1021/bc900170e. [DOI] [PubMed] [Google Scholar]
- 54.Caccia D, Ronda L, Frassi R, et al. PEGylation promotes hemoglobin tetramer dissociation. Bioconjug Chem. 2009;20(7):1356–66. doi: 10.1021/bc900130f. [DOI] [PubMed] [Google Scholar]
- 55.Portoro I, Kocsis L, Herman P, et al. Towards a novel haemoglobin-based oxygen carrier: Euro-PEG-Hb, physico-chemical properties, vasoactivity and renal filtration. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbapap.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 56.Iafelice R, Cristoni S, Caccia D, et al. Identification of the sites of deoxyhaemoglobin PEGylation. Biochem J. 2007;403(1):189–96. doi: 10.1042/BJ20061556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vandegriff KD, Malavalli A, Wooldridge J, et al. MP4, a new nonvasoactive PEG-Hb conjugate. Transfusion. 2003;43(4):509–16. doi: 10.1046/j.1537-2995.2003.00341.x. [DOI] [PubMed] [Google Scholar]
- 58.Johnson JL, Moore EE, Offner PJ, et al. Resuscitation of the injured patient with polymerized stroma-free hemoglobin does not produce systemic or pulmonary hypertension. Am J Surg. 1998;176(6):612–7. doi: 10.1016/s0002-9610(98)00275-x. [DOI] [PubMed] [Google Scholar]
- 59.Kasper SM, Walter M, Grune F, et al. Effects of a hemoglobin-based oxygen carrier (HBOC-201) on hemodynamics and oxygen transport in patients undergoing preoperative hemodilution for elective abdominal aortic surgery. Anesth Analg. 1996;83(5):921–7. [PubMed] [Google Scholar]
- 60.Asanuma H, Nakai K, Sanada S, et al. S-nitrosylated and pegylated hemoglobin, a newly developed artificial oxygen carrier, exerts cardioprotection against ischemic hearts. J Mol Cell Cardiol. 2007;42(5):924–30. doi: 10.1016/j.yjmcc.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 61.Drobin D, Kjellstrom BT, Malm E, et al. Hemodynamic response and oxygen transport in pigs resuscitated with maleimide-polyethylene glycol-modified hemoglobin (MP4) J Appl Physiol. 2004;96(5):1843–53. doi: 10.1152/japplphysiol.00530.2003. [DOI] [PubMed] [Google Scholar]
- 62.Young MA, Riddez L, Kjellstrom BT, et al. MalPEG-hemoglobin (MP4) improves hemodynamics, acid-base status, and survival after uncontrolled hemorrhage in anesthetized swine. Crit Care Med. 2005;33(8):1794–804. doi: 10.1097/01.ccm.0000172648.55309.13. [DOI] [PubMed] [Google Scholar]
- 63.Young MA, Riddez L, Kjellstrom BT, Winslow RM. Effect of maleimide-polyethylene glycol hemoglobin (MP4) on hemodynamics and acid-base status after uncontrolled hemorrhage in anesthetized swine: Comparison with crystalloid and blood. J Trauma. 2007;63(6):1234–1244. doi: 10.1097/TA.0b013e31815bd7b0. 10.1097/TA.0b013e31815bd7b0. [DOI] [PubMed] [Google Scholar]
- 64.Young MA, Malavalli A, Winslow N, et al. Toxicity and hemodynamic effects after single dose administration of MalPEG-hemoglobin (MP4) in rhesus monkeys. Transl Res. 2007;149(6):333–42. doi: 10.1016/j.trsl.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 65.Cabrales P, Sun G, Zhou Y, et al. Effects of the molecular mass of tense-state polymerized bovine hemoglobin on blood pressure and vasoconstriction. J Appl Physiol. 2009;107(5):1548–58. doi: 10.1152/japplphysiol.00622.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hess JR, Reiss RF. Resuscitation and the limited utility of the present generation of blood substitutes. Transfus Med Rev. 1996;10(4):276–85. doi: 10.1016/s0887-7963(96)80003-4. [DOI] [PubMed] [Google Scholar]
- 67.Natanson C, Kern SJ, Lurie P, et al. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. Jama. 2008;299(19):2304–12. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greenburg AG, Pitman A, Pearce B, Kim HW. Clinical contextualization and the assessment of adverse events in HBOC trials. Artif Cells Blood Substit Immobil Biotechnol. 2008;36:477–486. doi: 10.1080/10731190802554000. [DOI] [PubMed] [Google Scholar]
- 69.Estep T, Bucci E, Farmer M, et al. Basic science focus on blood substitutes: a summary of the NHLBI Division of Blood Diseases and Resources Working Group Workshop, March 1, 2006. Transfusion. 2008;48(4):776–82. doi: 10.1111/j.1537-2995.2007.01604.x. [DOI] [PubMed] [Google Scholar]
- 70.Mozzarelli A. Hemoglobin-based oxygen carriers as blood substitutes. Biochim Biophys Acta. 2008;1784(10):1363–4. doi: 10.1016/j.bbapap.2008.09.001. [DOI] [PubMed] [Google Scholar]