Blood platelets play a pivotal role in thrombosis and haemostasis. Platelet transfusions are life-saving medical procedures for patients undergoing major surgery or suffering from diseases such as cancer or thrombocytopenia. Research efforts in transfusion science are applied to the banking of platelets in areas ranging from donor genotyping to the improvement of the blood component processing procedure and determination of product quality. After production, platelet concentrates are stored at room temperature (20–24 °C) with agitation for only 4–7 days. This shelf-life restriction is imposed by health regulatory authorities in order to ensure safety and quality of the blood product owing to the risk of bacterial growth and the loss of platelet viability during storage commonly referred to as the “platelet storage lesion” (PSL).
The symptoms of platelet storage lesion
According to the PubMed scientific citation database, alterations occurring in platelets during storage were first mentioned in 1957 by Mustard et al. describing the influence of blood collecting techniques on platelet numbers during blood collection and storage1,2. Almost 15 years later, Murphy et al. introduced the term “platelet storage lesion” linked to observations such as lactate accumulation, disc to sphere transformation and reduced responsiveness to ADP for platelet aggregation3. These studies also revealed that some but not all of these features seem to be restored after transfusion suggesting a partial reversibility of the negative effects of storage. During the last four decades, several excellent studies have contributed to an improved understanding of PSL as highlighted in numerous review articles4–8. Triggered mainly by blood processing and the duration of storage, the PSL is best defined as the sum of all deleterious changes leading to progressive damage in platelet structure and function that arise from the time blood is drawn from a donor to the time platelets are transfused to a recipient5. These changes are found spanning several platelet physiological compartments including cytoskeletal reorganization, loss of glycoprotein expression on the platelet surface, derangement of metabolic activity, changes in the lipid membrane, activation of signaling cascades, apoptosis-like symptoms and protein translation. Most of these aspects are characteristic of platelet activation9,10 except for the deterioration effect contributing to the PSL mediated by the lactate accumulation which seems to be platelet activation independent11. The reduction of glycoproteins, particular GPIb - the subunit of the GPIb-IX-V complex responsible for the vWF interaction - on the platelet surface during storage is most likely due to proteolysis12. This process can be decelerated by treatment with inhibitors against matrix metalloproteinases13. Most of these alterations during storage can be monitored using a variety of in vitro measures14 as well as determination of in vivo recovery and survival in normal volunteers thereby providing a valuable constellation of tools for the estimation of platelet viability15. However, the changes occurring during a 5-day storage period do not result in a decreased clinical efficacy as measured by the corrected count increment (CCI)16.
Similar to studies of agonist-stimulated fresh platelets, moderate platelet activation during storage is revealed by assessing the surface expression of P-selectin using CD62P binding which confirms the conclusion described by Bode9. Based on this evidence of platelet activation many inhibitor studies were aiming to reduce the PSL development by adding compounds such as prostaglandin E1, theophylline, thrombin inhibitors or L-carnitine that resulted in an improved platelet function and integrity compared to the untreated sample17,18. Furthermore, protein-free physiologic salt solutions fortified with citrate, bicarbonate and glucose19 as well as supplementation of platelet concentrates with either second messengers or pharmacological inhibitors of different platelet function including amiloride, adenosine, sodium nitroprusside, prostaglandin E1, dipyridamole, ticlopidine, and quinacrine20 as well as magnesium and potassium21, triggered the development of a variety of platelet additive solutions (PAS)22 designed to slow down several aspects of PSL progression23. Lastly, mouse model studies identified a potential mechanism leading to the clearance of platelet concentrates after transfusion of platelets stored at 4 °C24 and suggested that enzymatic glycosylation of chilled platelets could prolong circulation of cold-stored platelets25. However, these results obtained from the mouse model did not agree with human platelets since modification by galactosylation did not prevent the accelerated platelet clearance and thus revealed the existence of two different mechanisms for short-and long-term cold-stored platelets26.
Proteomics to assess protein changes during storage
In order to slow the progression of PSL in a targeted manner, signaling events triggered by PSL needed to be explored. This was achieved by the application of proteomics27 to analyze changes in the platelet proteome during storage28,29. Complementary proteomic approaches employing both peptide-centric (isotope Tagging for Relative and Absolute Quantitation and Isotope Coded Affinity Tagging) and protein-centric (qualitative and quantitative two-dimensional [2D] gel electrophoresis) methods were applied in order to attain both optimal proteome coverage as well as to capture alterations in posttranslational modifications, respectively, which revealed the discovery of several hundred protein changes. Many protein alterations occurring during storage are similar to a proteomic study that monitored protein changes during platelet activation by agonists30 confirming the earlier observations that platelets are activated during storage9. A subset of the proteins that changed significantly in protein concentration based on confidence and consistency in the proteomic results were subjected to further biochemical analysis. This study revealed one potential mechanism for the development of PSL involving activation of the GTPase Rap1 that contributed to GPIIb/IIIa activation31. Therefore, the proteomic studies began to unravel targets for the interference of PSL-related signaling events. Inhibitor studies targeting PI3-kinase which among other kinases mediates Rap1 activation, showed diminished Rap1 and GPIIbIIIa activation as well as a deceleration of in vitro storage-induced platelet deterioration. This reduction in PSL development was demonstrated by reduced glycolytic activity as well as improved responsiveness to the agonist ADP in an extent of shape change assay31. Furthermore, although observed in a mouse model rather than in human platelets, a recent study revealed that inhibiting p38 MAPK improved post-translational survival and haemostatic function of stored platelets providing an additional opportunity for intervention in PSL progression since p38 MAPK signaling is not a central component in platelet integrin activation32.
Conclusion and future perspective
These recent results suggest that protein kinases might represent one important group of proteins involved in the development of PSL and provide a potential target for inhibition in order to reduce development of PSL. Further studies are necessary to fine-tune the inhibition effect and unravel potential “side effects”. Furthermore, demonstration of an effect is only a first step. It is unlikely that most of the known inhibitors would be acceptable additions to platelet concentrates from a patient safety perspective. With the recent implementation of pathogen reduction technologies (PRT) a new dimension of challenges has appeared on the horizon33. The treatment of platelet concentrates with either UV-A and a photo sensitizer or UV-C revealed acceleration of PSL development34–37. This is corroborated by a proteomic analysis discovering significant increases in concentration for several proteins triggered by irradiation37. These results point directly to a potential synergistic research effort among PSL, PRT and PAS development (Figure 1). Future efforts must address a better understanding of PRT impacts on the PSL and the subsequent identification of ways to modify PAS towards the main goal as formulated by Murphy et al. in 1971: “if a ‘storage lesion’ can be defined, its correction might allow further prolongation of effective storage”3.
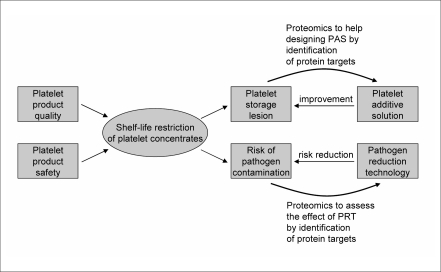
Figure 1.
Platelet product quality and product safety determine the restriction of the platelet shelf-life. Platelet storage lesion and risk of pathogen contamination are the main targets for investigation towards prolongation of the shelf-life. Proteomics provides an excellent tool to address these issues in order to identify protein targets for intervention. Subsequent development of PAS and improvement of PRT will lead to the deceleration of PSL progression as well as a reduction in PRT-mediated effects in platelet concentrates. This synergy will hopefully achieve reduced PSL development while maintaining pathogen risk reduction towards a potential increase in the shelf-life of platelet concentrates.
References
- 1.Mustard JF, Walker CB. The influence of blood collecting techniques on platelet numbers during blood storage. Br J Haematol. 1957;3:50–4. doi: 10.1111/j.1365-2141.1957.tb05770.x. [DOI] [PubMed] [Google Scholar]
- 2.Mustard JF. A study of changes in platelets, antihaemophilic globulin, factor V and factor VII during blood collection and storage by different techniques. Br J Haematol. 1957;3:202–14. doi: 10.1111/j.1365-2141.1957.tb05787.x. [DOI] [PubMed] [Google Scholar]
- 3.Murphy S, Gardner FH. Platelet storage at 22 degrees C; metabolic, morphologic, and functional studies. J Clin Invest. 1971;50:370–7. doi: 10.1172/JCI106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shrivastava M. The platelet storage lesion. Transfus Apher Sci. 2009;50:105–13. doi: 10.1016/j.transci.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Thon JN, Schubert P, Devine DV. Platelet storage lesion: a new understanding from a proteomic perspective. Transfus Med Rev. 2008;22:268–79. doi: 10.1016/j.tmrv.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Cauwenberghs S, van Pampus E, Curvers J, et al. Hemostatic and signaling functions of transfused platelets. Transfus Med Rev. 2007;21:287–94. doi: 10.1016/j.tmrv.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman RM. Platelets: testing, dosing and the storage lesion-recent advances. Hematology Am Soc Hematol Educ Program. 2006:492–6. doi: 10.1182/asheducation-2006.1.492. [DOI] [PubMed] [Google Scholar]
- 8.Seghatchian J. Platelet storage lesion: an update on the impact of various leukoreduction processes on the biological response modifiers. Transfus Apher Sci. 2006;34:125–30. doi: 10.1016/j.transci.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Bode AP. Platelet activation may explain the storage lesion in platelet concentrates. Blood Cells. 1990;16:109–25. [PubMed] [Google Scholar]
- 10.Rinder HM, Snyder EL. Activation of platelet concentrate during preparation and storage. Blood Cells. 1992;18:445–56. [PubMed] [Google Scholar]
- 11.Bertolini F, Porretti L, Lauri E, et al. Role of lactate in platelet storage lesion. Vox Sang. 1993;65:194–8. doi: 10.1111/j.1423-0410.1993.tb02147.x. [DOI] [PubMed] [Google Scholar]
- 12.Ribeiro A, Swann JC, Berndt MC. Alterations of the level of glycoproteins Ib-IX and IIb-IIIain platelets stored at 22 degrees C. Thromb Res. 1992;66:619–27. doi: 10.1016/0049-3848(92)90038-c. [DOI] [PubMed] [Google Scholar]
- 13.Bergmeier W, Burger PC, Piffath CL, et al. Metalloproteinase inhibitors improve recovery and hemostatic function of in vitro-aged or –injured mouse platelets. Blood. 2003;102:4229–35. doi: 10.1182/blood-2003-04-1305. [DOI] [PubMed] [Google Scholar]
- 14.Maurer-Spurej E, Chipperfield K. Past and future approaches to assess the quality of platelets for transfusion. Transfus Med Rev. 2007;21:295–306. doi: 10.1016/j.tmrv.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Holme S, Murphy S. Platelet storage at 22 degrees C for transfusion: interrelationship of platelet density and size, medium pH, and viability after in vivo infusion. J Lab Clin Med. 1983;101:161–74. [PubMed] [Google Scholar]
- 16.Leach MF, AuBuchon JP. Effect of storage time on clinical efficacy of single-donor platelet units. Transfusion. 1993;33:661–4. doi: 10.1046/j.1537-2995.1993.33893342748.x. [DOI] [PubMed] [Google Scholar]
- 17.Bode AP, Miller DT. Preservation of in vitro function of platelets stored in the presence of inhibitors of platelet activation and a specific inhibitor of thrombin. J Lab Clin Med. 1988;111:118–24. [PubMed] [Google Scholar]
- 18.Sweeney JD, Blair AJ, Cheves TA, et al. L-carnitine decreases glycolysis in liquid-stored platelets. Transfusion. 2000;40:1313–9. doi: 10.1046/j.1537-2995.2000.40111313.x. [DOI] [PubMed] [Google Scholar]
- 19.Holme S, Heaton WA, Courtright M. Improved in vivo and in vitro viability of platelet concentrates stored for seven days in a platelet additive solution. Br J Haematol. 1987;66:233–8. doi: 10.1111/j.1365-2141.1987.tb01304.x. [DOI] [PubMed] [Google Scholar]
- 20.Rivera J, Lozano ML, Corral J, et al. Quality assessment of platelet concentrates supplemented with second-messenger effectors. Transfusion. 1999;39:135–43. doi: 10.1046/j.1537-2995.1999.39299154726.x. [DOI] [PubMed] [Google Scholar]
- 21.Diedrich B, Sandgren P, Jansson B. In vitro and in vivo effects of potassium and magnesium on storage up to 7 days of apheresis platelet concentrates in platelet additive solution. Vox Sang. 2008;94:96–102. doi: 10.1111/j.1423-0410.2007.01002.x. [DOI] [PubMed] [Google Scholar]
- 22.Gulliksson H. Platelet additive solutions: current status. Immunohematology. 2007;23:14–9. [PubMed] [Google Scholar]
- 23.Zhang JG, Carter CJ, Devine DV, et al. Comparison of a novel viscous platelet additive solution and plasma: preparation and in vitro storage parameters of buffy-coat-derived platelet concentrates. Vox Sang. 2008;94:299–305. doi: 10.1111/j.1423-0410.2007.01029.x. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmeister KM, Felbinger TW, Falet H, et al. The clearance mechanism of chilled blood platelets. Cell. 2003;112:87–97. doi: 10.1016/s0092-8674(02)01253-9. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmeister KM, Josefsson EC, Isaac NA, et al. Glycosylation restores survival of chilled blood platelets. Science. 2003;301:1531–4. doi: 10.1126/science.1085322. [DOI] [PubMed] [Google Scholar]
- 26.Wandall HH, Hoffmeister KM, Sørensen AL, et al. Galactosylation does not prevent the rapid clearance of long-term, 4 degrees C-stored platelets. Blood. 2008;111:3249–56. doi: 10.1182/blood-2007-06-097295. [DOI] [PubMed] [Google Scholar]
- 27.Schubert P, Devine DV. Proteomics meets blood banking: Identification of protein targets for the improvement of platelet quality. J Proteomics. 2010;73:436–444. doi: 10.1016/j.jprot.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Thiele T, Steil L, Gebhard S, et al. Profiling of alterations in platelet proteins during storage of platelet concentrates. Transfusion. 2007;47:1221–33. doi: 10.1111/j.1537-2995.2007.01255.x. [DOI] [PubMed] [Google Scholar]
- 29.Thon JN, Schubert P, Duguay M, et al. Comprehensive proteomic analysis of protein changes during platelet storage requires complementary proteomic approaches. Transfusion. 2008;48:425–35. doi: 10.1111/j.1537-2995.2007.01546.x. [DOI] [PubMed] [Google Scholar]
- 30.García A, Prabhakar S, Hughan S, et al. Differential proteome analysis of TRAP-activated platelets: involvement of DOK-2 and phosphorylation of RGS proteins. Blood. 2004;103:2088–95. doi: 10.1182/blood-2003-07-2392. [DOI] [PubMed] [Google Scholar]
- 31.Schubert P, Thon JN, Walsh GM, et al. A signaling pathway contributing to platelet storage lesion development: targeting PI3-kinase-dependent Rap1 activation slows storage-induced platelet deterioration. Transfusion. 2009;49:1944–55. doi: 10.1111/j.1537-2995.2009.02224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Canault M, Duerschmied D, Brill A, et al. p38 mitogen-activated protein kinase activation during platelet storage: consequences for platelet recovery and hemostatic function in vivo Blood 2010. in press; DOI 10.1182/blood-2009-03-211706 [DOI] [PMC free article] [PubMed]
- 33.Solheim BG, Seghatchian J. The six questions of pathogen reduction technology: an overview of current opinions. Transfus Apher Sci. 2008;39:51–7. doi: 10.1016/j.transci.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Picker SM, Steisel A, Gathof BS. Cell integrity and mitochondrial function after Mirasol-PRT treatment for pathogen reduction of apheresis-derived platelets: Results of a three-arm in vitro study. Transfus Apher Sci. 2009;40:79–85. doi: 10.1016/j.transci.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 35.Lin L, Conlan MG, Tessman J, et al. Amotosalen interactions with platelet and plasma components: absence of neoantigen formation after photochemical treatment. Transfusion. 2005;45:1610–20. doi: 10.1111/j.1537-2995.2005.00554.x. [DOI] [PubMed] [Google Scholar]
- 36.Osselaer JC, Doyen C, Defoin L, et al. Universal adoption of pathogen inactivation of platelet components: impact on platelet and red blood cell component use. Transfusion. 2009;49:1412–22. doi: 10.1111/j.1537-2995.2009.02151.x. [DOI] [PubMed] [Google Scholar]
- 37.Mohr H, Steil L, Gravemann U, et al. A novel approach to pathogen reduction in platelet concentrates using short-wave ultraviolet light. Transfusion. 2009;49:2612–24. doi: 10.1111/j.1537-2995.2009.02334.x. [DOI] [PubMed] [Google Scholar]