Abstract
OBJECTIVE
Hyperandrogenemia, insulin resistance, and dyslipidemia demonstrate familial aggregation in the female first-degree relatives of women with polycystic ovary syndrome (PCOS), suggesting that these defects are heritable. Hyperandrogenemia also appears to be the male reproductive phenotype. We performed this study to test the hypothesis that brothers of women with PCOS have metabolic defects similar to those of their proband sisters.
RESEARCH DESIGN AND METHODS
This was a prospective case-control study performed at four academic medical centers in the U.S. Fasting blood was obtained from 196 non-Hispanic white brothers of women with PCOS and 169 control men of age, BMI, and ethnicity comparable to those of brothers. A separate analysis was performed by study site to assess potential regional variations in metabolic parameters.
RESULTS
Overall, brothers of women with PCOS had significantly higher total (P = 0.001) and LDL cholesterol (P = 0.01) as well as triglyceride levels (P = 0.01) compared with control men, although there were regional variations in these differences. There were significant positive correlations between brothers and their sisters with PCOS for total (ρ= 0.2, P = 0.009) and LDL cholesterol (ρ = 0.3, P = 0.001) and triglyceride (ρ = 0.2, P = 0.05) levels. Brothers also had significantly higher fasting insulin levels and homeostatic index of insulin resistance (P = 0.02 for both comparisons) compared with control men.
CONCLUSIONS
Brothers of women with PCOS have dyslipidemia as well as evidence for insulin resistance similar to that of their proband sisters with PCOS. These findings are consistent with the hypothesis that some metabolic abnormalities in PCOS are heritable and are not sex specific.
Polycystic ovary syndrome (PCOS) is a complex genetic disorder that affects ~7% of premenopausal women (1). It is the leading cause of anovulatory infertility and an important risk factor for type 2 diabetes and metabolic syndrome in young adult women as well as in adolescent girls (1). The major reproductive phenotype is hyperandrogenemia and up to 40% of premenopausal sisters are affected (2). Moreover, mothers (3) and brothers (4) also have elevated mean androgen levels compared with control subjects of comparable sex, age, weight, and ethnicity. We have identified one PCOS susceptibility allele within a dinucleotide repeat (D19S884) in intron 55 of the fibrillin-3 gene on chromosome 19p13.2 that is linked and associated with hyperandrogenemia (5).
Abnormalities in insulin action and secretion, glucose tolerance, and lipid levels also demonstrate familial aggregation in first-degree female relatives of women with PCOS (3,6–9). Elevated LDL cholesterol levels are the most consistent lipid abnormality in affected women as well as in their sisters and mothers (3,8–10). In addition, the prevalence of metabolic syndrome is increased in these groups (3,9). Further, increased LDL levels and metabolic syndrome are significantly associated with hyperandrogenemia, suggesting that the cardinal reproductive feature of the syndrome plays a direct role in the etiology of the associated metabolic abnormalities (3,9). Consistent with this hypothesis, the PCOS susceptibility allele is associated with markers of insulin resistance in women with PCOS (5). Features of the metabolic phenotype, however, have been less well characterized in male than in female relatives. These studies have also been constrained by lack of control data (11) and/or relatively small sample sizes (7,12). The most comprehensive studies have come from Chile and Turkey (7,8,13) and have suggested that male first-degree relatives have insulin resistance (7,13) and dyslipidemia (8). However, the relevance of these latter studies to a U.S.-based population is unclear because there can be ethnic and racial differences in insulin action and lipid metabolism (14,15). Moreover, none of these studies investigated predictors of male metabolic abnormalities. Accordingly, we performed this study of anthropometric and metabolic parameters in a large cohort of brothers of women with PCOS from the U.S. compared with simultaneously recruited control men of comparable age, BMI, ethnicity, and location. Further, we examined whether the male metabolic phenotypes were correlated with those in the PCOS probands, suggesting heritability, or were sex specific.
RESEARCH DESIGN AND METHODS
We prospectively enrolled 196 brothers aged 18–55 years of 158 Caucasian women of European origin with PCOS and 169 unrelated control men of comparable age, BMI, and ethnicity between January 1995 and October 2005. The study was conducted at the Pennsylvania State University College of Medicine–Hershey Medical Center (HMC), University of Pennsylvania Medical Center, Brigham and Women's Hospital (BWH), and Northwestern University Feinberg School of Medicine (NU) after approval by the institutional review board at each site. Written informed consent was obtained from all subjects before their participation in the study. Data on reproductive hormone levels from 119 of these brothers have previously been reported (4). The diagnosis of PCOS was made in the probands by an elevation of circulating testosterone and/or non–sex hormone– binding globulin (SHBG) bound (unbound testosterone) levels associated with chronic oligomenorrhea (2). Women with nonclassic 21-hydroxylase deficiency, hyperprolactinemia, and androgen-secreting tumors were excluded by appropriate tests (2). Two families had 4 brothers, 4 families had 3 brothers, 24 families had 2 brothers, and 128 families had 1 brother. The clinical and biochemical features of the probands have been reported as part of previous studies (2,4).
The selection criteria for control men were 1) no major medical or psychiatric illnesses, 2) no personal history of hypertension and no personal or first-degree family history of diabetes, and 3) normal glucose tolerance according to the World Health Organization criteria (16). Excluding the diagnosis of PCOS based on family history is problematic because it frequently remains undiagnosed, particularly in women who have variants of the syndrome with regular menstrual cycles. Further, if there are no female first-degree relatives of reproductive age, the presence of PCOS cannot be assessed. Finally, it has been our experience that men rarely know the reproductive histories of their female first-degree relatives. Thus, we do not screen our control subjects for first-degree relatives with PCOS; rather we assume in power calculations that ~7% (prevalence of PCOS in general population) of male control subjects will have a first-degree relative with PCOS compared with 100% of brothers. Inclusion of control men with a first-degree relative with PCOS would bias the study toward the null hypothesis. Neither brothers nor control men were taking any medications known to alter sex hormone metabolism or glucose homeostasis for at least 1 month before the study.
Data collection
Brothers were evaluated at one of the four study sites (on-site, n = 55) or in a local laboratory (off-site, n = 141). The majority of on-site brothers were studied at HMC (n = 53). All control men were studied on-site: 95 at HMC, 53 at NU, and 21 at BWH. Height, weight, blood pressure, and waist measurements were obtained as previously reported for the on-site subjects (2,4,9). For the off-site subjects, the height and weight were self-reported as previously validated (2,4,9). Waist circumference was self-reported for off-site brothers who were provided with a calibrated tape measure for this determination (n = 52). Self-measured waist circumference correlates well with measurements performed by a trained technician (3). There were no differences in height, weight, or waist circumference between brothers who were studied on-site and had these parameters measured by study personnel compared with those brothers studied off-site in whom these parameters were self-reported. Information on tobacco, alcohol, and exercise history was obtained by questionnaire in 95 brothers and 112 control men. A morning blood sample was obtained after an overnight fast from all subjects as reported previously (2,4,9). All control men and 27 brothers (14%) underwent 75-g oral glucose tolerance tests with fasting and 2-h postchallenge blood sampling for glucose and insulin levels.
Assays
Plasma glucose, insulin, proinsulin, testosterone, unbound testosterone, dehydroepiandrosterone sulfate (DHEAS), SHBG, total cholesterol, HDL cholesterol, LDL cholesterol, and triglyceride levels were measured as reported previously (2,4).
Data analyses
The homeostatic index of insulin resistance (HOMA-IR) was calculated according to the homeostasis model assessment, a structural computer model of the glucose-insulin feedback system in the homeostatic state (http://www.dtu.ox.ac.uk/php?maindoc=/homa/download.php/index). For analysis of the data, the family unit was the case; in families with multiple brothers, brothers' data were averaged to yield one mean value per family for brothers (4). Log transformation of the data was performed when necessary to achieve homogeneity of variance. Continuous variables were compared using ANCOVA adjusted for age. Analyses were repeated 1) after exclusion of brothers with a first-degree relative with type 2 diabetes (n = 46); 2) after adjustment for tobacco use, alcohol intake, and exercise history; 3) after exclusion of brothers with a history of hypertension (n = 8); and 4) after exclusion of brothers with glucose intolerance defined as fasting glucose >100 mg/dl (n = 17) or 2-h postchallenge glucose ≥140 mg/dl (impaired glucose tolerance n = 2, diabetes n = 1) (16). Associations between PCOS probands and their brothers for reproductive and metabolic hormones were assessed using Pearson or Spearman correlation coefficients. Predictors of HOMA-IR, LDL cholesterol, and triglyceride levels in brothers were determined using a general linear model. The relationship between SHBG levels and HOMA-IR was examined by Spearman correlation coefficient and was further adjusted for age and BMI. Dichotomous variables were compared using χ2 analysis.
Our previous studies have suggested that subjects from the central Pennsylvania area have an increased prevalence of dyslipidemia (9,10); thus, we investigated the impact of study site on outcomes. We had a sufficient number of subjects to perform the following comparisons: brothers studied on-site at HMC (HMC brothers) versus control men studied at HMC, HMC brothers versus brothers studied off-site or at other sites (non-HMC brothers), non-HMC brothers versus control men studied at NU, and control men studied at HMC versus control men studied at NU. Because the study was initiated in 1995, we also examined the impact of study time by comparing a subgroup of brothers and control men studied in 2001 or later. Analyses were performed using the 11.0 PC package of SPSS statistical software (SPSS, Chicago, IL). P < 0.05 was considered significant. Data are reported as geometric means ± SD.
RESULTS
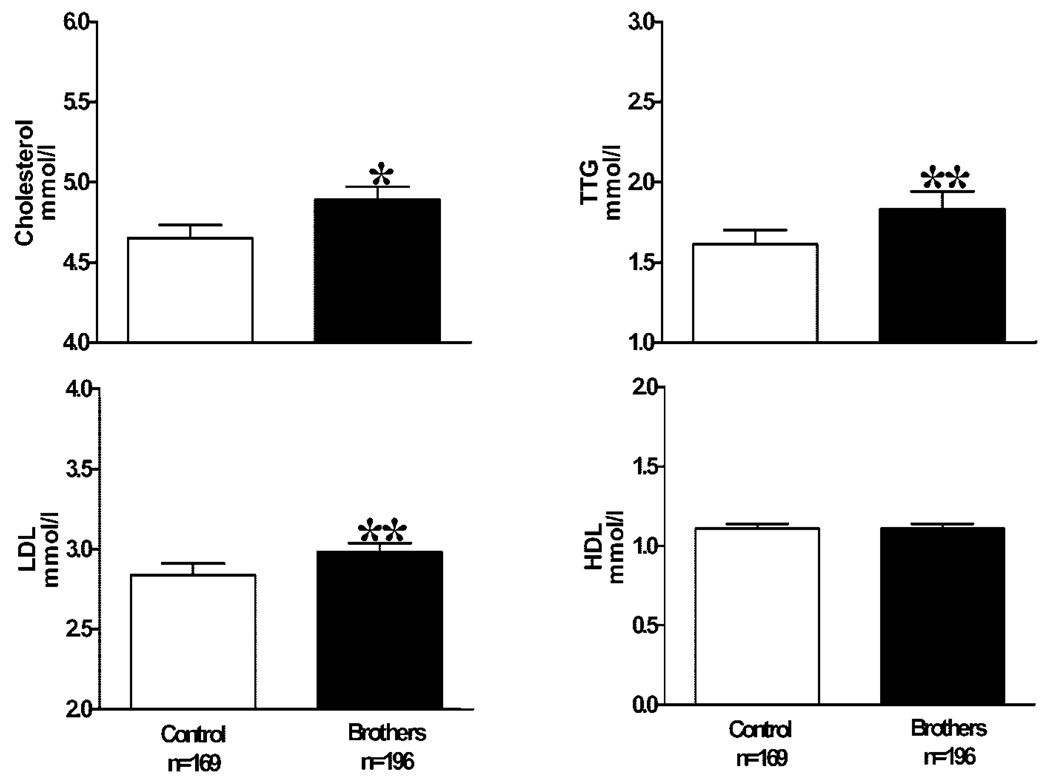
Brothers were slightly but significantly younger than control men despite recruitment of both groups in the same age range (Table 1). BMI and waist circumference were similar in both groups (Table 1). Overall, 71% of brothers and the same percentage of control men were overweight or obese as defined by a BMI ≥25 kg/m2. DHEAS levels were significantly higher in brothers than in control men (brothers 8.21 ± 3.34 µmol/l vs. control men 6.38 ± 3.00 µmol/l; P < 0.001), as reported previously (4), but there were no significant differences in either total testosterone, unbound testosterone, or SHBG levels (data not shown). Systolic blood pressure was similar, but diastolic blood pressure was significantly higher in brothers compared with control men (Table 1). Fasting insulin and proinsulin levels and HOMA-IR were significantly higher whereas fasting proinsulin/insulin ratios did not differ in brothers compared with control men (Table 1). Cholesterol, LDL cholesterol, and triglyceride levels were significantly higher in brothers compared with control men, whereas HDL cholesterol levels were similar in the two groups (Fig. 1). The differences in total and LDL cholesterol and fasting insulin levels and HOMA-IR remained significant after exclusion of brothers with a first-degree relative with diabetes (P < 0.05). The differences in total and LDL cholesterol also remained significant after exclusion of brothers with glucose intolerance or hypertension as well as after adjustment for alcohol intake, tobacco use, and exercise history (P < 0.05). Furthermore, the changes in total and LDL cholesterol levels remained significant in the subgroup analysis of brothers and control men recruited in 2001 and later (P < 0.01 for both comparisons). According to the Framingham study data, overall death increases by 5% and cardiovascular death by 9% for each 0.26 mmol/l increase in cholesterol levels at levels >4.62 mmol/l (17). The mean cholesterol level for brothers of 4.89 mmol/l was above this threshold and significantly more brothers (58%) compared with control men (47%) had total cholesterol levels above the 4.62 mmol/l threshold (P < 0.05 by χ2 analysis).
Table 1.
Clinical and metabolic features
| Variables | Brothers* | Control men | P value† |
|---|---|---|---|
| Age (years) | 30 ± 8 | 33 ± 10 | 0.004 |
| BMI (kg/m2) | 28.4 ± 5.7 | 28.0 ± 4.9 | 0.44 |
| Waist circumference (cm) | 93 ± 15 | 96 ± 14 | 0.40 |
| Systolic blood pressure (mmHg) | 125 ± 15 | 123 ± 12 | 0.15 |
| Diastolic blood pressure (mmHg) | 77 ± 10 | 75 ± 9 | 0.02 |
| Fasting glucose (mmol/l) | 4.9 ± 0.5 | 5.1 ± 0.4 | 0.14 |
| Fasting insulin (pmol/l) | 90 ± 60 | 78 ± 42 | 0.02 |
| Fasting proinsulin (pmol/l) | 13 ± 17 | 11 ± 7 | 0.02 |
| Fasting proinsulin/insulin | 0.13 ± 0.11 | 0.12 ± 0.08 | 0.70 |
| HOMA-IR | 1.9 ± 1.2 | 1.7 ± 0.9 | 0.02 |
Data are presented as geometric mean ± SD.
Data have been averaged for brothers from the same family.
ANCOVA adjusted for age.
Figure 1.
Data are presented as mean ± SE. *P = 0.001, brothers versus control subjects; **P = 0.01 brothers versus control subjects. TTG, triglycerides.
There were significant positive correlations between PCOS probands and their brothers for DHEAS (ρ = 0.4, P<0.001), SHBG (ρ = 0.2, P = 0.008), cholesterol (ρ = 0.2, P = 0.009), LDL cholesterol (ρ = 0.3, P = 0.001), triglyceride (ρ = 0.2, P = 0.05), fasting insulin (ρ = 0.2, P = 0.009), and proinsulin levels (ρ = 0.3, P = 0.002). BMI (β coefficient = 0.1, P < 0.001) was the only predictor of HOMA-IR. HOMA-IR was the strongest predictor of LDL cholesterol levels with an increase of‘ 0.21 mmol/l in LDL cholesterol for each unit increase in HOMA-IR after adjustment for age, BMI, and testosterone, SHBG, and DHEAS levels (Table 2). Age was also a predictor of LDL levels after adjustment for BMI, HOMA-IR, and DHEAS, testosterone, and SHBG levels. Age, SHBG, and HOMA-IR were predictors of triglyceride levels, independent of BMI (Table 2). SHBG levels were significantly negatively correlated with HOMA-IR (ρ = −0.23, P = 0.005), but the correlation was no longer significant after adjustment for BMI (ρ = −0.14, P = 0.09).
Table 2.
Multivariate analysis for predictors of LDL and triglyceride levels in brothers
| Variable | UnitΔ | LDL (mmol/l)Δ | Triglyceride (mmol/l)Δ |
|---|---|---|---|
| Age | 1 year | 0.024 (0.005–0.043) | 0.031 (0.002–0.060) |
| P | 0.02 | 0.04 | |
| BMI | 1 kg/m2 | 0.003 (−0.025 to 0.030) | 0.0001 (−0.056 to 0.025) |
| P | 0.86 | 0.44 | |
| HOMA-IR | 1 | 0.210 (0.057–0.363) | 0.658 (0.460–0.856) |
| P | 0.008 | <0.001 | |
| Testosterone | 1 nmol/ | 0.0003 (−0.001 to 0.001) | 0.0007 (−0.0005 to 0.002) |
| P | 0.35 | 0.23 | |
| SHBG | 1 nmol/l | −0.003 (−0.008 to 0.002) | −0.014 (−0.021 to −0.006) |
| P | 0.29 | 0.001 | |
| DHEAS | 1 µmol/l | 0.0003 (0.00003−0.0003) | 0.0001 (−0.0002 to 0.0002) |
| P | 0.18 | 0.84 |
Data are means (95% CI).
We evaluated the impact of study site on our findings. HMC brothers did not differ from non-HMC brothers, except that HDL cholesterol levels were significantly lower in HMC brothers (0.98 ± 0.26 mmol/l vs. 1.13 ± 0.03 mmol/l, respectively, P = 0.002). In contrast, total and LDL cholesterol levels as well as triglyceride levels were significantly higher in HMC control men compared with NU control men (P < 0.01). When brothers were compared with control men at HMC, triglyceride levels remained significantly higher (P = 0.04), but total and LDL cholesterol did not differ. HMC brothers also had significantly lower HDL cholesterol levels than HMC control men (0.98 ± 0.26 mmol/l vs. 1.11 ± 0.28 mmol/l, respectively, P = 0.02). The findings in the non-HMC brothers compared with the NU control men were similar to the findings for the overall group; total and LDL cholesterol and triglyceride levels were significantly higher (P < 0.01 for all three comparisons), whereas HDL cholesterol levels were similar.
CONCLUSIONS
We found that brothers of women with PCOS have dyslipidemia and evidence for insulin resistance. In the overall group of brothers, there were significant increases in total and LDL cholesterol levels and in triglyceride levels compared with those in control men. These metabolic abnormalities were similar to those reported in women with PCOS (10) and their first-degree female relatives (3,9). The LDL cholesterol elevations in brothers were independent of glucose intolerance and obesity and persisted after adjustment for lifestyle factors such as tobacco use, alcohol intake, or exercise. There were significant positive correlations between insulin, LDL cholesterol, and triglyceride levels in brothers and their proband sisters. Our findings support the hypothesis that some metabolic features of PCOS are heritable and are not sex specific.
Fasting insulin levels and HOMA-IR were higher in brothers compared with control men, suggesting that brothers were insulin resistant. In contrast, there was no significant differences in insulin sensitivity assessed by frequently sampled intravenous glucose tolerance tests in our recent study of 23 brothers compared with matched control men (12). However, this study lacked adequate power for the observed differences in insulin sensitivity to achieve statistical significance. Further, decreases in insulin clearance could have contributed to the differences in fasting insulin levels and HOMA-IR in brothers in the present study (18). We were not able to evaluate the prevalence of glucose intolerance in brothers because only a small number (n = 27) had oral glucose tolerance testing and brothers who were treated with antidiabetic medications were excluded from the study. We were also not able to compare the prevalence of metabolic syndrome in brothers with that in control men because, by design, features of metabolic syndrome were specifically excluded as part of the selection criteria for the control men.
In brothers, HOMA-IR, independent of BMI, was the strongest predictor of LDL elevations, suggesting that insulin resistance is an important factor in the pathogenesis of LDL cholesterol abnormalities in the male members of PCOS families. Neither DHEAS nor testosterone levels predicted LDL levels in brothers. In contrast to brothers, hyperandrogenemia rather than insulin resistance was the strongest predictor of LDL cholesterol levels in women with PCOS and their first-degree female relatives (3,9,10). Our findings are consistent with prior reports that, in contrast to women, endogenous sex hormone levels may have a lesser impact on circulating LDL levels in men (19). However, insulin resistance also usually does not alter circulating LDL levels, although it can influence LDL particle size (20). Thus, the relationship between markers of insulin resistance and LDL levels in brothers may reflect the association of both of these parameters with another factor not accounted for in our analysis. In addition to higher LDL cholesterol levels, brothers also had higher triglyceride levels compared with control men. Triglyceride levels were elevated only in obese women with PCOS and their obese first-degree relatives (9,10). HOMA-IR, independent of BMI, was a predictor of triglyceride levels in brothers, suggesting that insulin resistance per se was an important determinant of triglyceride elevations in brothers. SHBG levels also predicted triglyceride levels in brothers but were most likely a proxy for insulin resistance because hepatic SHBG production is downregulated by hyperinsulinemia (14). Consistent with this hypothesis, SHBG levels were significantly correlated with HOMA-IR in brothers. Metabolic abnormalities were a consistent finding in brothers across study sites. However, there were regional differences in control subjects such that some of the metabolic abnormalities noted in the brothers were attenuated compared with those in HMC control men. The HMC brothers and control subjects are from a fairly homogenous Caucasian population of German and Dutch ancestry. Our findings in the present as well as previous studies suggest that this population has an increased prevalence of dyslipidemia (9,10). Regional variations in the environment and ethnicity represent an important potential confounder of case-control studies and probably contributes to conflicting reports of PCOS phenotypes (14). Nevertheless, there were metabolic abnormalities when brothers were compared with control subjects of comparable location, supporting our hypothesis that brothers have a metabolic phenotype.
Brothers had a mean increase of 0.26 mmol/l in total cholesterol levels compared with control men. Cholesterol levels are directly related with 30-year overall and cardiovascular mortality, especially in men aged <50 years (17). The mean cholesterol level for brothers of 4.89 mmol/l was above the threshold of 4.62 mmol/l at which overall death increases by 5% and cardiovascular death by 9% for each 0.26 mmol/l increase in cholesterol levels (17). Further, significantly more brothers than control men had total cholesterol levels above this threshold. In addition, insulin resistance is an independent predictor of cardiovascular disease risk in men (21).
In summary, brothers of women with PCOS have a metabolic phenotype consisting of dyslipidemia and insulin resistance. The familial aggregation of these metabolic abnormalities is consistent with heritable traits. Given the high prevalence of PCOS, being a male first-degree relative may represent an important new risk factor for cardiovascular disease in men.
Acknowledgments
This work was supported by National Institutes of Health Grants U54 HD34449, R01 DK40605, P50 HD044405, M01 RR00048, M01 RR10732, K12 RR017707, and M01 RR02635.
The authors thank the women with PCOS and their brothers for participating in this study, Barbara Scheetz, Jennifer Schlinder, Elizabeth Podczerwinski, and Jennifer Steel for coordinating the study, and the staff of the General Clinical Research Centers at the Pennsylvania State University College of Medicine, Brigham and Women's Hospital, and North-western University Feinberg School of Medicine for their care of the study participants.
Abbreviations
- BWH
Brigham and Women’s Hospital
- DHEAS
dehydroepiandrosterone sulfate
- HMC
Pennsylvania State University College of Medicine–Hershey Medical Center
- HOMA-IR
homeostatic index of insulin resistance
- NU
Northwestern University Feinberg School of Medicine
- PCOS
polycystic ovary syndrome
- SHBG
sex hormone–binding globulin
References
- 1.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685–697. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 2.Legro RS, Driscoll D, Strauss JF, 3rd, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci USA. 1998;95:14956–14960. doi: 10.1073/pnas.95.25.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sam S, Legro RS, Essah PA, Apridonidze T, Dunaif A. Evidence for metabolic and reproductive phenotypes in mothers of women with polycystic ovary syndrome. Proc Natl Acad Sci USA. 2006;103:7030–7035. doi: 10.1073/pnas.0602025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legro RS, Kunselman AR, Demers L, Wang SC, Bentley-Lewis R, Dunaif A. Elevated dehydroepiandrosterone sulfate levels as the reproductive phenotype in the brothers of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:2134–2138. doi: 10.1210/jcem.87.5.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urbanek M, Sam S, Legro RS, Dunaif A. Identification of a PCOS susceptibility variant in fibrillin-3 and association with metabolic phenotype. J Clin Endocrinol Metab. 2007;92:4191–4198. doi: 10.1210/jc.2007-0761. [DOI] [PubMed] [Google Scholar]
- 6.Colilla S, Cox NJ, Ehrmann DA. Heritability of insulin secretion and insulin action in women with polycystic ovary syndrome and their first degree relatives. J Clin Endocrinol Metab. 2001;86:2027–2031. doi: 10.1210/jcem.86.5.7518. [DOI] [PubMed] [Google Scholar]
- 7.Yildiz BO, Yarali H, Oguz H, Bayraktar M. Glucose intolerance, insulin resistance, and hyperandrogenemia in first degree relatives of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2031–2036. doi: 10.1210/jc.2002-021499. [DOI] [PubMed] [Google Scholar]
- 8.Yilmaz M, Bukan N, Ersoy R, Karakoc A, Yetkin I, Ayvaz G, Cakir N, Arslan M. Glucose intolerance, insulin resistance and cardiovascular risk factors in first degree relatives of women with polycystic ovary syndrome. Hum Reprod. 2005;20:2414–2420. doi: 10.1093/humrep/dei070. [DOI] [PubMed] [Google Scholar]
- 9.Sam S, Legro RS, Bentley-Lewis R, Dunaif A. Dyslipidemia and metabolic syndrome in the sisters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:4797–4802. doi: 10.1210/jc.2004-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med. 2001;111:607–613. doi: 10.1016/s0002-9343(01)00948-2. [DOI] [PubMed] [Google Scholar]
- 11.Norman RJ, Masters S, Hague W. Hyperinsulinemia is common in family members of women with polycystic ovary syndrome. Fertil Steril. 1996;66:942–947. doi: 10.1016/s0015-0282(16)58687-7. [DOI] [PubMed] [Google Scholar]
- 12.Sam S, Sung Y, Legro RS, Dunaif A. Evidence for pancreatic β-cell dysfunction in brothers of women with PCOS. Metabolism. 2007;57:84–89. doi: 10.1016/j.metabol.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sir-Petermann T, Angel B, Maliqueo M, Carvajal F, Santos JL, Perez-Bravo F. Prevalence of type II diabetes mellitus and insulin resistance in parents of women with polycystic ovary syndrome. Diabetologia. 2002;45:959–964. doi: 10.1007/s00125-002-0836-3. [DOI] [PubMed] [Google Scholar]
- 14.Dunaif A, Sorbara L, Delson R, Green G. Ethnicity and polycystic ovary syndrome are associated with independent and additive decreases in insulin action in Caribbean-Hispanic women. Diabetes. 1993;42:1462–1468. doi: 10.2337/diab.42.10.1462. [DOI] [PubMed] [Google Scholar]
- 15.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B. Impaired fasting glucose and impaired glucose tolerance. Diabetes Care. 2007;30:753–759. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 17.Anderson KM, Castelli WP, Levy D. Cholesterol and mortality: 30 years of follow-up from the Framingham study. JAMA. 1987;257:2176–2180. doi: 10.1001/jama.257.16.2176. [DOI] [PubMed] [Google Scholar]
- 18.Gower BA, Granger WM, Franklin F, Shewchuk RM, Goran MI. Contribution of insulin secretion and clearance to glucose-induced insulin concentration in African-American and Caucasian children. J Clin Endocrinol Metab. 2002;87:2218–2224. doi: 10.1210/jcem.87.5.8498. [DOI] [PubMed] [Google Scholar]
- 19.Haffner SM, Mykkanen L, Valdez RA, Katz MS. Relationship of sex hormones to lipids and lipoproteins in nondiabetic men. J Clin Endocrinol Metab. 1993;77:1610–1615. doi: 10.1210/jcem.77.6.8263149. [DOI] [PubMed] [Google Scholar]
- 20.Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, Pugh K, Jenkins AJ, Klein RL, Liao Y. Effects of insulin resistance and type 2 on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52:453–462. doi: 10.2337/diabetes.52.2.453. [DOI] [PubMed] [Google Scholar]
- 21.Despres JP, Lamarche B, Mauriege P, Cantin B, Dagenais GR, Moorjani S, Lupien PJ. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996;334:952–957. doi: 10.1056/NEJM199604113341504. [DOI] [PubMed] [Google Scholar]