Abstract
Introduction
Receptors from the superfamily of pentameric ligand-gated ion channels including gamma aminobutyric acid type A (GABAA), glycine, 5HT3, and neuronal nicotinic acetylcholine receptors are modulated by anesthetics in concentrations that are used clinically. Recently, a prokaryotic member of this family (denoted GLIC) was cloned from the cyanobacterium Gloeobacter violaceus, its electrophysiology was characterized, and its three dimensional x-ray diffraction crystal structure was determined. Here we report its modulation by nine common anesthetics. Our goal was to identify agents that modulated GLIC and which therefore might merit further investigation by structural methods.
Methods
We studied the modulatory effect on GLIC of halothane, isoflurane, sevoflurane, desflurane, xenon, nitrous oxide, etomidate, propofol, and ethanol. GLIC was expressed in Xenopus laevis oocytes and studied using two-electrode voltage clamping.
Results
GLIC was potently inhibited by halothane, sevoflurane, isoflurane, desflurane, and propofol, with concentrations producing 50% inhibition (IC50s) of 0.07 mM, 0.15 mM, 0.06 mM, 0.03 mM, and 0.5 μM, respectively. Hill coefficients for these anesthetics were significantly less than 1: 0.27, 0.27, 0.32, 0.25, and 0.42, respectively. Hill coefficients for xenon and etomidate were not different from 1. IC50s for xenon and etomidate were 105 % atmospheres and 73 μM respectively. 200 mM ethanol did not modulate channel currents, nor did 100% nitrous oxide. A two-site model fit the data for desflurane and halothane better than a one site model.
Conclusion
GLIC is modulated by many anesthetics at clinically relevant concentrations. We observed three patterns of modulation. Halogenated volatile anesthetics (desflurane, halothane, isoflurane, sevoflurane) and propofol potently inhibited currents through GLIC, with Hill numbers averaging approximately 0.3 indicating negative cooperativity. A two site model fit the data for desflurane and halothane better than a one site model. Less potent drugs (xenon, etomidate) modulated GLIC at higher concentrations and over a narrower concentration range, with Hill numbers not different than 1. The modulation by xenon occurred at clinically relevant concentrations. The modulation by etomidate occurred above clinical concentrations. Ethanol and nitrous oxide did not modulate currents through GLIC at surgical anesthetic concentrations. These studies, together with the atomic scale structure of GLIC, lay the groundwork for further mechanistic studies of these allosteric effects.
Keywords: Volatile Anesthetics, cys-loop receptors, ligand-gated ion channels, Gloeobacter violaceus, GABAA receptor, nicotinic acetylcholine receptor, glycine receptor
Introduction
Chemical neurotransmission at synapses is mediated by ligand-gated ion channels, which are activated by neurotransmitters released from presynaptic vesicles. Pentameric ligand-gated ion channels from the superfamily containing gamma aminobutyric acid type A (GABAA), glycine, 5HT3, and neuronal nicotinic acetylcholine (nACh) receptors are allosterically modulated by clinical concentrations of anesthetics and are thus attractive candidates as mediators of anesthetic action. Mechanisms that involve specific binding of anesthetics to receptors, nonspecific binding to receptors, and indirect, bilayer-mediated modulation of receptor function have been proposed to account for this modulation (1). In the absence of structural information, it has been difficult to define the various mechanism(s) by which different anesthetics interact with these receptors.
Two prokaryotic members of this receptor superfamily family have recently been cloned, one from the cyanobacterium Gloeobacter violaceus (2) (denoted GLIC) and a homologous channel from the enterobacterium Erwinia chrysanthemi (3) (denoted ELIC). The function of GLIC has been characterized electrophysiologically. It is gated by protons and conducts cations. Functional information is not yet available for ELIC. X-ray diffraction crystal structures of both GLIC and ELIC have been determined. GLIC was crystallized in an apparently open conformation (4), and ELIC in a closed conformation (3). This structural information may improve our understanding of how drugs modulate receptors from the GABAA receptor superfamily.
In the present investigation, we studied the modulation of currents through GLIC by the anesthetics halothane, isoflurane, sevoflurane, desflurane, xenon, etomidate, propofol, ethanol, and nitrous oxide. We did this in order to identify anesthetics that modulate GLIC and which therefore might merit further investigation by structural methods (e.g, x-ray diffraction). We found that like GABAA receptors, GLIC is modulated by a large number of anesthetics. Like nACh receptors, it is very potently modulated by some of these drugs.
Materials and Methods
The Institutional Animal Care and Use Committee at the University of California, San Francisco, approved all studies on animals.
Materials
Female frogs (Xenopus laevis) were purchased from Nasco (Modesto, CA). Collagenase type 1, for defolliculating oocytes, was purchased from Worthington Biochemical Corporation (Lakewood, NJ). Isoflurane, sevoflurane, and desflurane were obtained from Baxter Healthcare Corporation (Deerfield, IL). Halothane was from Halocarbon labs (River Edge, NJ). Etomidate (>95%) was purchased from Chem Pacific (Baltimore, MD). Xenon was purchased from Synquest labs (Alachua, FL). USP grade nitrous oxide was from Airgas (San Francisco, CA). Propofol (97%) and other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Solutions containing xenon and nitrous oxide were made by equilibrating 50 mL of the anesthetic gas with 50 mL of buffer in a gas tight syringe that was vigorously shaken. This procedure was repeated a total of six times, with a final equilibration of buffer with xenon or nitrous oxide at 37°C for two hours. All other solutions were made up gravimetrically.
Oocyte Expression
Stage V and VI Xenopus laevis oocytes isolated from a surgically removed portion of ovary were defolliculated by gentle rotation in 500 U/mL collagenase type 1 (Worthingtom Biochemical Corporation, Lakewood, NJ) for 1 hour at room temperature. GLIC was expressed by microinjecting into each oocyte nucleus 1.5 ng total cDNA subcloned into a PMT3 vector. The injected oocytes were placed singly in wells of a 96-well tissue culture plate (Fisher Scientific, Pittsburgh, PA) containing modified Barth’s solution (88mM NaCl, 1mM KCl, 2.4mM NaHCO3, 20mM HEPES, 0.82mM MgSO4, 0.33mM Ca(NO3)2, 0.41mM CaCl2, with 5mM sodium pyruvate, 50 μg/mL gentamycin, 50 U/mL penicillin, and 50 mcg/mL streptomycin, filtered and adjusted to pH=7.4) and incubated at 15°C. One to two days after injection, the oocytes were used for electrophysiological recordings.
Two-electrode voltage clamp recording
Two-electrode voltage clamping (GeneClamp 500B; Molecular Devices, Axon Instruments, Foster City, CA) was performed on oocytes at room temperature using frog Ringer’s solution as perfusate (“FR”: 115 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, and 10 mM HEPES, filtered and adjusted to pH 7.4). An automated perfusion system was used to deliver solutions (Automate ValveBank perfusion system, San Francisco, CA). Recordings were obtained in a 250 μL recording chamber at flow rates of 2–3 mL/minute. Signals were filtered using a 4-pole low-pass Bessel filter set at a 50–100 Hz cutoff prior to sampling at 100–1000 Hz.
Oocytes were voltage clamped at −60mV using two glass electrodes filled with 3 M KCl (0.5–3 MΩ resistance). Ethanol, isoflurane, sevoflurane, desflurane, halothane, xenon, nitrous oxide, propofol, and etomidate were tested in our studies. Currents were elicited by application of superfusate at pH 5.5 (a value equivalent to 20 – 30% of the maximal current produced by pH 3.0 FR solution). For each oocyte, stable inward currents were verified by application of protons until a plateau in current was achieved, which typically required 20 seconds or more (see Fig 1 and reference (2)), followed by a 5 to 6 minute washout, which was repeated three times. Each anesthetic was then applied to oocytes for 100 seconds, followed by coapplication of protons with the anesthetic. Return to the baseline response to protons was confirmed after washout of the test compound for 4 minutes.
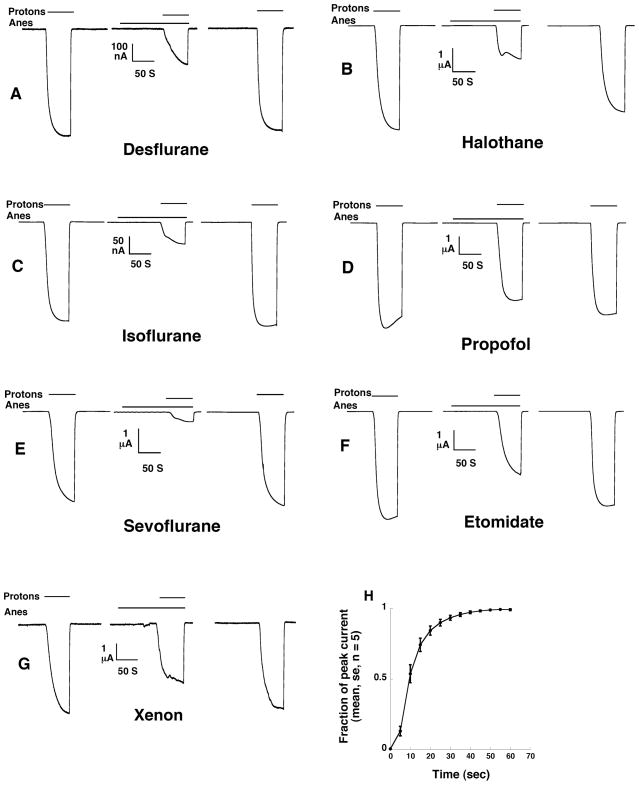
Fig 1.
Current tracings are shown for 0.5 mM desflurane (panel A), 0.25 mM halothane (panel B), 0.3 mM isoflurane (panel C), 1 μM propofol (panel D), 0.3 mM sevoflurane (panel E), 50 μM etomidate (panel F), and 80 % atm xenon (panel G), all of which inhibit currents through GLIC. The first current tracing in each group shows the activation of the channel by protons at pH 5.5. The second current tracing is obtained with a 100 second washin of anesthetic followed by coapplication of anesthetic and protons. The final tracing shows currents activated by protons after washout of anesthetic. “Anes” denotes the time span during which anesthetic was applied. “Protons” denotes the time when pH 5.5 superfusate was applied. Panel H shown the fraction of the plateau current achieved as a function of time (average of 5 oocytes). Mean currents and standard errors are displayed in panel H.
Data Analysis
Anesthetic effect was calculated as the percent change in current in oocytes clamped at −60mV during anesthetic and proton coadministration vs. currents when protons alone were applied. All data are presented as mean ± SE, with 4 to 9 oocytes tested at each anesthetic concentration. For each anesthetic, the change in channel currents upon application of anesthetic was fit to two models. The first was a Hill equation (i.e., a one-site model):
where x is the concentration of anesthetic, n is the Hill coefficient, and IC50 is the concentration of anesthetic that produces a 50% change in current. The second was a two-site model:
where IC501 and IC502 are the apparent affinities of the anesthetics for the two sites, I1 and I2 are the % inhibition attributable to each site, and B and A are the maximum and minimum asymptotes of the fitted curve.
Data were analyzed using SPSS v16.0 (Chicago, Il) and GraphPad Prism 5 (La Jolla, CA).
Results
Current tracings, and the time to maximal currents in response to application of FR at pH 5.5, are shown in Figure 1.
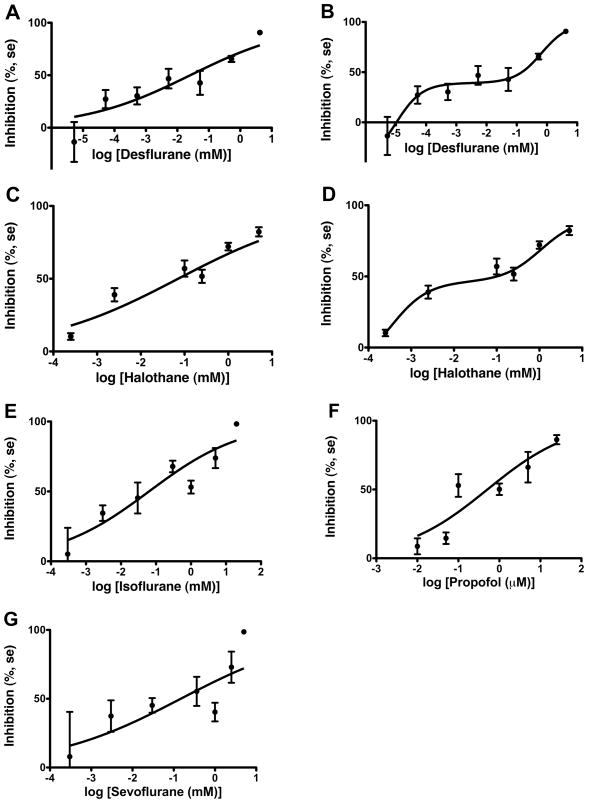
GLIC was potently inhibited by the halogenated inhaled anesthetics (2), with inhibition produced by 1% of MAC for halothane, sevoflurane, and isoflurane, and by 0.01% of MAC for desflurane. Propofol inhibited currents at concentrations as low as 2% of its anesthetic EC50. See Figure 2 and Table 1.
Fig 2.
Halogenated inhaled anesthetics and propofol concentration dependently inhibit current through GLIC. Hill curves are fit to the data for each anesthetic. A total of 40, 39, 41, 31, and 38 oocytes were studied for desflurane, halothane, isoflurane, propofol, and sevoflurane, respectively. Mean change in currents (in percent), and standard errors, are displayed at each concentration. For desflurane and halothane a 2-site model provided a better fit to the data. These are shown in panel B (for desflurane) and panel D (for halothane).
Table 1.
IC50 and Hill coefficients for modulators of GLIC, and anesthetizing concentrations of these drugs in animals
| Agent |
IC50 |
95% CI |
Hill Coefficient |
Anesthetic EC50 |
|---|---|---|---|---|
| Desflurane | 0.03 mM | 0.007 – 0.1 mM | 0.25 ± 0.05 | 0.56 mM (39) |
| Halothane | 0.07 mM | 0.03 – 0.15 mM | 0.27 ± 0.04 | 0.23 mM (39) |
| Isoflurane | 0.06 mM | 0.02 – 0.2 mM | 0.32 ± 0.07 | 0.28 mM (39) |
| Sevoflurane | 0.15 mM | 0.02 – 1.1 mM | 0.27 ± 0.1 | 0.33 mM (39) |
| Propofol | 0.51 μM | 0.21 – 1.3 μM | 0.42 ± 0.08 | 1.9 μM (21) |
| Etomidate | 73 μM | 48 – 110 μM | 1.1 ± 0.38 | 8.7 μM (40) |
| Xenon | 1.05 % atm | 80 – 138 % atm | 1.9 ± 0.47 | 0.71 atm (5) |
| Ethanol† | 117 – 190 mM (6) | |||
| Nitrous Oxide† | 1.04 atm (7) |
IC50 and Hill coefficients are expressed as mean ± se. CI = confidence interval
Ethanol and nitrous oxide did not modulate GLIC
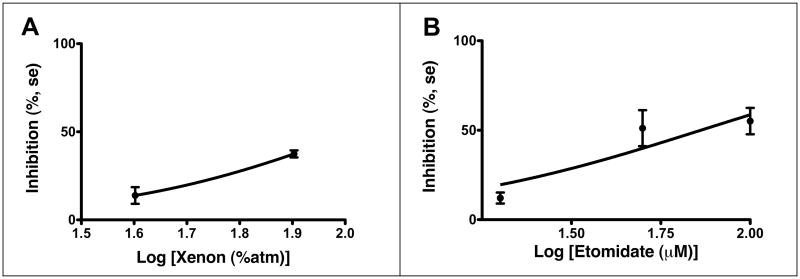
0.8 atm xenon, which is slightly above the MAC of xenon in humans (5), significantly inhibited currents. Etomidate was inhibitory only at concentrations above those used to achieve anesthesia. See Figure 3 and Table 1.
Fig 3.
Xenon and etomidate inhibit GLIC. 9 and 19 oocytes, respectively, were studied. Solid circles denote the mean change in current in percent at each concentration. Error bars display standard errors. Both anesthetics inhibit GLIC.
Ethanol had no effect on GLIC at 200 mM compared to controls (p > 0.05; data not shown). This is the upper end of anesthetizing concentrations in animals (6). 1 atm nitrous oxide also had no effect compared to 1 atm nitrogen (p > 0.05; data not shown). This concentration of nitrous oxide is approximately MAC in humans (7).
Inhibition of currents through GLIC spanned 3–4 orders of magnitude in concentration for the halogenated anesthetics and propofol. For these compounds, Hill numbers averaged approximately 0.3. Etomidate and xenon had higher Hill numbers that were not significantly different than 1. Hill numbers and IC50s for anesthetic modulation of GLIC, and anesthetic EC50 in animals, are listed in Table 1.
The two site model fit the data better than the Hill equation for desflurane (F3, 35 = 3.379, p = 0.029) and halothane (F3, 34 = 3.112, p = 0.039) (8). For desflurane, IC501 = 0.00001 mM (95% confidence interval, 0.0000004 to 0.0002 mM), IC502 = 0.3 mM (95% confidence interval, 0.15 to 3.0 mM), I1 = 0.15, I2 = 0.85. For halothane, IC501 = 0.0003 mM (95% confidence interval, 0.000002 to 0.06 mM), IC502 = 1.1 mM (95% confidence interval, 0.1 to 12 mM), I1 = 0.23, I2 = 0.77.
Discussion
The receptor superfamily containing GABAA, neuronal nicotinic acetylcholine, glycine, and 5HT3 receptors are plausible targets of anesthetic action because of their role in mediating synaptic transmission and their modulation by clinical concentrations of anesthetics. However, detailed structural information for these receptors has been elusive. GLIC is a prokaryotic member of this superfamily of receptors. Its x-ray diffraction structure has recently been determined (4,9). In the present report, we investigated the modulation of GLIC by anesthetics, with the expectation that this ion channel will provide a more relevant model system for examining anesthetic interactions with ion channels than do soluble proteins (10). We found that GLIC is modulated by many anesthetics, including several at much higher affinity than on other receptors. This increases the probability that an anesthetic binding site will be characterized on GLIC.
Anesthetics could be clustered into three groups, based on their pattern of modulation of GLIC.
One group consisted of halogenated anesthetics and propofol. These compounds modulated currents through GLIC at anesthetic and subanesthetic concentrations (Fig 2). Hill numbers were significantly less than 1, ranging from 0.25 for desflurane to 0.42 for propofol (Table 1).
It is interesting to compare this with other members of the superfamily. Currents through nACh receptors, like those of GLIC, are generally inhibited by anesthetics, but over a smaller concentration range, with Hill numbers of 1 or greater. Unlike GLIC, homomeric nACh receptors are not particularly anesthetic-sensitive, although heteromeric nACh receptors are very anesthetic-sensitive (11). For example, the concentration response relation for inhibition by isoflurane of α4 β2 nACh receptors from chickens had an IC50 of 85 μM and Hill number of 1.3. Propofol also inhibited these receptors, but above the clinical concentration range, with an IC50 of 19 μM and Hill number of 2.9 (11). Investigations in rat α4 β2 nicotinic acetylcholine receptors agree with these results (12). A study of α4β2, α3 β4, and α2 β4 isoforms reported IC50s of 82, 56, and 25 μM for isoflurane (13).
Anesthetics modulate 5HT3 receptors at higher concentrations than GLIC. Isoflurane enhances 5HT3 receptor function at MAC by less than 50% (14); more that 100% potentiation is observed as the anesthetic concentration is raised to 10 MAC. In one report, halothane showed potentiation of 5HT3 receptor function similar to isoflurane (14), but another study suggested that 5HT3A receptors were potentiated approximately 200% by 1 MAC of halothane (15). Sevoflurane is qualitatively different than isoflurane and halothane, inhibiting rather than enhancing currents through 5HT3 receptors at MAC (16). 5HT3 receptors are relatively insensitive to propofol, with an IC50 more than 50 times propofol’s anesthetizing concentration (17).
Halogenated anesthetics have positive modulatory actions on GABAA and glycine receptors (18,19). Enhancement of channel currents depends on the agonist concentration and can reach several hundred percent at EC5–10. Both receptors respond to anesthetics at concentrations that produce anesthesia, but the threshold for modulation is generally above that which modulates GLIC. There are many excellent reviews of inhaled anesthetic pharmacology on GABAA and glycine receptors (20). Propofol strongly potentiates GABAA receptor currents at anesthetizing concentrations (21). In contrast, propofol enhances glycine currents only at supraanesthetic concentrations, with EC50 of 16 and 27 μM for glycine α1 and α1 β receptors (22).
The second group of compounds consisted of xenon and etomidate (Fig 3). These compounds inhibited agonist activated currents through GLIC, but their concentration-response curves had Hill numbers of 1. For comparison, xenon also has moderate effects on α4β4 nACh receptors (23), and small modulatory effects on GABAA, glycine, and 5HT3 receptors. Etomidate inhibits currents through GLIC, as well as nACh receptors, at concentrations above those that produce anesthesia (24). Etomidate’s IC50 on 5HT3 receptors is approximately 20 times its anesthetic concentration (17). Etomidate produces only a 29% potentiation of currents through glycine receptors at 300μM etomidate (22). GABAA receptors are strongly and stereoselectively modulated by etomidate at clinically relevant concentrations (25), with Hill coefficients of 1.9 for the R isomer and 2.9 for the S isomer, in contrast to the supraanesthetic effects of etomidate on GLIC, nACh, glycine, and 5HT3 receptors.
A third group of compounds consisted of ethanol and nitrous oxide. At anesthetizing concentrations, these agents did not modulate GLIC. This differs from other receptors in the superfamily. 75mM ethanol potentiates currents through α2β4, α4β4, α2β2, and α4β2 neuronal nicotinic acetylcholine receptors, although α3β2 and α3β4 are not sensitive to ethanol. 25–50 mM ethanol inhibits homomeric α7 receptors, but this response is variable (26). Subunit composition also determines potentiation of glycine and GABAA receptor function by ethanol. Homomeric α1 glycine receptors are potentiated more than homomericα2 receptors (27). Extrasynaptic GABAA receptors containing a δ subunit have been reported to be potentiated by concentrations of ethanol as low as 3 mM (28). This compares to a threshold between 30 and 100 mM for receptors containing γ subunits (29). 5HT3 receptor function is potentiated by ethanol in concentrations from 50 to 200 mM, but the enhancement is less than for other anesthetics, reaching at most a 50% increase in currents (14). In contrast to GLIC, nitrous oxide modulates mammalian members of this superfamily similarly, but the modulation is small except forα4β2 nACh receptors (23). Nitrous oxide at 0.6 atm potentiated the function of glycine receptors by approximately 30% and GABA receptors by 20%. α4β2 nACh receptors were inhibited by 39% butα4β4 nACh receptors were inhibited by only 7%. 5HT3 receptors were inhibited approximately 15% (23).
The Hill coefficient is often used to describe the cooperativity of binding of two or more ligands to proteins. As noted above, concentration response curves for anesthetics generally have Hill coefficients of 1 (no cooperativity) or greater than 1 (positive cooperativity). This is consistent with either binding (30) or nonbinding (31) mechanisms. In this study, Hill numbers were significantly less than 1 for desflurane, halothane, isoflurane, sevoflurane, and propofol, an indication of negative cooperativity. In this regard, GLIC differs from its anesthetic-sensitive mammalian homologs. Negative cooperativity can arise between subunits having identical binding sites if binding of one ligand decreases the binding affinity of a subsequent ligand. This mechanism has been demonstrated for the bacterial homodimeric aspartate receptor, which bind ligands with negative cooperativity, and has identical binding sites for aspartate as revealed by the x-ray structure of the receptor in the absence of ligand. The x-ray structure of the receptor with a bound ligand, however, shows that only half of the binding sites are occupied, with the second site distorted (32). This contrasts with positive cooperativity, where the doubly liganded state would be populated at the expense of the singly liganded state, which in turn would be relatively depleted (33). If anesthetics can be cocrystallized with GLIC, it should be possible to determine whether a half-of-the-sites mechanism of negative cooperativity applies to GLIC.
An alternative explanation for negative cooperativity of halogenated volatile anesthetics and propofol with GLIC is that it is due to the presence of two or more protein binding sites of different affinity. Photolabelling of multiple tyrosines in nACh receptors from Torpedo californica with [14C]halothane supports the idea that more than one anesthetic binding site can be present on a receptor that is homologous to GLIC (34). Yet another explanation is that negative cooperativity is the result of two or more different mechanisms by which anesthetics interact with GLIC, such as a binding (30) and a nonbinding mechanism (35). Either of these explanations would be consistent with a two-site model.
For desflurane and halothane a two-site model fit the data better than a one-site model. Do desflurane and halothane modulate GLIC by a different mechanism than the other halogenated agents and propofol? It is not known how many anesthetic binding sites are on GLIC, whether they are identical or not, or whether the number of binding sites differs among volatile anesthetics. We note that at approximately the anesthetic IC50, there is a plateau in the inhibitory effect and a small (but in this study, a statistically insignificant) decrease in inhibition for all four halogenated anesthetics and propofol (Fig 2). This is suggestive of a biphasic response of GLIC to these agents and if it is real, it would also support a two-site mechanism for the volatile anesthetics and propofol.
The modulation of GLIC by volatile anesthetics, at anesthetizing concentrations, suggests that the anesthetic sensitivity of the GABAA receptor superfamily is prokaryotic in origin. This adds to the growing list of ion channels with anesthetic-sensitive homologs in one-celled organisms. Among these are voltage-gated Na+ channels from bacteria (36) and two-pore domain K+ channels from yeast (37). The sensitivity of their mammalian homologs is likely the result of common descent from ancestral, anesthetic-sensitive channels in one-celled organisms. This supports the conjecture that the capacity to respond to inhaled anesthetics arose in organisms that lack nervous systems (38). In this regard, the negative cooperativity of GLIC for anesthetics may make sense. If anesthetics mimic compounds in Gloeobacter’s environment which are used for growth or metabolism, responding to these compounds over a large concentration range may be more beneficial than responding over a narrow range (32).
In summary, GLIC is modulated by many anesthetics. Halogenated volatile anesthetics (desflurane, halothane, isoflurane, sevoflurane) and propofol potently inhibited currents through GLIC. This modulation occurred over a broad concentration range, from subanesthetic to anesthetic concentrations, with Hill numbers averaging approximately 0.3, which indicates negative cooperativity. For desflurane and halothane, a two-site model fits the data better than a one-site model, suggesting more than one site or mechanism by which those anesthetics interact with GLIC. Less potent drugs (xenon, etomidate) modulated GLIC at higher concentrations and over a narrower concentration range, with Hill numbers not different than 1. The modulation by xenon occurred at clinically relevant concentrations. The modulation by etomidate occurred above clinical concentrations. Ethanol and nitrous oxide did not modulate currents through GLIC at surgical anesthetic concentrations. We anticipate that the atomic scale structure of GLIC will lead to an understanding of the mechanism underlying these allosteric effects.
Acknowledgments
This work was supported in part by NIGMS R01 GM069379
Author’s role in the manuscript: YW and LY helped design the study, conducted the study, reviewed the manuscript. PJC was involved in study design and manuscript preparation. JMS participated in study design, data analysis, and manuscript preparation.
Footnotes
Reprints will not be available
References
- 1.Eger EI, 2nd, Raines DE, Shafer SL, Hemmings HC, Jr, Sonner JM. Is a new paradigm needed to explain how inhaled anesthetics produce immobility? Anesth Analg. 2008;107:832–48. doi: 10.1213/ane.0b013e318182aedb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bocquet N, de Carvalho P, Cartaud J, Neyton J, Le Poupon C, Taly A, Grutter T, Changeux JP, Corringer PJ. A prokaryotic proton-gated channel from the nicotinic acetylcholine receptor family. Nature. 2007;445:116–9. doi: 10.1038/nature05371. [DOI] [PubMed] [Google Scholar]
- 3.Hilf RJ, Dutzler R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. nature. 2007;452:375–9. doi: 10.1038/nature06717. [DOI] [PubMed] [Google Scholar]
- 4.Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux JP, Corringer PJ. X-ray structure of a pentameric ligand-gated channel in an apparently open conformation. Nature. 2009;457:111–4. doi: 10.1038/nature07462. [DOI] [PubMed] [Google Scholar]
- 5.Cullen SC, Eger EI, 2nd, Cullen BF, Gregory P. Observations on the anesthetic effect of the combination of xenon and halothane. Anesthesiology. 1969;31:305–9. doi: 10.1097/00000542-196910000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Fang Z, Ionescu P, Chortkoff BS, Kandel L, Sonner J, Laster MJ, Eger EI., 2nd Anesthetic potencies of n-alkanols: results of additivity and solubility studies suggest a mechanism of action similar to that for conventional inhaled anesthetics. Anesth Analg. 1997;84:1042–8. doi: 10.1097/00000539-199705000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Hornbein TF, Eger EI, 2nd, Winter PM, Smiter G, Wetstone D, Smith KH. The minimum alveolar concentration of nitrous oxide in man. Anesth Analg. 1982;61:553–6. [PubMed] [Google Scholar]
- 8.Motulsky H, Christopoulos A. A practical guide to curve fitting. Oxford: Oxford University Press; 2004. Fitting models to biological data using linear and nonlinear regression. [Google Scholar]
- 9.Hilf RJ, Dutzler R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature. 2009;457:115–8. doi: 10.1038/nature07461. [DOI] [PubMed] [Google Scholar]
- 10.Franks NP, Lieb WR. Do general anaesthetics act by competitive binding to specific receptors? Nature. 1984;310:599–601. doi: 10.1038/310599a0. [DOI] [PubMed] [Google Scholar]
- 11.Flood P, Ramirez-Latorre J, Role L. Alpha 4 beta 2 neuronal nicotinic acetylcholine receptors in the central nervous system are inhibited by isoflurane and propofol, but alpha 7-type nicotinic acetylcholine receptors are unaffected. Anesthesiology. 1997;86:859–65. doi: 10.1097/00000542-199704000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Violet JM, Downie DL, Nakisa RC, Lieb WR, Franks NP. Differential sensitivities of mammalian neuronal and muscle nicotinic acetylcholine receptors to general anesthetics. Anesthesiology. 1997;86:866–74. doi: 10.1097/00000542-199704000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Cardoso RA, Yamakura T, Brozowski SJ, Chavez-Noriega LE, Harris RA. Human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes predict efficacy of halogenated compounds that disobey the Meyer-Overton rule. Anesthesiology. 1999;91:1370–7. doi: 10.1097/00000542-199911000-00029. [DOI] [PubMed] [Google Scholar]
- 14.Machu TK, Harris RA. Alcohols and anesthetics enhance the function of 5-hydroytryptamine3 receptors expressed in Xenpus laevis oocytes. J Pharmacol Exp Ther. 1994;271:898–905. [PubMed] [Google Scholar]
- 15.Solt K, Stevens R, Davies PA, Raines DE. General anesthetic-induced channel gating enhancement of 5-hydroxytryptamine type 3 receptors depends on receptor subunit composition. J Pharmacol Exp Ther. 2005;315:771–6. doi: 10.1124/jpet.105.090621. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki T, Koyama H, Sugimoto M, Uchida I, Mashimo T. The diverse actions of volatile and gaseous anesthetics on human-cloned 5-hydroxytryptamine3 receptors expressed in Xenopus oocytes. Anesthesiology. 2002;96:699–704. doi: 10.1097/00000542-200203000-00028. [DOI] [PubMed] [Google Scholar]
- 17.Rusch D, Braun HA, Wulf H, Schuster A, Raines DE. Inhibition of human 5-HT3A and 5-HT3AB receptors by etomidate, propofol and pentobarbital. Eur J Pharmacol. 2007;573:60–4. doi: 10.1016/j.ejphar.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishikawa K, Harrison NL. The actioins of sevoflurane and desflurane on the gamma-aminobutyric acid receptor type A: effects of TM2 mutations in the alpha and beta subunits. Anesthesiology. 2003;99:678–84. doi: 10.1097/00000542-200309000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Downie DL, Hall AC, Lieb WR, Franks NP. Effects of inhalational general anaesthetics on native glycine receptors in rat medullary neurones and recombinant glycine receptors in Xenopus oocytes. Br J Pharmacol. 1996;118:493–502. doi: 10.1111/j.1476-5381.1996.tb15430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris RA, Mihic SJ, Dildy-Mayfield JE, Machu TK. Actions of anesthetics on ligand-gated ion channels: role of receptor subunit composition. FASEB. 1995;9:1454–62. doi: 10.1096/fasebj.9.14.7589987. [DOI] [PubMed] [Google Scholar]
- 21.Krasowski MD, Jenkins A, Flood P, Kung AY, Hopfinger AJ, Harrison NL. General anesthetic potencies of a series of propofol analogs correlate with potency for potentiation of gamma-aminobutyric acid (GABA) current at the GABAA receptor but not with lipid solubility. J Pharmacol Exp Ther. 2001;297:338–51. [PubMed] [Google Scholar]
- 22.Pistis M, Belelli D, Peters JA, Lambert JJ. The interaction of general anaesthetics with recombinant GABAA and glycine receptors expressed in Xenopus laevis oocytes: a comparative study. Br J Pharmacol. 1997;122:1707–19. doi: 10.1038/sj.bjp.0701563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamakura T, Harris RA. Effects of gaseous anesthetics nitrous oxide and xenon on ligand-gated ion channels. Comparison with isoflurane and ethanol. Anesthesiology. 2000;93:1095–101. doi: 10.1097/00000542-200010000-00034. [DOI] [PubMed] [Google Scholar]
- 24.Nirthanan S, Garcia G, Chiara DC, Husain SS, Cohen JB. Identification of binding sites in the nicotinic acetylcholine receptor for TDBzl-etomidate, a photoreactive positive allosteric effector. J Biol Chem. 2008;283:22051–62. doi: 10.1074/jbc.M801332200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomlin SL, Jenkins A, Lieb WR, Franks NP. Stereoselective effects of etomidate optical isomers on gamma-aminobutyric acid type A receptors and animals. Anesthesiology. 1998;88:708–17. doi: 10.1097/00000542-199803000-00022. [DOI] [PubMed] [Google Scholar]
- 26.Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, Harris RA. Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1999;289:774–80. [PubMed] [Google Scholar]
- 27.Mascia MP, Mihic SJ, Valenzuela CF, Schofield PR, Harris RA. A single amino acid determines differences in ethanol actions on strychnine-sensitive glycine receptors. Mol Pharm. 1996;50:402–6. [PubMed] [Google Scholar]
- 28.Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha4 beta3 delta and alpha6 beta3 gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA. 2003;100:15218–23. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris RA, Mihic SJ, Brozowski SJ, Hadingham K, Whiting PJ. Ethanol, flunitrazepam, and pentobarbital modulation of GABAA receptors expressed in mammalian cells and Xenopus oocytes. Alcohol Clin Exp Res. 1997;21:444–51. doi: 10.1111/j.1530-0277.1997.tb03789.x. [DOI] [PubMed] [Google Scholar]
- 30.Bertaccini EJ, Shapiro J, Brutlag DL, Trudell JR. Homology modeling of a human glycine alpha 1 receptor reveals a plausible anesthetic binding site. J Chem Inf Model. 2005;45:128–35. doi: 10.1021/ci0497399. [DOI] [PubMed] [Google Scholar]
- 31.Cantor RS. Solute modulation of conformational equilibria in intrinsic membrane proteins: apparent “cooperativity” without binding. Biophys J. 1999;77:2643–7. doi: 10.1016/S0006-3495(99)77098-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koshland DE. The structural basis of negative cooperativity: receptors and enzymes. Curr Opin Struct Biol. 1996;6:757–61. doi: 10.1016/s0959-440x(96)80004-2. [DOI] [PubMed] [Google Scholar]
- 33.Dill KA, Bromberg S. Statistical thermodynamics in chemistry and biology. New York: Garland Science; 2003. Molecular driving forces; pp. 539–544. [Google Scholar]
- 34.Chiara DC, Dangott LJ, Eckenhoff RG, Cohen JB. Identification of nicotinic acetylcholine receptor amino acids photolabeled by the volatile anesthetic halothane. Biochemistry. 2003;42:13457–67. doi: 10.1021/bi0351561. [DOI] [PubMed] [Google Scholar]
- 35.Cantor R. The lateral pressure profile in membranes: a physical mechanism of general anesthesia. Biochemistry. 1997;36:2339–44. doi: 10.1021/bi9627323. [DOI] [PubMed] [Google Scholar]
- 36.Ouyang W, Jih TY, Zhang TT, Correa AM, Hemmings HC., Jr Isoflurane Inhibits NaChBac, a Prokaryotic Voltage-Gated Sodium Channel. J Pharmacol Exp Ther. 2007;322:1076–83. doi: 10.1124/jpet.107.122929. [DOI] [PubMed] [Google Scholar]
- 37.Gray AT, Winegar BD, Leonoudakis DJ, Forsayeth JR, Yost CS. TOK1 is a volatile anesthetic stimulated K+ channel. Anesthesiology. 1998;88:1076–84. doi: 10.1097/00000542-199804000-00029. [DOI] [PubMed] [Google Scholar]
- 38.Sonner JM. A hypothesis on the origin and evolution of the response to inhaled anesthetics. Anesth Analg. 2008;107:849–54. doi: 10.1213/ane.0b013e31817ee684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franks NP, Lieb WR. Which molecular targets are most relevant to general anaesthesia? Toxicology Letters. 1998;100–101:1–8. doi: 10.1016/s0378-4274(98)00158-1. [DOI] [PubMed] [Google Scholar]
- 40.Krasowski MD, Harrison NL. General anaesthetic actions on ligand-gated ion channels. Cell Mol Life Sci. 1999;55:1278–303. doi: 10.1007/s000180050371. [DOI] [PMC free article] [PubMed] [Google Scholar]