Abstract
Introduction
Elevated concentrations of isovaleric, methylmalonic, and propionic acid are associated with impaired consciousness in genetic diseases (organic acidemias). We conjectured that part of the central nervous system depression observed in these disorders was due to anesthetic effects of these metabolites. We tested three hypotheses. First, that these metabolites would have anesthetic-sparing effects, possibly being anesthetics by themselves. Second, that these compounds would modulate glycine and GABAA receptor function, increasing chloride currents through these channels as potent clinical inhaled anesthetics do. Third, that these compounds would affect physical properties of lipids.
Methods
Anesthetic EC50’s were measured in Xenopus laevis tadpoles. Glycine and GABAA receptors were expressed in Xenopus laevis oocytes and studied using two-electrode voltage clamping. Pressure-area isotherms of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) monolayers were measured with and without added organic acids.
Results
Isovaleric acid was an anesthetic in tadpoles, while methylmalonic and propionic acid decreased isoflurane’s EC50 by half. All three organic acids concentration-dependently increased current through α1 glycine receptors. There were minimal effects on α1β2γ2s GABAA receptors. The organic acids increased total lateral pressure (surface pressure) of DPPC monolayers, including at mean molecular areas typical of bilayers.
Conclusion
Isovaleric, methylmalonic, and propionic acid have anesthetic affects in tadpoles, positively modulate glycine receptor fuction, and affect physical properties of DPPC monolayers.
Keywords: Organic acidemia, isovaleric acid, methylmalonic acid, propionic acid, Anesthesia, Glycine receptor, GABA type A receptor, Lipid monolayer, Langmuir-Blodget film, interfacial activity
Introduction
General anesthesia is characterized by central nervous system depression leading to a variety of behavioral responses including immobility in response to noxious stimuli, amnesia, and unconsciousness, which are reversible upon discontinuation of the anesthetic (1). We have conjectured that certain metabolites, which are elevated in diseases associated with impaired consciousness, may have anesthetic properties (2–5). In previous studies, we focused on metabolites elevated in end-stage organ failure. We showed that beta hydroxybutyric acid was an anesthetic in tadpoles, and that ammonia reduced isoflurane minimum alveolar concentration (MAC) by 60% in rats. Both compounds modulated the function of particular anesthetic sensitive channels in a manner qualitatively similar to volatile anesthetics (2, 5).
This study focuses on anesthetic properties of organic acids. Organic acidemias are genetic metabolic diseases arising from mutations in genes coding for enzymes involved in amino acid metabolism (6). Isovaleric acidemia is caused by a mutation in the gene coding for isovaleryl CoA dehydrogenase (7). Methylmalonic acidemias are caused by mutations leading to a deficiency of methyl malonyl CoA (8). Propionic acidemia is caused by mutations in propionyl-CoA carboxylase (9, 10). Severe forms of all three disorders are associated with lethargy and unconsciousness.
We hypothesized that part of the central nervous system depression observed in these disorders was due to anesthetic effects of metabolites. Our reasoning was as follows. We noted that interfacial activity (the accumulation of a compound at interfaces, defined as the boundary between two immiscible fluid phases, such as the bilayer/water interface) has been shown to be a necessary condition of anesthetic action for a wide range of volatile anesthetics via molecular dynamic simulations and nuclear magnetic resonance spectroscopy, which showed that volatile anesthetics accumulated at interfaces but volatile compounds that were not anesthetics did not accumulate at interfaces (11–17). We reasoned that interfacial activity may also be a sufficient condition, and if so then other interfacially active compounds may have anesthetic properties. Our study showing that surfactants modulate receptor function in a manner similar to inhaled anesthetics supported this hypothesis (3).
In addition, we noted that a mechanism by which anesthetics affect lipid physical properties which are mechanically coupled to ion channel function has been proposed (18), and this could explain anesthetic effects of interfacially active molecules. This theory notes that because the membrane bilayer is in contact with water, there is tension at the bilayer/water interface. Interfacial tension decreases the area per lipid in the membrane owing to the free energy cost of increasing the area of membrane in contact with water. Opposing this is the entropic drive toward random acyl chain conformations, which increases the area per lipid molecule. As a result, ion channels will see depth-dependent laterally directed pressures (stresses) trying to squeeze the channel shut in some places, and pull it open in others. When an ion channel changes between its closed and open conformation, it will do work against the stresses imposed by the bilayer. This work contributes to the free energy difference between the open and closed conformations of the channel. Normally, this effect is not apparent because channels are adapted to the stresses on them. Volatile anesthetics, and other interfacially active compounds, will affect the packing of lipids at the bilayer/water interface, thereby altering the lateral pressures there (and, indirectly, throughout the bilayer). Channels will accordingly do a different amount of work in going between their closed and open states when volatile anesthetics, and other interfacially active compounds, are present, because the channels are doing work against a changed lateral pressure distribution. This provides a mechanism by which interfacially active molecules could have anesthetic actions. We expected that organic acids would interact with the interfacial region of the bilayer, and for the preceding reasons might have anesthetic effects.
We tested three hypotheses about isovaleric, methylmalonic, and propionic acid. First, that they would have anesthetic-sparing effects, possibly being anesthetics by themselves. Second, that these compounds would modulate glycine and GABAA receptor function, increasing chloride currents through these channels as potent clinical inhaled anesthetics do, and that they would have a reduced modulatory effect on glycine and GABAA receptors containing mutations which reduced the modulatory effect of isoflurane and ethanol. Third, that these compounds would affect physical properties of lipids. We tested this hypothesis by applying isovaleric, methylmalonic, and propionic acid to lipid monolayers (19) while measuring the change in surface pressure (total lateral pressure) of the monolayer. We used 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) monolayers because they are particularly well characterized and the phosphocholine headgroup is the most common headgroup in eukaryotic cell membranes (20, 21).
Materials and Methods
Studies on animals were approved by the institutional animal care and use committee of the University of California, San Francisco.
Studies on expressed receptors
Materials
Wild-type and mutant α1(S270I)β2γ2s GABAA receptor and wild-type and mutant α1(S267I) glycine receptor clones were a gift of Professor R. Adron Harris (University of Texas, Austin). The cDNAs encoding GABAA receptor α1 wild-type and mutant subunits were cloned into a pBK-CMV vector. cDNAs encoding GABAA receptor β2 and γ2s and glycine receptor α1 wild-type and mutant subunits were cloned into a pCIS2 vector. Collagenase type 1, for defolliculating oocytes, was purchased from Worthington Biochemical Corporation (Lakewood, NJ). The propionic acid, methylmalonic acid, isovaleric acid and other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Oocyte harvest and defolliculation
A female Xenopus laevis frog was anesthetized in 0.35% tricaine methane sulphonate solution (w/v). A portion of an ovary was surgically removed and defolliculated by gentle rotation in OR2 buffer (82.5 mM NaCl, 2.0 mM KCl, 1 mM MgCl2, 5 mM HEPES, pH 7.5) containing 500 U/ml collagenase type 1 for 1 to 1.5 h at room temperature. The individual oocytes were washed in OR2 buffer 8 to 10 times, and maintained in modified Barth buffer (88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 0.82 mM MgSO4, 0.33 mM Ca(NO3)2, 20 mM HEPES, 0.41 mM CaCl2, 5 mM sodium pyruvate, pH 7.5) containing 100 U/ml penicillin, 100 mg/ml streptomycin and 50 mg/ml gentamycin at 15°C.
DNA injection and expression
2.5 ng cDNA of the glycine receptor α1 or mutant α1 (S267I) subunit, or a total of 2.5 ng cDNA of the GABAA receptor wild type or mutant (in a 1:1:6 ratio of α:β:γ subunits) were injected into the Xenopus oocyte nuclei via blind injection into the animal pole of the oocytes. Oocytes were kept in Barth’s buffer at 15°C and studied 1 to 2 days after cDNA injection.
Two-electrode voltage clamping
Two-electrode voltage clamping was performed following previously described methods (5,37). EC5 concentrations of glycine, and EC10 concentrations of GABA, were used in these studies. For each oocyte, stable inward currents in response to application of agonist were verified by application of agonist for 20 seconds followed by a 5 minute washout, repeated three times. Each study compound was then applied to oocytes for 100 seconds, followed by coapplication of agonist(s) with the study compound for 20 seconds. Return to baseline response to agonist after washout of the test compound for 5 minutes was confirmed.
Tadpole Anesthetic EC50
Tadpoles were raised by in vitro fertilization using previously described methods (22, 23).
Anesthetic potency was tested in tadpoles one week after fertilization using standard methods (24). Tadpoles were placed in 0.1× Marc’s modified ringer’s (MMR) (10mM NaCl, 0.18mM KCl, 0.2mM CaCl2, 0.1mM MgCl2, 0.5 mM HEPES, adjusted to pH 7.0) with the drugs under study for 1.5 hours, then prodded on their side for thirty seconds with a glass rod, or until they moved, whichever occurred first. Tadpoles that responded to any amount of prodding were classified as moving. Tadpoles that did not respond to prodding were classified as non-moving. For tadpoles tested with propionic or methylmalonic acid, 0.3% isoflurane was bubbled into the 0.1× MMR prior to testing. Equilibration of the buffer with isoflurane was confirmed by gas chromatography. Non-moving tadpoles were removed and placed in 0.1X MMR for recovery; only data for animals that recovered were analyzed.
Monolayer Isotherms
Isotherms were measured using a KSV Langmuir-Blodgett minitrough (Monroe, CT). The Teflon trough and Delrin barriers were cleaned prior to use with HPLC grade butyl acetate followed by ethanol and MilliQ water. The surface pressure balance used a platinum Wilhelmy plate which was cleaned with the same procedure as above and then heated in a flame before use. MilliQ water, with or without organic acid, was used for the subphase. The absence of contaminants in the subphase which affected surface pressure was confirmed by measuring surface pressure during compression of the barriers prior to spreading the monolayer.
The spreading agent was HPLC grade chloroform. DPPC (Avanti Polar Lipids, Alabaster, AL) at a concentration of 1 mg/ml was applied to the surface of the subphase using a Hamilton syringe. Once the spreading agent had fully evaporated (15 to 20 minutes), the monolayer was compressed at a rate of approximately 2 Å2/molecule/minute. A Wilhelmy balance recorded the resulting surface pressure every second. Two isotherms were obtained for each concentration of each acid, averaged, and compared to a reference isotherm for pure DPPC.
Data Analysis
Change in currents was calculated in oocytes clamped at −80mV during drug and agonist coadministration vs. agonist alone. Data for oocytes are presented as mean ± SE. Statistical significance was determined using Student’s t-test. The fraction of tadpoles not moving as a function of drug concentration was analyzed by nonlinear regression to a Hill equation. EC50s and Hill coefficients were calculated and used to compare the curves. The surface pressure at a mean molecular area of 60 Å2 (similar to that found in bilayers (25)) was calculated at each concentration of organic acid. Linear regression was performed to determine if there was an increase in surface pressure with organic acid concentration. P<0.05 was considered significant.
Results
Glycine Receptor
There was a concentration dependent increase in currents through wild-type α1 glycine receptors for all three organic acids (Fig 1A). Propionic acid decreased currents at 1.25 mM.
Fig 1.
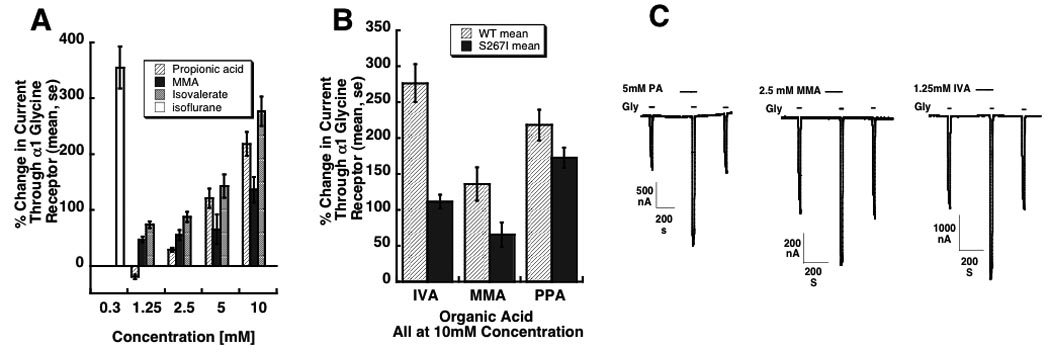
Modulation of α1 glycine receptor function by isovaleric acid, methylmalonic acid, and propionic acid. Panel A: 100 second exposures to propionic, methylmalonic (MMA), and isovaleric acid increases currents through homomeric α1 glycine receptors in response to EC5 glycine. Receptors were expressed in Xenopus laevis oocytes and studied by two-electrode voltage clamping. Currents through glycine receptors increase in a concentration-dependent manner. The effect of 0.3mM isoflurane is shown for comparison. Panel B: Mutant S267I glycine receptors engineered to have a reduced modulatory response to isoflurane and ethanol also have a reduced response to isovaleric (IVA) and methylmalonic (MMA) acid (p < 0.05). The effect of propionic acid (PPA) is not statistically significant. Panel C: current tracings showing the effect of 5mM propionic acid (PA), 2.5 mM methylmalonic acid (MMA), and 1.25 mM isovaleric acid (IVA). Three peaks are shown for each acid: the first is in response to agonist (glycine) alone, the second to acid and agonist, and the third to agonist after washout of the acid.
10 mM methylmalonic acid and isovaleric acid produced a significantly larger increase in currents through wild-type α1 glycine receptors than in mutant α1 (S267I) glycine receptors, as seen with isoflurane and ethanol (26). There was a greater enhancement of the wild-type α1 glycine receptor compared to the mutant α1 glycine (S267I) receptor by 10 mM propionic acid (Fig 1B), but this result did not reach statistical significance.
Representative current tracings are in Fig. 1C.
GABAA Receptor
10 mM methylmalonic acid (MMA) and isovaleric acid (IVA) produced only small increases in current through GABAA α1β2γ2s receptors. There was no significant effect of 10 mM propionic acid. The change in current was smaller, but nevertheless significant, in mutant α1(S270I)β2γ2s receptors for isovaleric and methylmalonic acids. Propionic acid inhibited currents through the mutant receptor. See Table 1.
Table 1.
| Change in Current Through: | ||
|---|---|---|
| Wild type GABAA α1β2γ2s receptor |
Mutant GABAA α1(S270I)β2γ2s |
|
| 0.3mM Isoflurane | 84.6 ± 7.2 % | 60.0 ± 5.9 % |
| 10mM Propionic Acid |
1.6 ± 2.6 % | −6.8 ± 1.6 % |
| 10mM Methylmalonic Acid |
14.8 ± 1.9 % | 4.6 ± 1.4 % |
| 10mM Isovaleric Acid |
17.4 ± 2.2 % | 5.6 ± 1.2 % |
Tadpoles
Isovaleric acid was an anesthetic. Its EC50 ± se was 44.8 ± 3.7 mM (bath concentration), with a Hill number n = 4.1 ± 1.2. Both methylmalonic acid and propionic acid had to be coadministered with isoflurane or the tadpoles would not recover over the concentration range that prevented movement. When coadministered with 0.3% isoflurane (a concentration equal to half isoflurane’s EC50 (22)), 75.0 ± 9.1 mM propionic acid (Hill number n = 1.4 ± 0.4), or 149.6 ± 2.1 mM methylmalonic acid (Hill number n = 12.4 ± 2.0) had to be present in the bath to reversibly anesthetize the tadpoles. See Fig 2.
Fig 2.
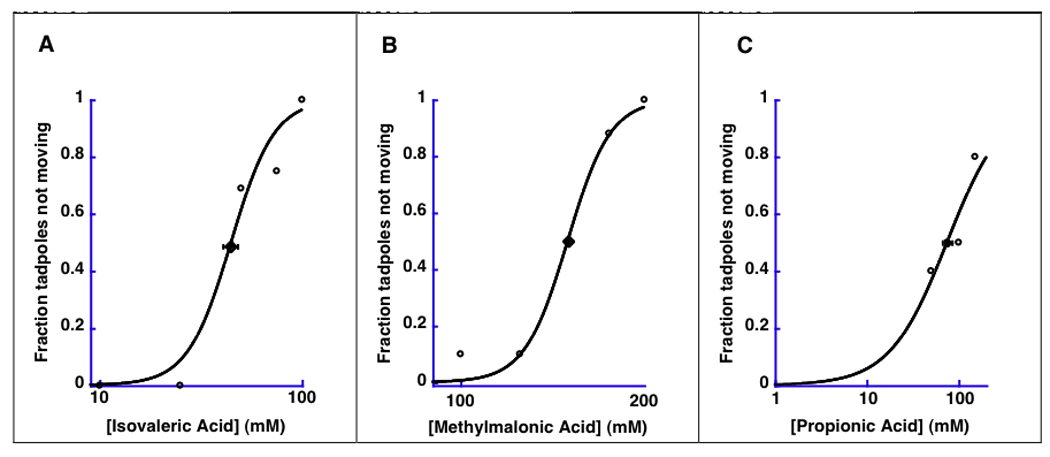
Tadpoles. Anesthetic effects of isovaleric acid, methylmalonic acid, and propionic acid in tadpoles. The fraction of tadpoles which did not move in response to prodding is shown as a function of the concentration of isovaleric acid (Panel A) methylmalonic acid (Panel B) and propionic acid (Panel C). Concentrations on the x-axis refer to the concentration in the aquarium. 0.3% isoflurane (half of isoflurane’s EC50) was coapplied for methylmalonic and propionic acid. Isovaleric acid is an anesthetic in tadpoles. Methylmalonic and propionic acid reduced isoflurane requirement by 50%.
DPPC Isotherms
All three organic acids increased surface pressure of DPPC monolayers at 23.1°C (Fig 3 panels A, B, and C) including at a mean molecular area of 60Å2, which is approximately that obtained in lipid bilayers (25). The slope of the regression lines for pressure vs concentration at 60 Å2 were 0.22 ± 0.05 (p = 0.018), 0.62 ± 0.07 (P < 0.001), and 0.19 ± 0.02 (P < 0.001) milliNewton/(meter*millimolar) for isovaleric, methylmalonic, and propionic acid, respectively. See Fig 3D.
Fig 3.
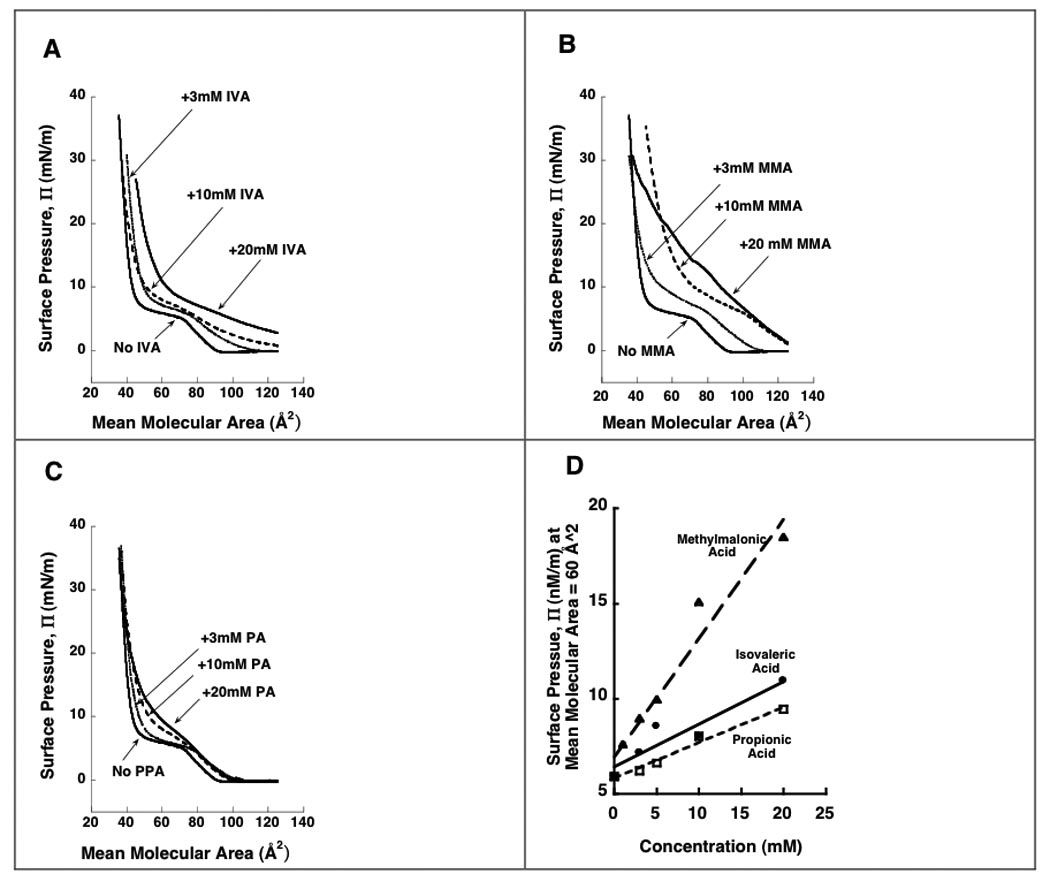
Pressure-area isotherms for DPPC alone and with isovaleric acid (Panel A), methylmalonic acid (Panel B), or propionic acid (Panel C) added to the subphase. IVA = isovaleric acid. MMA = methylmalonic acid. PPA = Propionic acid. Surface pressure, Π (total lateral pressure) is expressed in units of milliNewtons/meter (mN/m) on the y-axis. Mean molecular area is in units of Å2 on the x-axis. The organic acids shift the isotherms to higher pressures, decrease the plateaus (two-phase coexistence region), and change the slopes (compressibility) of the monolayers. Panel D shows the surface pressure at a mean molecular area of 60 Å2, which is approximately that obtained in bilayers (25). The acids increase the pressures in a concentration dependent manner, with methylmalonic acid having the greatest effect and propionic acid the least effect. All experiments were performed at 23.1°C.
Discussion
Our results show that isovaleric acid is ananes the ticintad poles. Methylmalonic and propionic acid have anesthetic-sparing effects in tadpoles. All three compounds show a concentration-dependent enhancement of homomeric α1 glycine receptor function at concentrations that are observed in disease (up to 5mM ) (27, 28) as well as at the higher concentrations we explored because of the anesthetic potential of these compounds. Minimal effects were observed on α1β2γ2s GABAA receptors.
To determine whether the allosteric effects were similar to those of volatile anesthetics, we applied the organic acids to mutant α1 glycine and α1β2γ2s GABAA receptors that were less sensitive to the modulatory effect of isoflurane and ethanol than wild type receptors (3). With the exception of propionic acid, which had a decreased modulatory effect on the glycine receptor that was not statistically significant, all of these compounds had a smaller effect on the mutant compared to the wild type channels. This is consistent with the hypothesized shared mechanism of action with isoflurane and ethanol.
We conjectured that the organic acids exerted their anesthetic effects indirectly, by modulating lipid properties. Measurement of surface pressures of lipid monolayers has been performed for over a century (29–31). We tested whether these compounds affected lipid properties by measuring pressure-area isotherms of DPPC monolayers with and without added organic acid. We used monolayers because their physical chemistry has been extensively studied (19, 20), the composition of the monolayer can be controlled, and its properties varied (in particular the mean area per molecule). For the pure DPPC monolayer, the shape of the isotherm can be explained as follows (Fig 4). At high mean molecular areas DPPC molecules interact weakly, forming a so-called gaseous phase. With compression, the molecules eventually interact to form a liquid expanded phase, at which point surface pressure rises. Further compression leads to the formation of a second, rigid, liquid condensed phase. The appearance of this second phase leads to the loss of a degree of freedom (a consequence of Gibb’s phase rule), with the result that surface pressure becomes constant in the two phase coexistence region and a plateau is observed. Continued compression produces a monolayer that is entirely in the liquid condensed phase. This rigid phase is relatively incompressible, and surface pressures rise rapidly. Further compression eventually leads to collapse of the monolayer. A molecular dynamic simulation shows the structure of the monolayer at various points on the isotherm (20).
Fig 4.
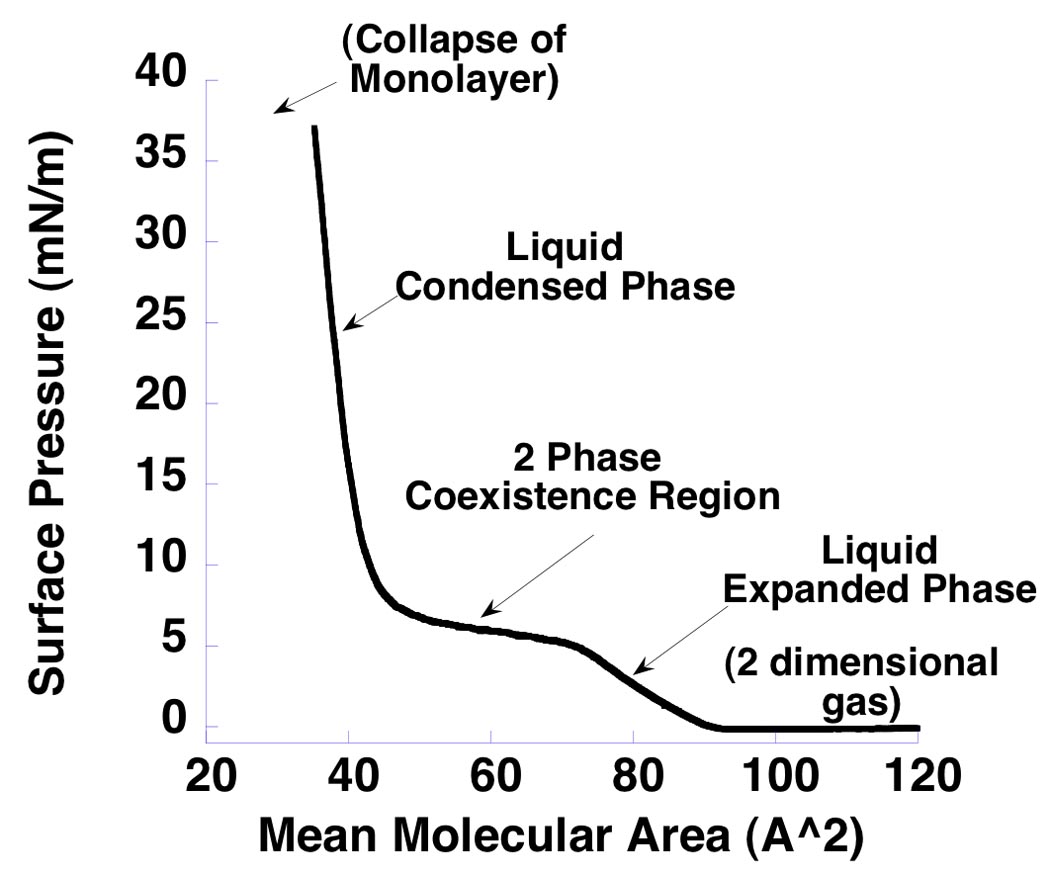
Isotherm for pure DPPC monolayer. Surface pressure (total lateral pressure) is expressed in units of milliNewtons/meter (mN/m) on the y-axis. Mean molecular area is in units of Å2 on the x-axis. The monolayer is initially compressed from high mean molecular areas. The surface pressure is constant at this point. With further compression the monolayer undergoes phase transitions, initially into a liquid expanded state during which surface pressure rises, then into a two phase coexistence region in which both liquid expanded and liquid condensed phases coexist and the surface pressure is constant, and finally into a rigid, liquid condensed phase.
Addition of each of the organic acids to the water subphase shifted the isotherm to higher pressures, and changed the shape of the isotherm. Increases in surface pressure of monolayers have been reported for volatile anesthetics (32–34). Surfactants can also shift DPPC pressure-area isotherms (35). In this study, the shift to higher surface pressures was most pronounced for methylmalonic acid, and least for propionic acid. In all cases these effects on the isotherms result from incorporation of the acids into the monolayers, which also changes the phase behavior of the DPPC. Of particular relevance, the acid was incorporated into the lipid monolayers at mean molecular areas characteristic of bilayers (fig 3D) of 60 A2 (25). Only at very low mean molecular areas (high surface pressures) were the acids squeezed out of the monolayer, with the isotherms approaching that of a pure DPPC monolayer.
That the organic acids interact with lipids does not rule out the possibility that these compounds may modulate ion channel function by binding directly to the channel protein. It does, however, open the door to a lipid-mediated mechanism for some of their clinical effects. The results are consistent with, but do not prove, that membrane stresses can account for the effect of the organic acids on receptor function.
Although the organic acids produce anesthetic or anesthetic-sparing effects in tadpoles, we do not know the concentrations in the tadpole that produce this effect. Our results only show that these compounds have anesthetic effects. Because we expected the animals would rapidly metabolize the organic acids, these drugs were introduced into aquarium water, which provided a large reservoir for the drug. Since only the unionized form of the acid should cross epithelial barriers, this acid is only a small fraction of the total concentration of the metabolite, and any metabolite entering the tadpole would be quickly metabolized, we expect that the concentrations of organic acid in the tadpoles are much less than the concentration we needed to apply in the aquarium.
Like ammonia (2) and beta hydroxybutyric acid (5), isovaleric acid, methylmalonic acid, and propionic acid are all endogenous compounds. Our results show that all are reversible central nervous system depressants. Why would an organism respond to an endogenous compound in this manner? Responding to an endogenous compound with central nervous system depression should be harmful and, hence, selected against. Yet, this does not seem to have happened. We have speculated that the basic mechanism accounting for this response may have arisen in one-celled organisms responding to interfacially active compounds in the environment (4). The observation that inhaled anesthetics (and surfactants (3)) enhance currents through inhibitory receptors, and inhibit currents through excitatory receptors, seems an odd physiologic response for a neuron. However, for a one-celled organism, this response makes sense: only this pattern of actions on ion channels will protect a one-celled organism’s transmembrane potential, by limiting entry of positive charges into the cell, in response to interfacially active compounds which are ubiquitous in the environment and which can modulate channel function through their effect on bilayer properties. By responding in this manner, anesthetic-sensitive ion channel architectures established in microbes could be passed on, ultimately to their descendants in multicellular organisms. It has been suggested that there is also ongoing selection for this response in animals (36).
We have identified twelve new modulators of glycine receptor function over the past two years. This was accomplished primarily using as guides the known interfacial activity of surfactants (37) and their effects on monolayer properties (35), the predicted effect of neurotransmitters on bilayer properties (36), molecular dynamic simulations (38) and radioligand binding studies (39) which show that various amino acids adsorb onto membranes. These modulators include two anionic surfactants (3), two cationic surfactants (3), one zwiterionic surfactant (3), a plasticizer (40), a neurotransmitter (GABA) (41), and five metabolites including the three organic acids reported here (ammonia (2), beta hydroxybutyric acid (5), isovaleric acid, methylmalonic acid, and propionic acid). We expect that several other small, interfacially active, endogenous molecules elevated in metabolic diseases may also be allosteric modulators of ion channel function.
In summary, we have shown that isovaleric acid, methylmalonic acid, and propionic acid have anesthetic effects in animals, positively modulate glycine receptor function, and affect pressure-area isotherms of DPPC lipid monolayers. These observations may provide mechanistic insight into the central nervous system depression observed in organic acidemias, and identify new structures with anesthetic properties.
Acknowledgments
This work was supported in part by NIGMS R01 GM069379
Footnotes
Reprints will not be available
References
- 1.Eger EI, 2nd, Sonner JM. Anaesthesia defined (gentlemen, this is no humbug) Best Pract Res Clin Anaesthesiol. 2006;20:23–29. doi: 10.1016/j.bpa.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Brosnan RJ, Yang L, Milutinovic PS, Zhao J, Laster MJ, Eger EI, Sonner JM. Ammonia has anesthetic properties. Anesth Analg. 2007;104:1430–1433. doi: 10.1213/01.ane.0000264072.97705.0f. [DOI] [PubMed] [Google Scholar]
- 3.Yang L, Sonner JM. Anesthetic-like modulation of receptor function by surfactants: A test of the interfacial theory of anesthesia. Anesth Analg. 2008 doi: 10.1213/ane.0b013e31817ee500. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonner JM. A hypothesis on the origin and evolution of the response to inhaled anesthetics. Anesth Analg. 2008 doi: 10.1213/ane.0b013e31817ee684. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L, Zhao J, Milutinovic PS, Brosnan RJ, Eger EI, 2nd, Sonner JM. Anesthetic properties of the ketone bodies beta-hydroxybutyric acid and acetone. Anesth Analg. 2007;105:673–679. doi: 10.1213/01.ane.0000278127.68312.dc. [DOI] [PubMed] [Google Scholar]
- 6.de Baulny HO, Saudubray JM. Branched-chain organic acidurias. Semin Neonatol. 2002;7:65–74. doi: 10.1053/siny.2001.0087. [DOI] [PubMed] [Google Scholar]
- 7.Budd MA, Tanaka K, Holmes LB, Efron ML, Crawford JD, Isselbacher KJ. Isovaleric acidemia. Clinical features of a new genetic defect of leucine metabolism. N Engl J Med. 1967;277:321–327. doi: 10.1056/NEJM196708172770701. [DOI] [PubMed] [Google Scholar]
- 8.Matsui SM, Mahoney MJ, Rosenberg LE. The natural history of the inherited methylmalonic acidemias. N Engl J Med. 1983;308:857–861. doi: 10.1056/NEJM198304143081501. [DOI] [PubMed] [Google Scholar]
- 9.Childs B, Nyhan W, Borden M, Bard L, Cooke R. Idiopathic hyperglycinemia and hyperglycinurea: New disorder of amino acid metabolism I. Pediatrics. 1961;27:522. [PubMed] [Google Scholar]
- 10.Hsia YE, Scully KJ, Rosenberg LE. Defective propionate carboxylation in ketotic hyperglycinaemia. Lancet. 1969;1(7598):757–758. doi: 10.1016/s0140-6736(69)91757-7. [DOI] [PubMed] [Google Scholar]
- 11.Pohorille A, Wilson MA, New MH, Chipot C. Concentrations of anesthetics across the water-membrane interface; the Meyer-Overton hypothesis revisited. Toxicol Lett. 1998;100–101:421–430. doi: 10.1016/s0378-4274(98)00216-1. [DOI] [PubMed] [Google Scholar]
- 12.Chipot C, Wilson MA, Pohorille A. Interactions of anesthetics with the water-hexane interface. A molecular dynamics study. J Phys Chem B Condens Matter Mater Surf Interfaces Biophys. 1997;101:782–791. doi: 10.1021/jp961513o. [DOI] [PubMed] [Google Scholar]
- 13.North C, Cafiso DS. Contrasting membrane localization and behavior of halogenated cyclobutanes that follow or violate the Meyer-Overton hypothesis of general anesthetic potency. Biophys J. 1997;72:1754–1761. doi: 10.1016/S0006-3495(97)78821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang P, Yan B, Xu Y. Different distribution of fluorinated anesthetics and nonanesthetics in model membrane: a 19F NMR study. Biophys J. 1997;72:1676–1682. doi: 10.1016/S0006-3495(97)78813-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koubi L, Tarek M, Klein ML, Scharf D. Distribution of halothane in a dipalmitoylphosphatidylcholine bilayer from molecular dynamics calculations. Biophys J. 2000;78:800–811. doi: 10.1016/S0006-3495(00)76637-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koubi L, Tarek M, Bandyopadhyay S, Klein ML, Scharf D. Effects of the nonimmobilizer hexafluroethane on the model membrane dimyristoylphosphatidylcholine. Anesthesiology. 2002;97:848–855. doi: 10.1097/00000542-200210000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Cantor RS. Breaking the Meyer-Overton rule: predicted effects of varying stiffness and interfacial activity on the intrinsic potency of anesthetics. Biophys J. 2001;80:2284–2297. doi: 10.1016/S0006-3495(01)76200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cantor R. The lateral pressure profile in membranes: a physical mechanism of general anesthesia. Biochemistry. 1997;36:2339–2344. doi: 10.1021/bi9627323. [DOI] [PubMed] [Google Scholar]
- 19.Petty MC. Langmuir-Blodgett films. Cambridge, UK: Cambridge University Press; 1996. [Google Scholar]
- 20.Baoukina S, Monticelli L, Marrink SJ, Tieleman DP. Pressure-area isotherm of a lipid monolayer from molecular dynamics simulations. Langmuir. 2007;23:12617–12623. doi: 10.1021/la702286h. [DOI] [PubMed] [Google Scholar]
- 21.Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, Muller SA, Rammner B, Grater F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmuller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006;127(4):831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 22.Milutinovic PS, Zhao J, Sonner JM. Tolerance to isoflurane does not occur in developing Xenopus laevis tadpoles. Anesth Analg. 2008 doi: 10.1213/ane.0b013e31818ca33e. In Press MS TMP-07-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sive H, Grainger R, Harland R. Early Development of Xenopus laevis. Cold Spring Harbor, New York: Cold Spring Harbor Press; 2000. [Google Scholar]
- 24.Mohr JT, Gribble GW, Lin SS, Eckenhoff RG, Cantor RS. Anesthetic potency of two novel synthetic polyhydric alkanols longer than the n-alkanol cutoff: evidence for a bilayer-mediated mechanism of anesthesia? J Med Chem. 2005;48:4172–4176. doi: 10.1021/jm049459k. [DOI] [PubMed] [Google Scholar]
- 25.White SH, King GI. Molecular packing and area compressibility of lipid bilayers. Proc Natl Acad Scie USA. 1985;82:6532–6536. doi: 10.1073/pnas.82.19.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Sonner JM. The anesthetic-like effects of diverse compounds on wild-type and mutant gamma-aminobutyric acid type A and glycine receptors. Anesth Analg. 2008;106:838–845. doi: 10.1213/ane.0b013e31816095bd. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro CA, Balestro F, Grando V, Wajner M. Isovaleric acid reduces Na+, K+-ATPase activity in synaptic membranes from cerebral cortex of young rats. Cell Mol Neurobiol. 2007;27:529–540. doi: 10.1007/s10571-007-9143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuck PF, Rosa RB, Pettenuzzo LF, Sitta A, Wannmacher CM, Wyse AT, Wajner M. Inhibition of mitochondrial creatine kinase activity from rat cerebral cortex by methylmalonic acid. Neurochem Int. 2004;45(5):661–667. doi: 10.1016/j.neuint.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Pockels A. On the relative contamination of the water surface by equal quantities of different substances. Nature. 1892;46:418–419. [Google Scholar]
- 30.Langmuir I. The mechanism of the surface phenomenon of flotation. Trans Faraday Soc. 1920;15:62–74. [Google Scholar]
- 31.Blodgett KD, Langmuir I. Built-up films of barium stearate and their optical properties. Phys Rev. 1937;51:964–982. [Google Scholar]
- 32.Dean RB, Hayes KE, Neville RG. The sorption of vapors by monolayers. VII. The effect of anesthetic vapors on some monolayers of biological interest. Journal of Colloid Science. 1953;8:377–384. [Google Scholar]
- 33.Dean RB, Li F-S. The sorption of vapors by monolayers. II. Organic vapors on stearic acid monolayers. J Am Chem Soc. 1950;72:3979–3982. [Google Scholar]
- 34.Clements JA, Wilson KM. The affinity of narcotic agents for interfacial films. Proc Natl Acad Sci U S A. 1962;48:1008–1014. doi: 10.1073/pnas.48.6.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McConlogue CW, Malamud D, Vanderlick TK. Interaction of DPPC monolayers with soluble surfactants: electrostatic effects of membrane perturbants. Biochimica et Biophysica Acta. 1998;1372:124–134. doi: 10.1016/s0005-2736(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 36.Cantor R. Receptor desensitization by neurotransmitters in membranes: are neurotransmitters the endogenous anesthetics? Biochemistry. 2003;42:11891–11897. doi: 10.1021/bi034534z. [DOI] [PubMed] [Google Scholar]
- 37.Rosen MJ. Surfactants and Interfacial Phenomena. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- 38.Chipot C, Pohorille A. Conformational equilibria of terminally blocked single amino acids at the water-hexane interface. A molecular dynamics study. J Phys Chem B Condens Matter Mater Surf Interfaces Biophys. 1998;102:281–290. doi: 10.1021/jp970938n. [DOI] [PubMed] [Google Scholar]
- 39.Pico C, Pons A, Palou A. In vitro adsorption of amino acids onto isolated rat erythrocyte membranes. Int J Biochem Cell Biol. 1995;27:761–765. doi: 10.1016/1357-2725(95)00049-u. [DOI] [PubMed] [Google Scholar]
- 40.Yang L, Milutinovic PS, Brosnan RJ, Eger EI, 2nd, Sonner JM. The plasticizer di(2-ethylhexyl) phthalate modulates gamma-aminobutyric acid type A and glycine receptor function. Anesth Analg. 2007;105:393–396. doi: 10.1213/01.ane.0000267336.37735.d7. [DOI] [PubMed] [Google Scholar]
- 41.Milutinovic PS, Yang L, Cantor RS, Eger EI, 2nd, Sonner JM. Anesthetic-like modulation of a gamma-aminobutyric acid type A, strychnine-sensitive glycine, and N-methyl-d-aspartate receptors by coreleased neurotransmitters. Anesth Analg. 2007;105:386–392. doi: 10.1213/01.ane.0000267258.17197.7d. [DOI] [PubMed] [Google Scholar]


