Introduction
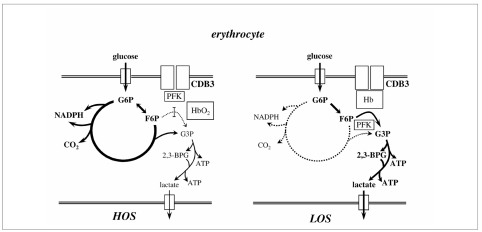
About fifteen years ago a number of experimental data on the specific interaction of haemoglobin (Hb) and various enzymes of the glycolytic pathway with the cytoplasmatic domain of band 3 protein emerged13. These data induced our research group to formulate the hypothesis of a modulation of the erythrocyte (RBC) metabolism driven by the free energy connected to the R to T Hb transition4. The general scheme of this simple model is reported in figure 1. In this model band 3 protein plays a pivotal role: when the erythrocyte is at high oxygenation state (HOS) band 3 interacts with some glycolytic enzymes (inhibiting their activity) and more glucose is addressed towards the pentose phosphate pathway (PPP). When the erythrocyte is at low oxygenation state (LOS) the high affinity of deoxy-Hb for the N-terminal cytoplasmatic domain of band 3 induces the release of glycolytic enzymes, and thus glucose flux towards the glycolytic pathway increases. Some recent results obtained by our and other groups are in agreement with this simple model, even though they indicate, that other factors might play a significant role in this erythrocyte modulation. The aim of this brief review is to offer to the reader a critical examination of the significance of these recent results in light of the model framework.
Figure 1.
Simplified scheme representing the modulation of erythrocyte metabolism by the O2 transition of Hb and its competition with glycolytic enzymes (mainly phosphofructokinase, PFK) for the cytoplasmic domain of band 3 (CDB3).
Band 3 protein
Band 3 is a transmembrane protein that accounts for about 25% of the total RBC membrane proteins. It is characterized by three distinct functional domains: (1) the membrane spanning domain, which transverses 12 times the bilayer and serves to catalyze the exchange of anions (mainly Cl− and HCO3−) across the membrane5; (2) the short C-terminal cytoplasmatic domain that binds carbonic anhydrase II6,7, and (3) the N-terminal cytoplasmatic domain (CDB3) that binds a variety of proteins8 and anchors the RBC membrane to the underlying cytoskeleton via ankyrin and protein 4.29.
Glycolysis and pentose phosphate pathway flux measurements
Initially, to confirm the reported hypothesis, metabolic behavior of human RBC under conditions in which they are fully oxygenated (HOS conditions) or greatly deoxygenated (LOS conditions) has been investigated. The results evidenced that glucose consumption is strongly pH dependent, but almost independent upon the oxygenation state10,11. However, the ratio between 14 CO2 production and glucose consumption corresponding to the fraction of glucose utilized through the PPP resulted 2-fold higher in HOS than in LOS erythrocytes at any pH value, showing an increase for both oxygenation states as pH decreased. Moreover, measurement by 13C-NMR spectroscopy of the rate of [13C-3] lactate production in RBCs incubated with 13C-1-labelled glucose that is linked only to glycolysis, resulted significantly higher in erythrocytes kept in LOS. On the whole, the data obtained evidenced that HOS and LOS erythrocytes displayed a significant difference in the amount of glucose addressed towards PPP and glycolysis and that PPP is about two times more active in HOS samples. This modulation did not alter total glucose consumption, which is only pH-dependent. The results obtained were in perfect agreement with the hypothesis that the glycolytic flux is modulated via a competition between glycolytic enzymes and deoxy-Hb for CDB3. In fact, in HOS erythrocytes the inhibition of glycolytic enzymes, due to their binding to CDB3, should result in a reduced glucose flux through glycolysis. As a consequence, since glucose consumption is constant, more glucose is metabolized by the PPP in order to ensure adequate levels of NADPH necessary to protect the RBCs from oxidative stress deriving from the high oxygen load. Conversely, in the LOS state the displacement of glycolytic enzymes from CDB3, induced by the binding of deoxy-Hb, results in an increased glucose flux throughout glycolysis, thereby increasing ATP and 2,3-BPG production.
To evaluate the effect of CDB3 tyrosine phosphorylation on glucose metabolism, metabolic pathway fluxes have been recently measured in intact RBCs after pervanadate treatment12. Pervanade inhibits phosphotyrosine phosphatase, responsible for the dephosphorylation of tyrosine residues, and increases the activity of two tyrosine kinases, the p72syk and a kinase belonging to the Src family (most probably Lyn)13,14, resulting in increased tyrosine phosphorylation. Phosphorylation of the tyrosine residues of band 3 at position 8 and 21, both involved in the N-terminal binding site, caused an increase of the oxygenated RBC glycolytic flux (45%) and a reduction (66%) of PPP flux with respect to control flux12. Confocal microscopy experiments evidenced also that pervanadate treatment of RBC caused the displacement of glycolytic enzymes from the membrane15. However, considering that deoxy-Hb binding site for band 3 is involved also in 2,3-BPG binding16, and that the increased number of negative charges on the N-terminal domain of band 3, due to its phosphorylation, may greatly enhance Hb binding to band 3, it is conceivable to hypothesize that several Hb molecules (located in proximity of the RBC membrane) may undertake a deoxygenation-linked binding to phosphorylated band 3, with the consequent displacement of the glycolytic enzymes from CDB3. Anyway, the possibility of proving by in vivo experiments that this is the case is really difficult. Moreover, as far as we are aware of, no papers reporting an increased affinity of Hb for the tyrosine-phosphorylated CDB3 or for any tyrosine-phosphorylated peptide mimicking the N-terminus of band 3 have been published up to now.
To obtain further evidences on the involvement of the Hb-CDB3 interaction in the oxygen-linked modulation of erythrocyte metabolism, rate of 13C-3 lactate production in RBCs incubated with 13C-1-labelled glucose was measured by 13C-NMR spectroscopy on RBCs saturated with carbon monoxide (CO), and on RBCs incubated at low oxygen pressure and treated with 4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (DIDS), a strong inhibitor of band 3 function10. In both cases the difference between the two oxygenation states almost vanished. In conclusion, the experiments on DIDS-treated erythrocytes supported the involvement of band 3 in the metabolic differences observed between oxygenated and deoxygenated RBCs, while those obtained on CO-saturated erythrocytes strongly suggested that the interaction with CDB3 involved the T conformation of Hb. In the presence of CO the Hb molecule is indeed blocked in the fully liganded R conformation even at very low oxygen tension. However, it should be underlined that no CDB3 phosphorylation level measurement was carried out in these experiments.
Haemoglobin and the band 3 complex
The differential interaction between band 3, oxygenated and deoxygenated Hb was evidenced by an experiment performed in the presence of increasing concentrations of the acetylated peptide corresponding to the first 11 residues of human band 3, that showed that the oxygen affinity of human Hb is progressively decreased16. Furthermore, the X-ray crystallographic analysis of this complex indicated that the peptide did not simply lie across the 2,3-BPG binding site (as it has been speculated in previous studies) but it penetrated deeply into the deoxy-Hb tetramer extending through the 2,3-BPG binding site, even if only from 5 to 7 of its 11 residues appeared in direct contact with β subunits. The other residues that extended out of the central cavity appeared highly disordered and thus, not visible in the electronic density map16.
To confirm that this interaction is present also in different vertebrates, a series of peptides corresponding to the 10 N-terminal CDB3 residues of trout, chicken and human have been tested in their interaction with Haplosternum littorale cathodic Hb, Andean frog Hb, avian Hbs A and D, and the results confirmed that this interaction is ubiquitous in vertebrates17.
In order to determine the precise binding site to band 3 in deoxy-Hb, a series of site-directed mutants of band 3 have been expressed in Escherichia coli and the measured shift of the oxygen binding curve of Hb has been compared to that obtained in the presence of the entire recombinant band 3 (ΔlogP50 = 18 mmHg)18. Band 3-lacking the first 12–23 residues (or a higher number of residues) did not significantly perturb the O2 dissociation curve with respect to the measurements carried out in the absence of band 3, suggesting that residues 12–23 are essential for deoxy-Hb binding18. In this respect, the effect exerted on the oxygen affinity of Hb by the peptide corresponding to the first 11 residues of band 3 can be explained by the high similarity of the sequences 1–11 and 12–23 of band 3. However, experiments performed by using wild type and mutant isoforms of CDB3 demonstrated that the presence of residues 1–11 failed to enhance CDB3 interaction with deoxy-Hb, suggesting that the first 11 residues somehow inhibit the access of deoxy-Hb to its higher affinity site on residues 12–23 of CDB3. Moreover, mutation of the acidic amino acid residues surrounding Tyr21 to their uncharged counterparts caused a significant reduction of band 3 affinity for deoxy-Hb, and the additional substitution Tyr21→Ala reduced the affinity even more. Moreover, deletion of sequences on both sides of residues 12–23 of band 3 functioned to suppress, rather than to enhance, Hb-band 3 affinity18.
Band 3 interactions
Although band 3 binding sites for a number of proteins have been precisely described, the whole network of interactions has not been studied in detail yet8,19. However, different studies described the interaction of many glycolytic enzymes to CDB3. The major band 3 binding site for aldolase has been shown to be localized within the first N-terminal 23 residues19,20. Two binding sites of glyceraldehyde 3-phosphate dehydrogenase (G3PD) are located at residues 1–11 and 12–23. The phosphofructokinase (PFK) binding site involves residues 12–2319, while a direct interaction between band 3 and either pyruvate kinase (PK) and lactate dehydrogenase (LDH) has not been documented up to now19, even if evidences of LDH-interaction with band 3 were already shown by Harris and Winzor 20 years ago21.
The association between membrane and cytoskeleton is obtained through the interaction of band 3 (in tetrameric form) with ankyrin at the β-hairpin loop comprising residues 175–18522, and also through the interaction of CDB3 (in tetrameric form) with protein 4.1. This interaction is alternative to that of protein 4.1 with glycophorins, that requires the presence of polyphosphoinositides as cofactors23. Another important site of interaction between the membrane and the spectrin-based cytoskeleton is represented by the band 3 macrocomplex, consisting of Rh-associated glycoprotein, Rh polypeptides, CD47, LW, glycophorin B and Landsteiner-Wiener blood group protein24.
The binding site of protein 4.2 on the N-terminus of CDB3 is still unknown, even if this protein has been proven to directly bind CDB325,26. The binding site of glycophorin A for band 3 has been hypothesized to be located in the vicinity of the trans-membrane span 8 and 9 and also of the cytosolic C-terminus27. Carbonic anhydrase II, that catalyzes the hydratation of carbon dioxide to bicarbonate ions in RBC, is known to bind band 3 at the C-terminal domain, at the sequence L886DADD28. Bicarbonate ions are channelled to band 3, which immediately exports it from the cell.
The influence of Hb-CDB3 interaction on the oxygen affinity of Hb may appear of reduced importance considering that only 0.5% of RBC's Hb molecules may bind band 3 in RBCs at LOS, due to the presence of 270 millions of Hb molecules and 1.2 million of band 3 molecules29. However, the impact of Hb-CDB3 interaction on nitric oxide (NO) delivery from RBC may be substantial18. In fact, it has been shown that the oxygen-linked intra-molecular transfer of some NO molecules bound to the heme Fe2+ to Cysβ93 (in form of an S-nitrosothiol) is followed by transfer of NO (transnitrosation) to CDB3 Cys residues, to which Hb is bound at LOS30. Thus, CDB3 may act as an allosteric regulator that promotes the T state of Hb and as an acceptor of NO groups transferred by SNO-Hb upon R to T transition. The number of band 3 molecules per RBC is 10 fold higher than that of SNO-Hb molecules in arterial RBC, suggesting that the transfer of NO to CDB3 may represent a main route in NO trafficking30.
Moreover, considering the number of molecules of G3PD (500.000), aldolase (20.000) and PFK (6000) per RBC31, it strongly emerges that the band 3 molecules are sufficient for binding all the molecules of the reported enzymes at HOS, and for displacing them during RBC deoxygenation, because of the competition with deoxy-Hb for CDB3.
Glycolytic Enzymes Localization in vivo
Confocal microscopy of unstimulated, healthy, intact and freshly drawn RBCs showed that the localization of PK and LDH, as well as aldolase, PFK and G3PD is at the level of the membrane15, while after RBC deoxygenation, all these enzymes are displaced from the membrane to the cytoplasm, thus, confirming that the association of the enzyme complex to the membrane is regulated by the oxygenation state of RBC.
Furthermore, band 3 knock-out mice presented glycolytic enzymes localized throughout the cytoplasm and not membrane-bound, independently from the oxygenation state of the erythrocyte32.
Metabolomics and transporter regulation
A dynamic mathematic model of RBC metabolism, involving the O2 sensing mechanism of Hb and the hypoxia-induced activation of glycolytic enzymes correlated with their release from CDB3 was developed to predict temporal alterations in intracellular metabolites and cellular energetics in response to hypoxia33. It resulted that the alterations predicted by this updated version of the model are consistent with the metabolome analysis carried out by capillary electrophoresis mass spectrometry34,35. However, it is important to underline that a previous version of the model, that did not consider the effect of the Hb-CDB3 interaction, was unable to reproduce the experimental metabolic alterations observed33.
The oxygenation state of RBCs resulted effective also in regulating the activity of many membrane soluble transporters, in fact the activity of the K/Cl cotransporter increased 20 times in oxygenated RBCs36,37, while that of the Na-K-2Cl cotransporter and of the Na/H exchanger increased upon deoxygenation37,38.
Conclusions
The fact that RBCs regulate their metabolism depending on their oxygenation state, favoring glycolysis at LOS and PPP at HOS, appears consolidated by the long series of reported papers39,40. Many experimental data confirm that this O2-linked RBC modulation is played at the level of the RBC membrane involving the quaternary state of band 341. Although it is difficult to evaluate the role played by the different factors, it seems probable that several aspects are crucial for this mechanism:
The oxygenation state of Hb;
The phosphorylation level of the N-terminal domain of band 3.
Thus, through this review we hope to stimulate further research in this important area, in order to give new insight on the molecular events that regulate phosphorylation of band 3, and its connection with red blood cell metabolism and function.
References
- 1.Low PS, Rathinavelu P, Harrison ML. Regulation of glycolysis via reversible enzyme binding to the membrane protein, band 3. J Biol Chem. 1993;268:14627–31. [PubMed] [Google Scholar]
- 2.Moriyama R, Lombardo CR, Workman RF, et al. Regulation of linkages between the erythrocyte membrane and its skeleton by 2,3-diphosphoglycerate. J Biol Chem. 1993;268:10990–6. [PubMed] [Google Scholar]
- 3.Harrison ML, Rathinavelu P, Arese P, et al. Role of band 3 tyrosine phosphorylation in the regulation of erythrocyte glycolysis. J Biol Chem. 1991;266:4106–11. [PubMed] [Google Scholar]
- 4.Giardina B, Messana I, Scatena R, et al. The multiple functions of haemoglobin. Crit Rev Biochem Mol Biol. 1995;30:165–96. doi: 10.3109/10409239509085142. (Review). [DOI] [PubMed] [Google Scholar]
- 5.Tanner MJ. The structure and function of band 3 (AE1): recent developments. Mol Membr Biol. 1997;14:155–65. doi: 10.3109/09687689709048178. (Review). [DOI] [PubMed] [Google Scholar]
- 6.Vince JW, Reithmeier RA. Identification of the carbonic anhydrase II binding site in the Cl(−)/HCO(3)(−) anion exchanger AE1. Biochemistry. 2000;39:5527–33. doi: 10.1021/bi992564p. [DOI] [PubMed] [Google Scholar]
- 7.Sterling D, Reithmeier RA, Casey JR. A transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J Biol Chem. 2001;276:47886–94. doi: 10.1074/jbc.M105959200. [DOI] [PubMed] [Google Scholar]
- 8.Zhang D, Kiyatkin A, Bolin JT, et al. Crystallographic structure and functional interpretation of the cytoplasmic domain of erythrocyte membrane band 3. Blood. 2000;96:2925–33. [PubMed] [Google Scholar]
- 9.Lux SE, Palek J. Disorders of the red cell membrane. In: Handin RI, Lux SE, Stossel TP, editors. Blood: Principles and Practice of Hematology. JB Lippincott Co; Philadelphia: 1995. pp. 1701–1818. [Google Scholar]
- 10.Messana I, Orlando M, Cassiano L, et al. Human erythrocyte metabolism is modulated by the O2-linked transition of hemoglobin. FEBS Lett. 1996;390:25–8. doi: 10.1016/0014-5793(96)00624-2. [DOI] [PubMed] [Google Scholar]
- 11.Messana I, Ferroni L, Misiti F, et al. Blood bank conditions and RBCs: the progressive loss of metabolic modulation. Transfusion. 2000;40:353–60. doi: 10.1046/j.1537-2995.2000.40030353.x. [DOI] [PubMed] [Google Scholar]
- 12.Lewis IA, Campanella ME, Markley JL, et al. Role of band 3 in regulating metabolic flux of red blood cells. Proc Natl Acad Sci U S A. 2009;106:18515–20. doi: 10.1073/pnas.0905999106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bordin L, Brunati AM, Donella-Deana A, et al. Band 3 is an anchor protein and a target for SHP-2 tyrosine phosphatase in human erythrocytes. Blood. 2002;100:276–82. doi: 10.1182/blood.v100.1.276. [DOI] [PubMed] [Google Scholar]
- 14.Brunati AM, Bordin L, Clari G, et al. Sequential phosphorylation of protein band 3 by Syk and Lyn tyrosine kinases in intact human erythrocytes: identification of primary and secondary phosphorylation sites. Blood. 2000;96:1550–7. [PubMed] [Google Scholar]
- 15.Campanella ME, Chu H, Low PS. Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane. Proc. Natl. Acad. Sci. U. S. A. 2005;102:2402–7. doi: 10.1073/pnas.0409741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walder JA, Chatterjee R, Steck TL, et al. The interaction of Haemoglobin with the cytoplasmic domain of band 3 of the human erythrocyte membrane. J Biol Chem. 1984;259:10238–46. [PubMed] [Google Scholar]
- 17.Weber RE, Voelter W, Fago A, et al. Modulation of red cell glycolysis: interactions between vertebrate hemoglobins and cytoplasmic domains of band 3 red cell membrane proteins. Am J Physiol Regul Integr Comp Physiol. 2004;287:R454–64. doi: 10.1152/ajpregu.00060.2004. [DOI] [PubMed] [Google Scholar]
- 18.Chu H, Breite A, Ciraolo P, et al. Characterization of the deoxyhemoglobin binding site on human erythrocyte band 3: implications for O2 regulation of erythrocyte properties. Blood. 2008;111:932–8. doi: 10.1182/blood-2007-07-100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu H, Low PS. Mapping of glycolytic enzyme-binding sites on human erythrocyte band 3. Biochem J. 2006;400:143–51. doi: 10.1042/BJ20060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perrotta S, Borriello A, Scaloni A, et al. The N-terminal 11 amino acids of human erythrocyte band 3 are critical for aldolase binding and protein phosphorylation: implications for band 3 function. Blood. 2005;106:4359–66. doi: 10.1182/blood-2005-07-2806. [DOI] [PubMed] [Google Scholar]
- 21.Harris SJ, Winzor DJ. Interactions of glycolytic enzymes with erythrocyte membranes. Biochim Biophys Acta. 1990;1038:306–14. doi: 10.1016/0167-4838(90)90242-8. [DOI] [PubMed] [Google Scholar]
- 22.Chang SH, Low PS. Identification of a critical ankyrin-binding loop on the cytoplasmic domain of erythrocyte membrane band 3 by crystal structure analysis and site-directed mutagenesis. J Biol Chem. 2003;278:6879–84. doi: 10.1074/jbc.M211137200. [DOI] [PubMed] [Google Scholar]
- 23.Pasternack GR, Anderson RA, Leto TL, et al. Interactions between protein 4.1 and band 3. An alternative binding site for an element of the membrane skeleton. J Biol Chem. 1985;260:3676–83. [PubMed] [Google Scholar]
- 24.Bruce LJ, Beckmann R, Ribeiro ML, et al. A band 3-based macrocomplex of integral and peripheral proteins in the RBC membrane. Blood. 2003;101:4180–8. doi: 10.1182/blood-2002-09-2824. [DOI] [PubMed] [Google Scholar]
- 25.Toye AM, Ghosh S, Young MT, et al. Protein-4.2 association with band 3 (AE1, SLCA4) in Xenopus oocytes: effects of three natural protein-4.2 mutations associated with hemolytic anemia. Blood. 2005;105:4088–95. doi: 10.1182/blood-2004-05-1895. [DOI] [PubMed] [Google Scholar]
- 26.Korsgren C, Cohen CM. Purification and properties of human erythrocyte band 4.2. Association with the cytoplasmic domain of band 3. J Biol Chem. 1986;261:5536–43. [PubMed] [Google Scholar]
- 27.Young MT, Beckmann R, Toye AM, et al. Red-cell glycophorin A-band 3 interactions associated with the movement of band 3 to the cell surface. Biochem J. 2000;350:53–60. [PMC free article] [PubMed] [Google Scholar]
- 28.Vince JW, Reithmeier RA. Carbonic anhydrase II binds to the carboxyl terminus of human band 3, the erythrocyte C1-/HCO3- exchanger. J Biol Chem. 1998;273:28430–7. doi: 10.1074/jbc.273.43.28430. [DOI] [PubMed] [Google Scholar]
- 29.Low PS. Structure and function of the cytoplasmic domain of band 3: center of erythrocyte membrane-peripheral protein interactions. Biochim Biophys Acta. 1986;864:145–67. doi: 10.1016/0304-4157(86)90009-2. [DOI] [PubMed] [Google Scholar]
- 30.Pawloski JR, Hess DT, Stamler JS. Export by red blood cells of nitric oxide bioactivity. Nature. 2001;409:622–6. doi: 10.1038/35054560. [DOI] [PubMed] [Google Scholar]
- 31.Lux SE. In: Blood: Principles and Practice of Hematology. Handin RI, Lux SE, Stossel TP, editors. JB Lippincott Co; Philadelphia: 1995. [Google Scholar]
- 32.Campanella ME, Chu H, Wandersee NJ, et al. Characterization of glycolytic enzyme interactions with murine erythrocyte membranes in wild-type and membrane protein knockout mice. Blood. 2008;112:3900–6. doi: 10.1182/blood-2008-03-146159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinoshita A, Tsukada K, Soga T, et al. Roles of hemoglobin. Allostery in hypoxia-induced metabolic alterations in erythrocytes: simulation and its verification by metabolome analysis. J Biol Chem. 2007;282:10731–41. doi: 10.1074/jbc.M610717200. [DOI] [PubMed] [Google Scholar]
- 34.Monton MR, Soga T. Metabolome analysis by capillary electrophoresis-mass spectrometry. J Chromatogr A. 2007;1168:237–46. doi: 10.1016/j.chroma.2007.02.065. Review. [DOI] [PubMed] [Google Scholar]
- 35.Soga T, Ohashi Y, Ueno Y, et al. Quantitative metabolome analysis using capillary electrophoresis mass spectrometry. J Proteome Res. 2003;2:488–94. doi: 10.1021/pr034020m. [DOI] [PubMed] [Google Scholar]
- 36.Berenbrink M, Völkel S, Heisler N, et al. O(2)-dependent K(+) fluxes in trout red blood cells: the nature of O(2) sensing revealed by the O(2) affinity, cooperativity and pH dependence of transport. J Physiol. 2000;526:69–80. doi: 10.1111/j.1469-7793.2000.t01-1-00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gibson JS, Cossins AR, Ellory JC. Oxygen-sensitive membrane transporters in vertebrate red cells. J Exp Biol. 2000;203:1395–407. doi: 10.1242/jeb.203.9.1395. Review. [DOI] [PubMed] [Google Scholar]
- 38.Muzyamba MC, Cossins AR, Gibson JS. Regulation of Na+–K+–2Cl− cotransport in turkey red cells: the role of oxygen tension and protein phosphorylation. J Physiol Lond. 1999;517:421–9. doi: 10.1111/j.1469-7793.1999.0421t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Rosa MC, Carelli Alinovi C, Galtieri A, et al. Allosteric properties of hemoglobin and the plasma membrane of the erythrocyte: new insights in gas transport and metabolic modulation. IUBMB Life. 2008;60:87–93. doi: 10.1002/iub.15. Review. [DOI] [PubMed] [Google Scholar]
- 40.Tanner MJ. Band 3 anion exchanger and its involvement in erythrocyte and kidney disorders. Curr Opin Hematol. 2002;9:133–9. doi: 10.1097/00062752-200203000-00009. Review. [DOI] [PubMed] [Google Scholar]
- 41.De Rosa MC, Carelli Alinovi C, Galtieri A, et al. The plasma membrane of erythrocytes plays a fundamental role in the transport of oxygen, carbon dioxide and nitric oxide and in the maintenance of the reduced state of the heme iron. Gene. 2007;398:162–71. doi: 10.1016/j.gene.2007.02.048. Review. [DOI] [PubMed] [Google Scholar]