Abstract
Objectives
To determine the effects of a feeding assistance intervention on food and fluid intake and body weight.
Design
Crossover controlled trial.
Setting
Four skilled nursing homes.
Participants
Seventy-six long-stay residents at risk for unintentional weight loss.
Intervention
Research staff provided feeding assistance twice per day during or between meals, five days per week for 24 weeks.
Measurements
Research staff independently weighed residents at baseline and monthly during a 24-week intervention and 24-week control period. Residents’ food and fluid intake and the amount of staff time spent providing assistance to eat was assessed for two days at baseline, three and six months during each 24-week period.
Results
The intervention group showed a significant increase in estimated total daily caloric intake and maintained or gained weight, while the control group showed no change in estimated total daily caloric intake and lost weight over 24 weeks. The average amount of staff time required to provide the interventions was 42 minutes per person/meal and 13 minutes per person/between meal snack compared to usual care during which residents received, on average, 5 minutes of assistance per person/meal and less than one minute per person/snack.
Conclusion
Two feeding assistance interventions are efficacious in promoting food and fluid intake and weight gain among residents at risk for weight loss. Both interventions require more staff time than usual NH care. The delivery of snacks between meals requires less time than mealtime assistance and, thus, may be more practical to implement in daily NH care practice.
Keywords: nursing homes, weight loss, feeding assistance interventions
INTRODUCTION
Unintentional weight loss is a common problem among nursing home (NH) residents and one that is associated with adverse, costly clinical outcomes including increases in hospitalizations, morbidity and mortality.1–4 A clinically significant weight loss episode is defined for NH residents by the Minimum Data Set (MDS) as a loss equal to or greater than 5% within a 30-day period or 10% within a 180-day period.5 A recent study including 900 NH residents showed that 48% experienced at least one weight loss episode equal to or greater than 5% of their body weight within 30 days, and 18% experienced this magnitude of loss more than once based on seven consecutive monthly weight values. This study also showed that a weight loss episode equal to or greater than 5% within 30 days was associated with an increased risk of death.4 A separate longitudinal study with 335 institutionalized elderly showed that patients who lost weight had a lower survival rate than those who gained weight or remained stable.3 Another cross-sectional study with 6,832 NH residents showed that poor oral intake and eating dependency increased the likelihood of both a weight loss episode meeting MDS criteria and a body mass index (BMI) indicative of under-nutrition.2 Similar to weight loss, a low BMI value (e.g., < 20) also has been shown to be associated with morbidity and mortality.6,7
Despite the prevalence of unintentional weight loss in the NH setting, there have been few controlled trials to evaluate interventions to affect weight status in this population. Most studies have focused on the effect of oral liquid nutritional supplements on weight loss outcomes, with mixed results.8–11 In practice, NH staff often do not provide supplements consistent with physician or dietitian orders nor do they provide adequate assistance or encouragement to promote consumption.12,13 Regardless, it remains questionable if weight loss can be prevented in NH residents due to medical instability and comorbidity common in this population.1–4
Two intervention studies showed that improvements in feeding assistance care during regularly scheduled meals (i.e., breakfast, lunch and dinner) or through the delivery of additional foods and fluids between meals (i.e., snacks at 10am, 2pm and 7pm) resulted in significant gains in oral food and fluid intake for 90% of residents with low intake.14,15 These feeding assistance interventions required a significant increase in staff time from an average of less than 10 minutes to 35 minutes per resident per meal and one minute or less to 12 minutes per resident per snack. In these studies, research staff implemented the interventions for only a two-day period to determine the immediate effect of the interventions on residents’ oral food and fluid intake. Thus, these studies did not determine the effect of maintaining the feeding assistance interventions on weight or BMI outcomes.14,15
The purpose of this controlled trial was to evaluate the effect of two feeding assistance interventions (mealtime assistance and between meal snack delivery) on residents’ oral food and fluid intake, BMI and weight status when maintained by research staff for 24 weeks. Two primary questions were addressed:
What effect did the feeding assistance interventions have on residents’ oral food and fluid intake, weight status and BMI?
How much staff time was required to implement the interventions?
METHODS
Setting and Recruitment
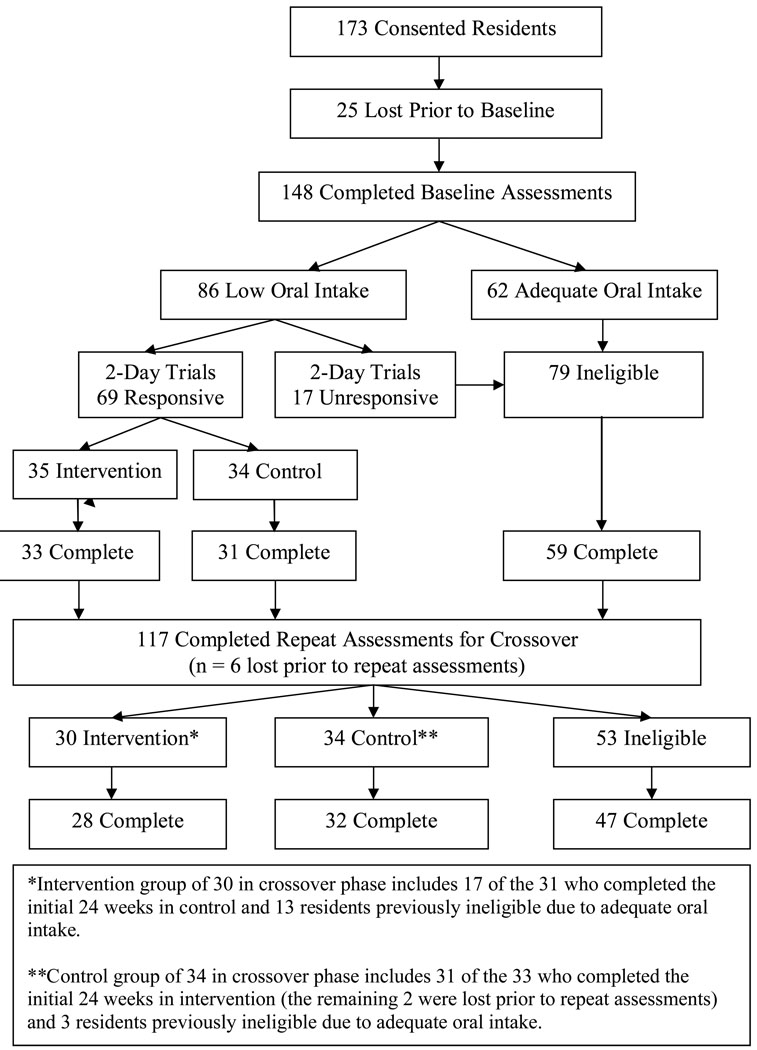
Participants were recruited from four NHs, three of which were proprietary, housing a total of 433 residents. Nurse-aide level staff-to-resident ratios across the four NHs, as reported by the directors-of-nursing, ranged from 6 to 11 residents per nurse aide on the 7am to 3pm shift and 11 to 15 residents per nurse aide on the 3pm to 11pm shift. A total of 310 (72%) residents met study inclusion criteria, which required residents to be long-stay (non-Medicare), free of a feeding tube, not receiving palliative care (hospice) and not on a planned weight loss diet at the time of the study. Written consent was obtained from either the resident or the resident’s responsible party designated in the medical record for 173 (56%) of the 310 eligible residents. Study recruitment procedures were approved by the university-affiliated institutional review board. Following consent, 25 participants were lost due to consent withdrawal (n=6), transfer out of the facility (n=5) or death (n=14). The remaining 148 participants completed baseline assessments, and 69 residents who met study criteria were randomized into intervention or control groups for the initial 24-week period (see Study Design and Figure 1).
Figure 1.
Flow of Subjects in Trial
Study Design
This study used a crossover design wherein participants were randomized at the facility level into intervention or control groups for an initial period of 24 weeks. To randomize at the facility level, the four NHs were identified as either intervention or control (in pairs of two) using a toss of the coin. To be assigned to either group, participants were required to have low oral food and fluid intake (see oral food and fluid intake) and be responsive to one of two feeding assistance interventions (see mealtime feeding assistance and between meal snack interventions) according to baseline assessments, which were completed for all consented subjects in each pair of concurrently participating NHs (one intervention and one control site in each pair). Following the initial 24-week phase, all participants were reassessed for low oral intake and responsiveness to the feeding assistance interventions. Study participants who had received intervention during the initial 24-weeks crossed over into the control group for 24-weeks. Those in the control group during the initial 24-weeks crossed over into intervention for 24-weeks, if deemed eligible based on reassessment. Figure one shows the flow of subjects through the trial.
According to baseline assessments, participants with low oral intake who were responsive to one or both feeding assistance protocols were randomized into intervention (if residing in facility one) or control (if residing in facility two) groups. At the end of the initial 24-week period and following reassessment, participants in the control site (facility two) crossed over to intervention while participants in the intervention site (facility one) crossed over to control. This process was repeated for facilities three and four. This design ensured that all eligible study participants would receive intervention and avoided the possibility of contamination effects if randomization was conducted at the resident level within each participating site.
Measures
Demographic (age, length of stay, sex, ethnicity), medical (diagnoses) and nutritional information (diet and supplement orders) were retrieved from each participant’s medical record. Participants’ cognitive status was assessed by research staff using a standardized, performance-based assessment (Mini-Mental State Examination [MMSE]), with a score range from 0 (severely impaired or comatose) to 30 (cognitively intact).16
Weighing Procedures
Independent assessments of body weight were conducted monthly by research staff for 12 consecutive study months using a standardized protocol. This protocol required research staff to weigh residents in the morning, prior to breakfast but following incontinence care, while the resident remained in bed clothes. Research staff used the facility scale calibrated to zero. Assessments of participants’ body weights were used to calculate body mass index and resting energy expenditure (BMI and REE, Refer to Table 1 footnotes for formulas). A BMI value less than 20 was considered indicative of under-nutrition.17 Monthly weight values also were used to determine changes in weight from baseline to post intervention. The most recent monthly weight value was used as the resident’s final weight value for those lost from the study prior to completion.
Table 1.
Demographic, Medical, and Nutritional Characteristics of Participants Overall
| Measure | Intervention (n=61) Percent (n) or Mean (± SD)* |
Control (n=63) Percent (n) or Mean (± SD)* |
|---|---|---|
| Demographic Characteristics | ||
| Percent Female | 84% (51) | 86% (54) |
| Percent White | 80% (49) | 76% (48) |
| Age | 82.33 (± 11.93) | 83.49 (± 11.46) |
| Length of Stay in Years | 2.95 (± 3.36) | 2.92 (± 3.47) |
| Medical Characteristics | ||
| Percent diagnosis of Depression | 41% (25) | 35% (22) |
| Percent diagnosis of Dementia | 43% (26) | 41% (26) |
| MMSE Total Score (0–30) † | 15.41 (± 8.75) | 15.00 (± 8.59) |
| Nutritional Characteristics | ||
| Percent Supplement Order | 49% (30) | 56% (35) |
| Percent Special Diet Order‡ | 82% (50) | 87% (55) |
| Percent Body Mass Index (BMI) < 20§ | 20% (12) | 14% (9) |
| Estimated REE (daily calories)‖ | 1144 (± 197) | 1147 (± 183) |
SD = Standard Deviation;
MMSE = Mini Mental State Exam
Special diets included any restrictions (no added salt, no concentrated sugars) or altered texture (ground, mechanical soft, puree, thickened liquids).
Body Mass Index (BMI) formula = 0.454 weight in pounds / (0.254 height in inches)2
A BMI below 20 is considered indicative of under-nutrition.
Harris-Benedict Formulas for Resting Energy Expenditure (REE) Estimation
Male = 66.5 + 13.75weight in kilograms + 5.0height in centimeters − 6.78age.
Female = 655.1 + 9.56weight in kilograms + 1.85height in centimeters − 4.68age.
Baseline Assessment: Oral Food and Fluid Intake
Research staff conducted direct observations for two consecutive days during and between regularly-scheduled meals for all 148 study participants (see Figure 1) to identify residents with low oral intake, which was defined according to the Minimum Data Set (MDS) criterion (leaves 25% or more of food uneaten at most meals, or 4 or more of 6 observed meals).5 The reliability and validity of the observational protocol has been described elsewhere.18 Research staff observed each participant from the time of meal tray delivery until meal tray pick up by NH staff for all three scheduled meals (breakfast, lunch and dinner) and between meals from 9am to 11am, 1pm to 3pm and 6pm to 8pm. During each observation period (meal or snack), research staff documented the total amount of time (minutes and seconds) that NH staff spent providing any type of assistance (e.g., verbal encouragement or cueing, physical help, tray set-up) to the resident to promote consumption of the served food and fluid items. Research staff also estimated the resident’s percent consumed of each served food and fluid item (excluding coffee and water) and total percent consumed (all served food and fluid items). Percent estimates were used by research staff because the same method is used by NH staff to identify residents with low oral food and fluid intake.5,18 Nursing home guidelines suggest that residents receive an estimated total of 2000 calories per day during regularly scheduled meals.19 Thus, an estimate of each participant’s caloric intake from meals was calculated based on average total percent consumed across the six observed meals multiplied by an estimated mean of 2000 calories per day.
A total of 86 (58%) of the 148 participants who completed baseline assessments (Figure 1) were identified as having low intake (i.e., ate < 75% of 4 or more of 6 observed meals). Residents received additional foods and fluids between meals on average less than once per day under usual NH care conditions; thus, the amount of additional calories beyond regularly-scheduled meals did not alter the resident’s classification as having “low oral intake” based on meal intake alone. This same 2-day assessment protocol also was conducted at three and six months after the start of each intervention phase to document intervention effects on estimated caloric intake. During each assessment (baseline, post three and six months), a digital camera was used to take photographs before and after the meal for a sample of participants’ meal trays (2 meals per participant at each assessment point) to allow a rater different from the observer who was blind to group assignment (intervention versus control) to estimate total percent eaten for reliability purposes. Both the observational protocol and the photography method have been shown to be reliable methods for estimation of percent intake in NH residents.18 In this study, inter-rater reliability coefficients for total percent eaten (foods + fluids) between observation and photo estimates were .938 at baseline, and .943 and .928 at post three and six months, respectively (All P<.000).
Mealtime Feeding Assistance Evaluation: 2-Day Trial
All 86 participants with low oral intake (Figure 1) received a two-day or six-meal (i.e., breakfast, lunch, and dinner on two consecutive days), trial of mealtime feeding assistance implemented by trained, research staff using the same protocol applied in previous research.14,15 Briefly, the intervention consisted of individual assistance (1 staff member to 1 resident), proper positioning for eating, compliance with dining location preferences and optional meal tray substitutions. In addition, a graduated prompting protocol that enhanced the resident’s self-feeding ability, to the greatest extent possible, also was used during each episode of assistance.14,15 Food and fluid intake was estimated for each of the six intervention meals using the same observation protocol used at baseline.
Between Meal Snack Evaluation: 2-Day Trial
All 86 study participants with low oral intake (Figure 1) also received a two-day or six-snack (i.e., 10am, 2pm, and 7pm on two consecutive days) trial of the between meal intervention. The between meal intervention was similar to mealtime feeding assistance in that it was also implemented by trained, research staff and consisted of essentially the same intervention components.15 A moveable cart was brought to the participant three times per day by research staff. The participant was offered a variety of food and fluid items from which to choose purchased from a local grocery that included the following: assorted juices (e.g., apple, orange, cranberry), yogurts, ice cream, fresh fruit (bananas, grapes), puddings, pastries (doughnuts, muffins), cheese/peanut butter and crackers. Items appropriate for diabetics and other special diets, including added thickeners to fluids, were provided as needed according to diet specifications documented in participants’ medical records. Research staff documented each food and fluid item and the amount consumed by the participant during each snack episode. The total amount of calories consumed from snacks was estimated by different research staff based on the information printed on the package of purchased items. During the same two-day period that snacks were offered by research staff, the mealtime food and fluid consumption of all participants who received snacks was estimated with the same observational protocol used in baseline assessments and the mealtime feeding assistance intervention.
Intervention: 24-Weeks
Responsiveness to the mealtime feeding assistance and between meal snack interventions was defined as an increase in oral intake by 15% or more (i.e., ≥ 300 calories/day) based on a comparison between the 2-day assessments of oral intake under usual NH care conditions and the initial 2-day trials of the interventions. A gain equal to or greater than 15% was identified in previous research as clinically significant and reflects a gain in calories equal to or greater than one standard deviation above variation in usual daily intake.14,15,18 Previous research also has shown that the 2-day trials are a valid method for determining which intervention protocol is most appropriate for an individual resident.14,15 Of the 86 study participants with low oral food and fluid intake, 69 were responsive to either mealtime assistance (n=35), or between meal snacks (n=34) or both (n=30) and 17 were not responsive to either intervention protocol. These rates of responsiveness to the two feeding assistance interventions are consistent with the results of previous studies.14,15 Responsive participants (n=69) were randomized at the facility level into intervention (mealtime assistance or between meal snacks n=35) or control (n=34) groups for the initial 24-study weeks (Figure 1). For participants responsive to both intervention protocols (n=30), each resident was assigned to the intervention protocol that had resulted in the largest increase in estimated total daily calories based on both meal and between meal intake. The same trained, research staff who completed baseline assessments and the initial 2-day trials of the intervention protocols also provided the appropriate intervention (mealtime assistance or between meal snacks) twice per day (breakfast and lunch, 10am and 2pm snacks), five days per week, for 24 weeks.
Crossover: 24-weeks
At the end of the initial 24-weeks, trained research staff repeated all assessments (oral intake, 2-day trials of mealtime assistance and between meal snacks) for all study participants who remained in the study at that time point (intervention, control and those previously ineligible due to adequate oral intake or lack of response to the 2-day intervention trials according to baseline assessments). The purpose of repeating these assessments prior to the crossover phase was to identify initially eligible residents who were no longer eligible for intervention and initially ineligible residents who became eligible. The facilities initially assigned to intervention crossed over to control and the facilities initially assigned to control crossed over into intervention. Across all 148 study participants who completed baseline assessments, 31 were lost from the study during the first 24 weeks, primarily due to death (18 of 31, 58%). The remaining 117 received repeat assessments prior to the crossover phase. Based on the repeat assessments, there were 64 eligible residents and these were placed into intervention (n=30) or control (n=34) groups for an additional 24 weeks (Figure 1 Crossover Phase).
Data Analyses
Baseline demographic, medical and nutritional characteristics were compared between intervention and control group participants using chi-square analysis for categorical measures (percent female, White, diagnosis of dementia or depression, BMI < 20, supplement or special diet order) and t-tests for continuous measures (age, length of NH residency, MMSE total score, estimated REE). Intervention effects were measured by change in initial body weight and BMI value over each 24-week phase. Based on preliminary analyses (see technical appendix), data from the two phases were pooled and an ordinary least squares (OLS) linear regression of the changes on the following variables was conducted: NH site (to test for NH effects wherein NH 4 was used as the reference group due to having the largest sample size), group assignment (intervention versus control), and other potentially confounding variables (e.g., age, gender, depression diagnosis, death/dropout). For those who completed baseline or repeat assessments and were assigned to intervention or control groups but who did not complete all 24 weeks within each phase (death/dropout), the most recent monthly weight value collected by research staff was used to calculate change in weight and BMI value for the analyses. Significance of the intervention effect and other coefficients was adjusted for within-person correlation using the robust option in Stata. To better understand the mechanism of the intervention effect, the difference in total caloric intake (meals plus snacks) was evaluated from baseline to post three and six months during each phase (initial 24 weeks and crossover) by group (intervention versus control) with an analysis of variance for repeated measures.
RESULTS
Subjects and Setting
Table 1 shows the demographic, medical and nutritional characteristics of all residents who received intervention during either the initial 24-week or crossover phases (n=61) compared to all residents in the control group during either phase (n=63). These numbers reflect the pooled data used for the analysis of treatment effects (see Data Analyses) and represent a total of 76 unique participants. There were no significant differences between intervention and control group participants on any of the characteristics shown in Table 1, as is expected with a crossover study design wherein most participants are in each group once. Both groups were predominately female and White. Participants in both groups had an average age between 82 and 83 years and length of NH residency at baseline just under three years. Participants were moderately to severely cognitively-impaired, as indicated by both MMSE total score (15 ± 9 in both groups) and the prevalence of dementia diagnoses at baseline (41%–43%). Thirty-five percent to 41% had a physician-recorded chart diagnosis of depression at baseline (Table 1). Approximately half of the participants in each group had an order for an oral liquid nutritional supplement and the majority had some type of special diet order prior to intervention. Fourteen percent to 20% had a BMI value less than 20 at baseline; and, estimated REE was approximately 1150 calories per person per day for both groups. All characteristics shown in Table 1 also were compared between intervention and control group participants within each 24-week phase (initial and crossover phases, see Figure one for sample sizes). These comparisons showed no differences between intervention and control groups during the initial 24-week phase and only a difference in age during the crossover phase (78.54 ± 13.63 versus 86.84 ± 8.08, respectively; t = 2.91, p = .005).
Intervention Effects
Table 2 shows the effects of intervention and other covariates (age, sex, baseline diagnosis of depression) for final and average BMI change values (Table 2. Models 1 and 2) and final and average weight change values (Table 2. Models 3 and 4) as the dependent variables. The intervention group experienced a 0.72 unit gain in final BMI value (Table 2. Model 1) and a 4-pound gain in final body weight (Table 2. Model 3) relative to the control group. These findings remained significant if average change in BMI (Table 2. Model 2) and weight values (Table 2. Model 4) were used in the analyses. Participants with a baseline diagnosis of depression experienced a 3-pound weight loss relative to those without a diagnosis (Table 2. Column 3 Depression), although this finding was not significant (p = .055). Participants who did not complete all 24 weeks within each phase experienced significantly more weight loss (an average of 5.5 pounds based on their final weight measurement) relative to those who did complete all 24 study weeks within each phase (Table 2. Model 3. Death/Dropout). When the death/dropout indicator was excluded from the analysis, the intervention effects became slightly larger with a 0.75 unit change in final BMI value (R-squared = 0.073, p < .05) and a 4.2 pound gain in body weight (R-squared = 0.079, p < .05). Overall, 56% of participants maintained or gained weight during intervention compared to 28% during control. Of the remainder who lost weight, 16.4% experienced at least one weight loss episode that met MDS criteria (≥ 5% in 30 days or 10% in 180 days) during intervention (mean = 1.2 ± 0.421 episodes per person), while 23.8% experienced a weight loss episode that met MDS criteria during control (mean = 0.8 ± 0.414 per person). There were no significant differences between NH sites on BMI and weight change outcomes (Table 2. NH1-4 where NH 4 is the reference group), except participants in NH site 2 experienced more weight gain relative to those in NH site 4.
Table 2.
Intervention Effects on Body Mass Index and Weight Change using OLS Regression with Robust Adjustment
| Model (1) | Model (2) | Model (3) | Model (4) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| coeff. | t-value | p-value | coeff. | t-value | p-value | coeff. | t-value | p-value | coeff. | t-value | p-value | |
| Intervention | 0.716 | 2.690 | 0.009 | 0.608 | 3.520 | 0.001 | 4.011 | 2.690 | 0.009 | 3.320 | 3.470 | 0.001 |
| Age | −0.004 | −0.430 | 0.668 | −0.004 | −0.670 | 0.508 | −0.033 | −0.630 | 0.534 | −0.029 | −0.870 | 0.388 |
| Female | 0.150 | 0.410 | 0.681 | 0.146 | 0.560 | 0.579 | 0.977 | 0.430 | 0.665 | 0.977 | 0.600 | 0.549 |
| Depression | −0.527 | −1.910 | 0.060 | −0.223 | −1.410 | 0.163 | −2.994 | −1.950 | 0.055 | −1.147 | −1.310 | 0.194 |
| Death/Dropout | −0.926 | −2.200 | 0.031 | −0.447 | −1.620 | 0.110 | −5.506 | −2.260 | 0.027 | −2.771 | −1.720 | 0.090 |
| NH1 | −0.525 | −1.380 | 0.170 | −0.320 | −1.250 | 0.214 | −2.883 | −1.310 | 0.194 | −1.941 | −1.300 | 0.199 |
| NH2 | 0.510 | 1.900 | 0.061 | −0.015 | −0.090 | 0.928 | 3.096 | 2.040 | 0.045 | −0.048 | −0.050 | 0.959 |
| NH3 | −0.057 | −0.150 | 0.885 | −0.246 | −1.020 | 0.313 | −0.223 | −0.110 | 0.915 | −1.400 | −1.050 | 0.295 |
| NH4 | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- |
| Constant | −0.014 | −0.020 | 0.988 | 0.065 | 0.110 | 0.909 | 0.356 | 0.070 | 0.941 | 0.729 | 0.240 | 0.810 |
| R-squared | 0.111 | 0.113 | 0.122 | 0.120 | ||||||||
| N | 124 | 124 | 124 | 124 | ||||||||
- NH = Nursing Home site where NH4 is the reference group due to having the largest sample size.
- Model (1) final BMI change as dependent variable;
- Model (2) average BMI change as dependent variable;
- Model (3) final weight change as dependent variable;
- Model (4) average weight change as dependent variable.
Table 3 shows the effect of the intervention on participants’ estimated total daily caloric intake (meals plus snacks) at baseline, three and six months post intervention within each phase (initial 24 weeks and crossover) for the intervention and control groups. During the initial 24-week phase, the intervention group showed a significant increase in estimated total daily calories from baseline to post intervention at both three (t = −4.202, p = .000) and six months (t = −3.875, p = .000), while the control group showed no change (Table 3. Initial 24-Weeks). During the crossover phase, the intervention group, again, showed a significant increase in their estimated total daily calories from baseline to post intervention at both three (t = −2.131, p = .019) and six months (t = −3.726, p = .000), while the control group showed a significant decline over time (Table 3. Crossover) from baseline to three (t = 2.808, p = .003) and six months (t = 1.970, p = .027). Most of the individuals in the control group during the crossover phase were in the intervention group during the initial 24-weeks (31 of 34 persons); thus, the baseline value for this group at crossover reflects their estimated total daily calories while intervention was still in place for these individuals; while, the values at three and six months reflect their estimated total daily calories while reverting back to usual NH care. An analysis of variance for repeated measures confirmed the significant difference in caloric intake between the two groups during each 24-week phase (initial: F = 9.66, p = .000; crossover: F = 6.53, p = .000). The average amount of NH staff time spent providing assistance to eat during meals to residents in the control group showed no significant change during the initial 24-week or crossover phases (average < 10 minutes per person per meal at each measurement point for all meals).
Table 3.
Estimated Total Caloric Intake by Group and Phase
| Measure | Initial 24-Weeks Intervention N=33 Mean (± SD) |
Initial 24-Weeks Control N=31 Mean (± SD) |
Crossover Intervention N=28 Mean (± SD) |
Crossover Control N=32 Mean (± SD) |
|---|---|---|---|---|
| Baseline | 1237 (302) | 1189 (398) | 1204 (377) | 1504 (356) |
| Post 3 Months | 1615 (420) | 1243 (401) | 1451 (481) | 1281 (259) |
| Post 6 Months | 1539 (322) | 1316 (476) | 1583 (331) | 1323 (339) |
Average total daily calories is estimated based on an average of 2000 calorie/day diet provided by the facility across three scheduled meals plus any additional calories from food and fluid items provided between meals (2 day assessment at each of three time points). Daily caloric intake estimates are reported per person/day and rounded to the nearest whole number.
Of the 76 unique participants who completed intervention, 19 (25%) received mealtime assistance and 57 (75%) received snacks; thus, most of the gain in estimated total daily calories for the intervention group was due to snacks. A greater proportion of participants were assigned to snacks because over half of those responsive to both intervention protocols showed a greater gain in total daily calories as a result of the snack intervention; thus, these residents were assigned to snacks even though they also were responsive to mealtime assistance. Research staff spent an average of 42.19 (± 19.05) minutes per person per meal providing mealtime assistance. Most often (87% of care episodes), assistance was provided one-to-one; and, occasionally (13% of care episodes), research staff were able to group participants together in small groups of two to three for mealtime assistance to make the intervention more time efficient. The snack intervention required an average of 13.89 (± 10.28) minutes of research staff time per person per snack. Research staff were able to group residents together more often for snack delivery (24% of care episodes) and in larger groups (up to four residents per group).
DISCUSSION
The prevalence of unintentional weight loss reported for NHs is considered an indicator of nutritional care quality, although it has been unclear in previous work to what extent unintentional weight loss is preventable in long-stay NH residents.27 The results of this study show that sustained optimal feeding assistance can affect weight loss outcomes among residents at risk for weight loss due to low oral intake. This study is the first controlled intervention trial to evaluate the effects of sustained feeding assistance on NH residents’ oral food and fluid intake, BMI and weight outcomes. Results showed that optimal feeding assistance provided either during or between regularly-scheduled meals, twice per day, five days per week, for 24 weeks had a significant effect on all three outcome measures. Mealtime feeding assistance required an average of 42 minutes/resident/meal, while the delivery of snacks between meals required an average of 14 minutes/resident/snack. These staff time estimates to provide feeding assistance in a manner that promotes both oral food and fluid intake and independence in eating are consistent with the results of previous studies.14,15 Unfortunately, the amount of staff time spent providing feeding assistance under usual NH care conditions averaged less than 10 minutes per person per meal and less than one minute per person per snack both in this and previous studies.13–15 It is notable that over half of the participants in this study were responsive to both interventions (mealtime assistance and between meal snack delivery), and between meal snack delivery resulted in the largest caloric gain for many of these participants even though they were also responsive to mealtime assistance. Only occasionally (13% of care episodes) were research staff able to group residents together to make daily care provision more time-efficient. Previous studies have shown that group feeding assistance is equally effective for those who are responsive to one-to-one care.14,15 Given that most U.S. NHs do not have adequate nurse aide staffing to provide feeding assistance for all residents in need, it may be more feasible in daily care practice to group residents at risk for unintentional weight loss for snack delivery at least twice daily.14,15,20,21
The results of this study also showed that residents with a diagnosis of depression lost more weight than those without a diagnosis. Previous studies have shown that depression is a major cause of unintentional weight loss and depressive symptoms often are undetected and, thus, untreated among NH residents.22–24 Thus, future intervention studies should, therefore, combine feeding assistance interventions with optimal treatments for depression.
Participants who did not complete the study also experienced more weight loss than those who did, and the primary reason for not completing the study was death. Numerous studies have shown that unintentional weight loss and/or a low BMI value is predictive of mortality.2–4,6,7 Thus, it is not surprising that the findings from this study showed that most of those lost from the study due to death experienced weight loss.
This study has a few important limitations. First, it was conducted in only four NHs located in one geographic region, and participants were predominately female and White. Thus, these findings may not be generalizable to NHs in other geographic regions or to minority and/or male NH residents. In addition, the number of residents included in the trial was small relative to the total resident population in each facility, although the proportions of NH residents with low oral intake who were responsive to the feeding assistance interventions were comparable to that shown in previous studies.14,15 Finally, the interventions were provided by research staff. It is unknown if indigenous NH staff could maintain these interventions with sufficient consistency in daily care practice to have the same effects on residents’ oral intake, weight and BMI status. Standardized protocols have been developed based on previous research and made available on the web (http://borun.medsch.ucla.edu “Weight Loss Prevention” module, and http://www.cms.internetstreaming.com “How to Enhance the Quality of Dining Assistance in Nursing Homes” web cast) to assist supervisory NH staff in identifying residents appropriate for feeding assistance care during and/or between meals and ensuring that direct care staff consistently provides this care in practice.25,26
ACKNOWLEDGMENTS
This research was supported by Grant AG10415 from the National Institute on Aging and Grant AG01026-01A1 from the National Institute of Health, UCLA Claude D. Pepper Older Americans Independence Center. There are no financial conflicts of interest for any of the authors. Each author contributed to study concept, data analyes, interpretation of data, and/or manuscript preparation.
Sponsor’s Role: None
Technical Appendix for Data Analyses
The data passed most checks for potential complications, which allowed the use of simple methods. Following is a description of eight issues related to the data analyses and the results of some sensitivity checks.
Use of differences from initial weight as the dependent variable. Body weight and the height-adjusted Body Mass Index (BMI) were stable over time. As a result, adjustment for initial values improves the precision of the estimated intervention effects. The most common adjustment methods are regressing final values on initial values and intervention assignment or studying changes from initial values. Using changes from initial values is equivalent to assuming the regression coefficient on initial values is 1. In fact, the coefficients on initial values were in all cases between .9 and 1, so the two methods led to comparable estimates for the treatment effect. For simplicity, change from initial values was used as the dependent variable.
Nursing home fixed effects: Randomization was at the facility level, so some aspect of the NH (e.g, resident samples and/or staff) might have influenced treatment effects. Thus, fixed effects for the NH site (i.e. indicator variables for all but one NH) were included in the analyses to control for the effect of NH site on intervention effects. Because of the crossover design (each of the four NHs is a control-site in one six-month period and a treatment-site in the other six-month period) NH site is not identical with treatment.
Use of ordinary least squares (OLS) regression. The studentized residuals from simple OLS models were roughly normally distributed. Residuals were checked for heteroscedasticity for NH site, initial weight value, influential observations (e.g., outliers that increase or decrease the estimates to a great extent), and none were found. Because residuals from OLS regression were well-behaved, hierarchical models were not used and the dependent variable was not transformed.
Unit of analysis is the person/six month phase, with the data from participants in both phases counting as two observations. The correlations of residuals across phases for participants was small (-.05) and not significant. For this reason, neither repeated measures nor panel data methods were used, although robust tests of significance clustering on participants were used in the regressions.
Inclusion of deaths and dropouts. There were 6 deaths/dropouts among intervention participants and 9 among control participants with usable data. Deaths/dropouts may be controlled for in the analysis by either excluding these cases or including an indicator in the regression that adjusts for deaths/dropouts. Because a low BMI value and/or weight loss is predictive of death and may be affected by intervention, persons lost from the study due to death/dropout were included in the final analyses using their most recent monthly weight value as their final weight. Results without the death/dropout indicator made the intervention effects slightly larger (.2 pounds on average over the entire sample.) Intervention effects were similar in magnitude but slightly less significant when these cases were omitted from the sample.
Choice of covariates in the regression. Although this was a randomized trial and the analyses controlled for initial weight, other differences in the NH participants also might affect weight. Thus, the analyses also controlled for age, gender, and depression.
Average weight or final weight as the outcome. The intervention aimed at maintaining or increasing weight of NH residents at risk for unintentional weight loss due to low oral intake. Weight was measured monthly by research staff, and the impact of low weight on health status presumably depends on how long a resident is in that vulnerable state, so there is some reason to use average weight change as the dependent variable. In addition, fluctuations in weight over time make the one time final weight measure noisier than an average weight measure. On the other hand, weight change is a long slow process, and the differences in final weight values for a successful intervention will be larger and easier to interpret than changes in average weight. Ultimately, separate analyses were conducted for each measure (average and final weight as the dependent variable), as well as a measure of under-nutrition (average and final BMI value).
Interactions with treatment: Interactions of the treatment effect were tested with depression and death/dropout, which were the two covariates with the largest effect on weight loss. The treatment effects were similar in all groups with or without these indicators.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Lead author, Simmons, and last author, Schnelle, involved in all aspects of the study. Keeler and Zhuo conducted data analyses, interpretation of data, and prepared the analyses section, tables and appendices for paper. Sato and Hickey were involved in acquisition of subjects and data.
REFERENCES
- 1.Abassi AA, Rudman D. Undernutrition in the nursing home: Prevalence, consequences, causes, and prevention. Nutr Rev. 1994;52:113–122. doi: 10.1111/j.1753-4887.1994.tb01403.x. [DOI] [PubMed] [Google Scholar]
- 2.Blaum CS, Fries BE, Fiatarone MA. Factors associated with low body mass index and weight loss in nursing home residents. J Gerontol A:Bio Sci Med Sci. 1995;50A:M162–M168. doi: 10.1093/gerona/50a.3.m162. [DOI] [PubMed] [Google Scholar]
- 3.Dwyer JT, Coleman KA, Krall E, et al. Changes in relative weight among institutionalized elderly adults. J Gerontol A:Bio Sci Med Sci. 1987;42:246–251. doi: 10.1093/geronj/42.3.246. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan DH, Johnson LE, Bopp MM, et al. Prognostic significance of monthly weight fluctuations among older nursing home residents. J Gerontol A:Bio Sci Med Sci. 2004;59A:633–639. doi: 10.1093/gerona/59.6.m633. [DOI] [PubMed] [Google Scholar]
- 5.Health Care Financing Administration. Long Term Care Facility Resident Assessment Instrument (RAI) User’s Manual, Minimum Data Set, Version 2. Natick: MA: Eliot Press; 1999. [Google Scholar]
- 6.Sullivan DH, Bopp MM, Roberson PK. Protein-energy undernutrition and life-threatening complications among the hospitalized elderly. J Gen Int Med. 2002;17:923–932. doi: 10.1046/j.1525-1497.2002.10930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L, Bopp MM, Roberson PK, et al. Undernutrition and risk for mortality in elderly patients within 1 year of hospital discharge. J Gerontol A:Bio Sci Med Sci. 2002;57A:M741–M746. doi: 10.1093/gerona/57.11.m741. [DOI] [PubMed] [Google Scholar]
- 8.Fiaterone MA, O’Neill EF, Doyle N, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. New Eng J Med. 1994;330:1769–1175. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 9.Lauque S, Arnaud-Battandier F, Mansourian R, et al. Protein-energy oral supplementation in malnourished nursing home residents. Age and Ageing. 2000;29:51–56. doi: 10.1093/ageing/29.1.51. [DOI] [PubMed] [Google Scholar]
- 10.Young KWH, Greenwood CE, van Reekum R, et al. Providing nutrition supplements to institutionalized seniors with probable Alzheimer’s Disease is least beneficial to those with low body weight status. J Am Geriatr Soc. 2004;52:1305–1312. doi: 10.1111/j.1532-5415.2004.52360.x. [DOI] [PubMed] [Google Scholar]
- 11.Johnson L, Dooley PA, Gleick JB. Oral nutritional supplement use in elderly nursing home patients. J Am Geriatr Soc. 1993;41:947–952. doi: 10.1111/j.1532-5415.1993.tb06760.x. [DOI] [PubMed] [Google Scholar]
- 12.Kayser-Jones J, Schell ES, Porter C, et al. A prospective study of the use of liquid oral dietary supplements in nursing homes. J Am Geriatr Soc. 1998;46:1378–1386. doi: 10.1111/j.1532-5415.1998.tb06004.x. [DOI] [PubMed] [Google Scholar]
- 13.Simmons SF, Patel AV. Nursing home staff delivery of oral liquid nutritional supplements to residents at risk for unintentional weight loss. J Am Geriatr Soc. 2006;54:1372–1376. doi: 10.1111/j.1532-5415.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- 14.Simmons SF, Osterweil D, Schnelle JF. Improving food intake in nursing home residents with feeding assistance: A staffing analysis. J Gerontol A:Bio Sci Med Sci. 2001;56A:M790–M794. doi: 10.1093/gerona/56.12.m790. [DOI] [PubMed] [Google Scholar]
- 15.Simmons SF, Schnelle JF. Individualized feeding assistance care for nursing home residents: Staffing requirements to implement two interventions. J Gerontol A:Bio Sci Med Sci. 2004;59A:966–973. doi: 10.1093/gerona/59.9.m966. [DOI] [PubMed] [Google Scholar]
- 16.Molloy DW, Alemayehu E, Roberts R. A standardized Mini-Mental State Examination (SMMSE): Its reliability compared to the traditional Mini-Mental State Examination (MMSE) Amer J Psy. 1991;148:102–105. doi: 10.1176/ajp.148.1.102. [DOI] [PubMed] [Google Scholar]
- 17.Thomas DR, Ashmen W, Morley JE, et al. Nutritional management in long term care: Development of a clinical guideline. J Gerontol A:Bio Sci Med Sci. 2000;55A:M725–M734. doi: 10.1093/gerona/55.12.m725. [DOI] [PubMed] [Google Scholar]
- 18.Simmons SF, Reuben D. Nutritional intake monitoring for nursing home residents: A comparison of staff documentation, direct observation, and photography methods. J Am Geriatr Soc. 2000;48:209–213. doi: 10.1111/j.1532-5415.2000.tb03914.x. [DOI] [PubMed] [Google Scholar]
- 19.Long term care standards manual. Chicago: Joint Commission on Accreditation of Hospitals; 1986. pp. 9–14. [Google Scholar]
- 20.Schnelle JF, Cretin S, Saliba D, et al. Health Care Financing Administration. Vol. 2. Cambridge, MA: Abt Associates, Inc; 2000. Summer. Minimum nurse aide staffing required to implement best practice care in nursing homes. Chapter in report to congress: Appropriateness of Minimum Nurse Staffing Ratios in Nursing Homes. Chapter 14; pp. 14.1–14.68. [Google Scholar]
- 21.Schnelle JF, Simmons SF, Harrington C, et al. Relationship of nursing home staffing to quality of care. Hlth Serv Res. 2004;39:225–250. doi: 10.1111/j.1475-6773.2004.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morley JE, Kraenzle D. Causes of weight loss in a community nursing home. J Am Geriatr Soc. 1994;42:583–585. doi: 10.1111/j.1532-5415.1994.tb06853.x. [DOI] [PubMed] [Google Scholar]
- 23.Morley JE, Silver AJ. Nutritional issues in nursing home care. Ann Intern Med. 1995;123:850–859. doi: 10.7326/0003-4819-123-11-199512010-00008. [DOI] [PubMed] [Google Scholar]
- 24.Simmons SF, Cadogan MP, Carbonera G, et al. The Minimum Data Set depression quality indicator: Does it reflect differences in care processes? Gerontol. 2004;44:554–564. doi: 10.1093/geront/44.4.554. [DOI] [PubMed] [Google Scholar]
- 25.Simmons SF, Babinou S, Garcia E, et al. Quality assessment in nursing homes by systematic direct observations: Feeding assistance. J Gerontol A:Bio Sci Med Sci. 2002;57A:M665–M671. doi: 10.1093/gerona/57.10.m665. [DOI] [PubMed] [Google Scholar]
- 26.Simmons SF. Quality improvement for feeding assistance care in nursing homes. Supplement, Medical Directors Role in Nursing Home Care Quality Improvement: An Educational Symposium of the New York Medical Directors Association. J Am Med Dir Assoc. 2007;8:S12–S17. doi: 10.1016/j.jamda.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Simmons SF, Garcia EF, Cadogan MP, et al. The Minimum Data Set weight loss quality indicator: Does it reflect differences in care processes related to weight loss? J Am Geriatr Soc. 2003;51:1410–1418. doi: 10.1046/j.1532-5415.2003.51459.x. [DOI] [PubMed] [Google Scholar]