Abstract
Hepatitis B virus (HBV), hepatitis C virus (HCV), autoimmune hepatitis (AIH), and non-alcoholic fatty liver disease (NAFLD) can induce chronic liver disease. The PD-1 inhibitory pathway assists in T cell response regulation during acute and chronic inflammation and participates in the progression of inflammatory liver disease. To examine whether PD-1 and its ligands, B7-H1 and B7-DC, are modulated during chronic necroinflammatory liver disease, we investigated expression profiles in normal patients and patients with the aforementioned conditions. Relative to liver biopsies from normal individuals, those from patients with chronic necroinflammatory liver diseases (HBV, HCV, and AIH) contain increased numbers of PD-1 expressing lymphocytes. Kupffer cells, liver sinusoidal endothelial cells (LSECs), and leukocytes express PD-1 ligands. We also detect PD-1 ligands on hepatocytes within biopsies and on isolated cells. All forms of chronic necroinflammatory liver disease examined correlate with increased B7-H1 and B7-DC expression on Kupffer cells, LSECs, and leukocytes. The degree of necroinflammation correlates with expression levels of PD-1 family members. These results demonstrate that expression of PD-1/PD-1 ligands links more directly with the degree of inflammation than with the underlying etiology of liver damage. The PD-1 pathway may assist the liver in protecting itself from immune-mediated destruction.
Keywords: HCV, HBV, AIH, NAFLD, Cytotoxic T cells, PD-1, B7-H1, B7-DC, B7-H3
PD-1, the receptor for the negative costimulatory molecules B7-H1 (PD-L1) and B7-DC (PDL2), may play a role in dampening acute and chronic liver insults. Specifically T, B, myeloid, and NKT cells express PD-1; activation correlates with increased expression levels (1-5). Ligation of PD-1 on lymphocytes inhibits their activation, proliferation, and cytokine secretion (6). Normal leukocytes, a number of soft tissues, and endothelial cells express low levels of B7-H1 and inducibly express B7-DC (1-3). Inflammatory cytokines, including IFN-gamma, up-regulate B7-H1 and B7-DC expression on a variety of epithelial cells and leukocytes (2, 7-9).
Modulation of expression levels of PD-1 and its receptors has been implicated in chronic infections, autoimmune disease, and cancer. The PD-1 signaling cascade may inhibit CD8+ T cell effector function during chronic murine viral infections (4). Additionally, PD-1 knockout mice develop autoimmune diseases (6, 8, 10-12). Involvement of the PD-1 pathway in human disease development (i.e. autoimmune diseases, cancer) has been recently reported; however, it remains unclear if PD-1 expression in human diseases results from or contributes to disease pathogenesis. PD-1 expression is altered in autoimmune diseases, including Sjogren's syndrome, Type I diabetes, multiple sclerosis, ankylosing spondylitis, and allergy. PD-1 ligand expression is seen in a variety of cancers, often correlating with worse outcome (6, 13).
To examine the pathogenic role of PD-1 pathway in the progression of chronic liver diseases, we investigated modulation of the inhibitory B7 family members in chronically inflamed livers. Specifically, HBV and HCV persistence correlate with weak, narrowly focused CD8+ T cell responses during acute infection (14). Hepatic bacterial and/or viral infections are implicated in triggering AIH (15-17). Excessive reactive oxygen species present in NAFLD livers are thought to directly damage hepatocytes leading to leukocyte activation and lymphocyte recruitment (18). All of these diseases involve T cell mediated hepatic destruction and correlate with increased risk of HCC (21, 22). Chronically damaged livers provide ample opportunity for lymphocyte modulation via PD-1 ligation. Indeed primary human hepatocytes as well as Kupffer, stellate, T, myeloid and sinusoidal endothelial cells (LSECs) express B7-H1 and B7-DC (4, 7, 19-23). At the mRNA level, chronic HCV and AIH patients have increased levels of B7-H1 and B7-DC mRNA as compared to normal livers (20). In contrast, elevated B7-H1 protein expression was detected in acute but not chronically HBV infected livers (24). The mechanism of up-regulation in these livers was not established; IFN-gamma has been implicated (20). Although peripheral CD8+ T cells in HBV and HCV patients up-regulate PD-1 expression, it remains ambiguous whether PD-1 is up-regulated on bulk or only virus-specific CD8+ T cells and if up-regulation correlates with chronicity of viral infection (25-33). Multiple studies found that blocking PD-1-B7-H1 interactions on leukocytes from HBV or HCV infected patients restores T cell function in vitro (24, 25, 28-31, 34).
In this report, the milieu in which T cells see antigen in normal and inflamed livers was examined by staining liver biopsies from chronic HBV, HCV, AIH, and NAFLD patients as well as individuals with normal liver histologies for expression of CD3, PD-1, B7-H1, B7-DC, and B7-H3. Our comparative study is unique in its focus on the location of expression of PD-1 and its ligands. The normal control group enabled us to differentiate baseline tolerogenic features of the liver from those modulated during chronic liver damage. We detected statistically significantly increased numbers of CD3+ lymphocytes in HBV, HCV, and AIH livers. A significant portion of intrahepatic lymphocytes from these patient groups expressed PD-1. LSECs, Kupffer cells, and intrahepatic leukocytes express B7-H1 and B7-DC. Hepatocytes can also express B7-H1 and B7-DC. The necroinflammatory levels associated with HBV, HCV, and AIH correlated with increased B7-H1 and B7-DC on leukocytes, Kupffer cells, and LSECs. Our early stage NAFLD patients did not demonstrate significant increases in CD3+ lymphocyte infiltrates, PD-1, or B7-H1 expression, suggesting that inflammation rather than liver damage itself leads to expression of PD family members. We examined MHC Class I expression; TCR engagement is a pre-requisite to PD-1 signaling (1, 3). MHC Class I was ubiquitously expressed on LSECs and Kupffer cells and significantly up-regulated on leukocytes and hepatocytes within HCV and AIH livers. This confirms that B7-H1 and B7-DC on parenchymal and nonparenchymal cells can signal to T cells. To distinguish hepatocyte and neighboring cell phenotypes, we paralleled these studies using primary hepatocytes and hepatoma cell lines.
Materials and Methods
Reagents
CellzDirect (Pittsboro, NC) and Apath, LLC (St. Louis, MO) provided primary hepatocytes and parental Huh 7.5 cells, respectively. IHC/ICC antibody sources were as follows: R&D Systems (Minneapolis, MN): PD-1, B7-H1 (primary hepatocytes), B7-DC, B7-H3; LifeSpan BioSciences (Seattle, WA): B7-H1 (liver biopsies); MBL (Woburn, MA): HLA-ABC; Dako (Carpinteria, CA): CD3, albumin; Jackson ImmunoResearch (West Grove, PA): anti-species specific secondary antibodies. Flow cytometry antibody sources were as follows: eBioscience (San Diego, CA): B7-H1; BD Biosciences (San Jose, CA): HLA-ABC (MHC Class I).
Patient Demographics
The UVA Institutional Review Board reviewed and approved the study. Liver biopsies were collected from 74 patients examined by the hepatology service at the University of Virginia between 2006 and 2007: chronic HBV (n=11), HCV (n=17), AIH (n=14), NAFLD (n=13), and normal (n=19) (Table 1). Study exclusion criteria included: HIV seropositivity, acute HAV, HCV antiviral therapy, acute hepatitis, cirrhosis, and current ethanol abuse. All HCV patients have genotype 1 infections. Normal patients include patients with elevated liver enzymes but lacking pathologies and liver transplant donors. Laboratory values, including liver enzymes and viral loads, were obtained from patient charts.
Table 1.
Summary of patient population.
| n | Age | ALT | AST | ALK Phos | HAI A | HAI B | HAI C | HAI D | HAI or NAS Sum | Fibrosis Score | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | 19 | 47.7 | 69.3 | 63.3 | 90.4 | 0 | 0 | 0.8 | 0.3 | 1.1 | 0 |
| HBV | 11 | 39.4 | 52.6 | 34.6 | 118.9 | 0.9 | 0 | 1.9 | 1.1 | 3.9 | 1.1 |
| HCV | 17 | 51.2 | 79.7 | 63.5 | 106.1 | 1.1 | 0 | 1.7 | 1.6 | 4.4 | 2 |
| AIH | 14 | 53 | 274 | 279 | 174.6 | 2 | 0 | 2.3 | 2.4 | 5.5 | 3.6 |
| NAFLD | 13 | 44 | 86 | 63.2 | 92.6 | NA | NA | NA | NA | 3.7 | 1.3 |
Blinded analyses of stained biopsies were performed by pathologists (JCI and MWC). Using the Ishak modified Knodell histology activity index (HAI), H&E stained slides were evaluated for necroinflammatory activity, and trichrome stained slides were evaluated for fibrosis and cirrhosis in biopsies from HCV, HBV, AIH and normal patients (35). The extent of damage in NAFLD livers was quantified using the NAFLD activity score (NAS) (36). Specific antigen expression levels were graded by quartiles, reflecting frequency and intensity of expression. The antigen expression was reported from 0-3 where 0=no expression and 3=maximal staining. The grading was set by evaluating a sub-group of the stained biopsies and determining a final scale, which incorporated antigen abundance with size and cellularity of biopsy. The scale led to excellent concordance between the pathologists. All slides stained for the same antigen were evaluated on the same date and scores were given for the various zones and indicated in the figures below.
Tissue Culture
Primary hepatocytes were cultured per CellzDirect's recommendations on Matrigel (BD, San Jose, CA) coated 6 well plates in Williams E media without phenol red (Sigma-Aldrich, St. Louis, MO) supplemented with L-glutamine, penicillin/streptomycin, hepes (Gibco BRL, Gaithersburg, MD), hydrocortisone (Sigma-Aldrich), Insulin Transferrin Selenium (ITS+; BD) at 37°C with 5% CO2. Adhesion media was additionally supplemented with 5% v/v FBS (CellGro, Mediatech, Manassas, VA). Culture media was refreshed every two days. At the end of culture cells were harvested for formalin fixation followed by agarose-assisted paraffin embedding. Huh 7.5 cells were cultured per Blight et al. (37) and analyzed using a FACS Canto (BD Biosciences). Huh 7.5 cells were cultured with/without the addition of 0.1 mg/ml recombinant human IFN-gamma for 24-48 hours (rhIFN-gamma, R&D Systems).
Immunohistochemistry (IHC) and histological evaluation
Immunohisto(cyto)chemistry was performed on paraffin embedded liver biopsies and primary hepatocyte cell pellets. Heat-induced antigen retrieval was performed utilizing pH 6.0 citrate buffers. Non-specific protein and avidin/biotin activity were blocked, and slides were incubated with/without primary antibodies at 4°C overnight. Following reduction of endogenous peroxidase activity, all sections were incubated with secondary antibodies. Antibodies were detected using the Vector ABC and NovaRed reagents (Vector Laboratories, Burlingame, CA). Sections were counterstained with hematoxylin. Slides stained with secondary Ab only or isotype and secondary Ab served as staining controls. Further, the specificity of B7-H1 was confirmed using rB7-H1 protein, which resulted in a concentration dependent decrease in staining intensity (data not shown).
Statistical Analyses
Minitab (State College, PA) and GraphPad Prism (La Jolla, CA) were used for statistical analyses. Antigen expression on biopsies was graded on a 0-3 scale and binned to meet criteria of Fisher's exact test, which was used to determine significance of variation in expression patterns between normal and chronically damaged livers at the 95% confidence level. Pearson correlation coefficients were calculated.
Results
Chronically Inflamed Livers Have Higher Frequencies of PD-1 Expressing Lymphocytes
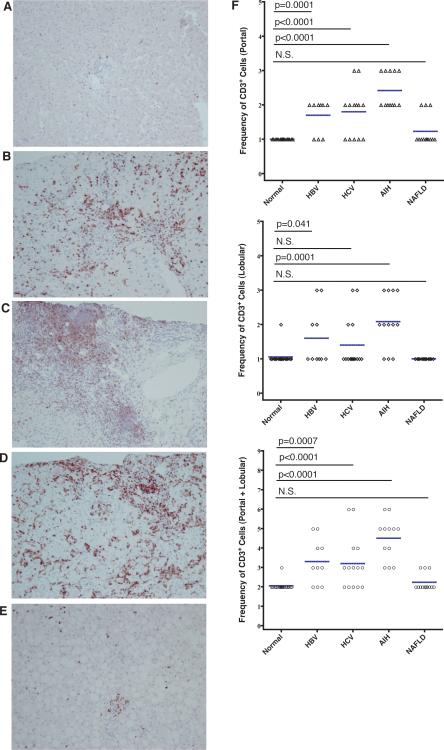
PD-1 ligation sends an inhibitory signal to T cells, limiting their effector functions. Effector T cells contribute to chronic necroinflammatory liver damage. We sought to determine how PD-1 expression on lymphocytes within the generally tolerant liver environment varies in and among chronic liver diseases. Serially sectioned liver biopsies were examined for CD3 and PD-1 expression by IHC. CD3 expressing lymphocytes are seen in portal/periportal and lobular regions (Figure 1A). In normal liver biopsies, a minimal number of CD3+ cells are admixed with other leukocytes in the portal/periportal and lobular areas. Our data indicate that patients with chronic necroinflammatory liver disease from HBV, HCV, and AIH (Figures 1B-D, respectively) have increased numbers of T cells in the portal/periportal region and large leukocyte aggregates in the lobular region, as compared to normal donors (Figure 1A). The large periportal aggregates are particularly pronounced in biopsies from HCV and AIH patients demonstrating lymphocytes extravasating from the peripheral blood and homing to inflamed sections of the liver.
Figure 1. HBV, HCV, and AIH induce lymphocyte homing to the liver.
Biopsies from patients with normal histology (A), HBV (B), HCV (C), AIH (D), and NAFLD (E) were stained with mouse anti-human CD3. Representative photomicrographs are displayed with an original magnification of 200x. The relative number of CD3+ T cells present in portal/periportal (P) and lobular regions (L) were tabulated on a quartile (0-3) scale. Overall liver scores (P+L) were obtained by summing portal and lobular scores. Individual scores and mean (horizontal line) scores in the portal/periportal and lobular regions are shown for each patient group. P-values for the Fisher's Exact test are listed above the graphs including N.S. (non-significant).
As shown in Figure 2A-E, leukocytes in the portal and lobular regions express cytoplasmic and membranous PD-1. Intrahepatic lymphocytes in HBV, HCV, and AIH inflamed livers up-regulate PD-1 expression, as compared to PD-1 expression by leukocytes in normal livers (Figure 2F). Chronically inflamed livers contain a higher number and percentage of leukocytes that express PD-1. Notably, PD-1 expression in chronic patients, compared to control patients, reaches significance in the portal region for HBV, HCV and AIH patients and HBV and AIH patients in the lobular region. The increased PD-1 expression does not reach statistical significance for HCV patients in the lobular region, correlating with a lack of significant infiltration of CD3+ lymphocytes in this region. The increased frequency of PD-1+ leukocytes suggests that chronically inflamed livers contain large numbers of leukocytes lacking the potential to stimulate direct cytolytic immune responses. However, the increased number of leukocytes can lead to secondary hepatic parenchymal destruction. Both hepatic and systemic inflammation are thought to induce lymphocyte homing to the liver and PD-1 expression. As such, we compared the trends in CD3 and PD-1+ leukocyte trafficking to the liver, which demonstrated a positive correlation (R2=0.62).
Figure 2. PD-1 Expression on intrahepatic leukocytes increases with inflammation.
Biopsies from patients with normal histology (A), HBV (B), HCV (C), AIH (D), and NAFLD (E) were stained with goat anti-human PD-1. Representative photomicrographs are displayed with an original magnification of 200x. The relative number of PD-1+ cells present in portal/periportal (P) and lobular regions (L) were tabulated on a quartile (0-3) scale. Leukocytes in both regions express PD-1. PD-1 is expressed on leukocytes within larger leukocyte aggregates. Individual scores and mean (horizontal line) scores in the portal/periportal and lobular regions are shown for each patient group. P values for the Fisher's Exact test are listed above the graphs. All pair wise comparisons of PD-1 expression levels are statistically significant except HCV v. NL in the lobular region and NAFLD v. NL in all regions.
Hepatocytes and Leukocytes up-regulate MHC Class I in livers experiencing chronic damage
We examined the intrahepatic expression pattern of MHC Class I, as effector T cell functions and PD-1 signaling require MHC Class I engagement by the TCR. Further, intrahepatic viruses and cytokines (ie, type I and II interferons) potentially modulate MHC Class I expression levels (38). As shown in Figure 3A-E, hepatocytes, leukocytes, Kupffer cells, and LSECs throughout the liver express HLA-ABC. Kupffer cells and LSECs express high levels of HLA-ABC, but these levels do not appear to be altered by chronic liver damage or inflammation. We detect strong surface expression of HLA-ABC on hepatocytes within biopsies from chronically damaged livers, on cultured cells, and on activated Huh 7.5 cells (Figure 3B-D, 6, supplemental figure 2; respectively). Hepatocytes and leukocytes in chronically damaged livers demonstrate increases in HLA-ABC expression (Figure 3F). These increases are statistically significant for HCV and AIH patients on hepatocytes and leukocytes, as compared to normal patients. Expression of MHC Class I correlates with high degrees of liver damage, as determined by HAI, as well large lobular leukocyte infiltrates (Figure 7C). The sensitivity of hepatocyte expression of MHC Class I to inflammatory stimuli was confirmed in vitro by rhIFN-gamma stimulation of primary hepatocytes and hepatoma cell lines (Figure 6).
Figure 3. Chronically damaged livers up-regulate MHC Class I expression on hepatocytes and leukocytes.
Biopsies from patients with normal histology (A), HBV (B), HCV (C), AIH (D), and NAFLD (E) were stained with mouse anti-human HLA-ABC (MHC Class I). Representative photomicrographs are displayed with an original magnification of 200x. Hepatocytes, leukocytes, LSECs, and Kupffer cells express HLA-ABC (MHC Class I) as determined by cellular morphology. F) The intensity and frequency of HLA-ABC expression on hepatocytes, leukocytes, LSECs and Kupffer cells, and composite scores are shown. P values for the Fisher's Exact test are listed above the graphs.
Figure 6. Determination of Class I MHC and PD-1 ligand expression in primary hepatocytes.
Primary hepatocytes were examined for HLA-ABC, B7-H1, and B7-DC expression by ICC following 8 days of culture on Matrigel as monolayers with or without the addition of rhIFN-gamma during the last 48 hours of culture.
Figure 7. B7 ligand expression increases with chronic inflammation.
Summed HAI (histology activity index) positively correlates with A) CD3, B) PD-1, C) MHC Class I, D) B7-H1, and E) B7-DC expression, but not with F) B7-H3 expression. Composite antigen expression was compared with the HAI score and the r-squared value is listed. The expression of CD3, PD-1, B7-H1, and B7-DC all correlate with HAI at a p value of <0.001. NAFLD livers were excluded from this analysis, as HAI does not adequately reflect liver damage in these patients.
B7-H1 and B7-DC are expressed in the Liver and Modulated by Chronic Inflammation
Up-regulation of PD-1 ligands in the liver would inhibit the function of PD-1 expressing lymphocytes, preventing them from mounting antiviral responses and further modulating their function. T cells displaying activated phenotypes as well as the abundant NK and NKT cell populations in the liver may secrete inflammatory cytokines that can induce B7-H1 and B7-DC expression. As shown in Figure 4A-E, Kupffer cells, LSECs, and leukocytes express B7-H1 on their surfaces. Cell morphology and previous research suggest dendritic cells and lymphocytes comprise liver leukocyte populations expressing B7-H1. Livers experiencing chronic necroinflammatory disease have significantly higher B7-H1 expression levels in the portal region on leukocytes, Kupffer cells, and LSECs, compared to patients with normal liver histologies (Figure 4). B7-H1 up-regulation only reaches statistical significance in the lobular region on these cell types in AIH patients. The more dramatic increase in B7-H1 expression in the portal area parallels this being the site of initial T cell infiltration in the liver and the development of large lymphoid aggregates. Interestingly, increased expression levels primarily result from an increase in the percentage of Kupffer cells and LSECs that express B7-H1, rather than an increase in expression levels by individual cells. In contrast, increased expression of B7-H1 by intrahepatic leukocytes results from increases in both frequency of expression and population size.
Figure 4. Chronically damaged livers have increased expression of B7-H1 on Kupffer cells, LSECs, and leukocytes.
Biopsies from patients with normal histology (A), HBV (B), HCV (C), AIH (D), and NAFLD (E) were stained with rabbit anti-human B7-H1. Antibody specificity was confirmed by reabsorbing the antibody with recombinant B7-H1 (not shown). Representative photomicrographs are displayed with an original magnification of 200x. (F) B7-H1 expression was scored on Kupffer cells, LSECs, and leukocytes in portal/periportal and lobular regions. Increases are statistically significant for LSECs, Kupffer cells, and leukocytes within livers affected by all conditions in the portal region, AIH in the lobular region, and HBV, HCV, and AIH in the overall liver. P values for the Fisher's Exact test are listed above the graphs.
Like B7-H1, B7-DC is expressed on cell membranes of Kupffer cells, LSECs, and leukocytes (Figure 5). This provides yet another source of ligation for PD-1, potentially contributing to down-regulation of intrahepatic immune response. B7-DC is significantly up-regulated throughout the liver of HCV and AIH patients, compared to expression levels in individuals with normal liver histologies (Figure 5). In contrast, there is only a small, statistically insignificant increase in B7-DC expression in chronic HBV and NAFLD livers, as compared to normal livers. In addition, the biliary epithelium expresses B7-DC, as supported by previous reports (22). The increased expression of B7-H1 and B7-DC mRNA was confirmed by qRT-PCR analysis on liver tissues (Supplemental Figure 1).
Figure 5. B7-DC is up-regulated on Kupffer cells, LSECs, and leukocytes in HBV, HCV, and AIH livers.
Representative biopsies from patients with normal histology (A), HBV (B), HCV (C), AIH (D), and NAFLD (E) were stained with goat anti-human B7-DC. Representative photomicrographs are displayed with an original magnification of 200x. (F) B7-DC expression scores (combined measures of intensity and frequency of expression) are shown for portal and lobular regions. These scores reflect expression on Kupffer cells, LSECs, and leukocytes. P values for the Fisher's Exact test are listed above the graphs.
Hepatocytes can Express MHC Class I, B7-H1, and B7-DC
We were particularly interested in hepatocyte expression of MHC Class I, B7-H1, and B7-DC as hepatocytes are the primary site of infection for HBV and HCV, damaged by NAFLD, and recipients of the mis-directed immune responses that produce AIH. Confirming biopsy-staining patterns, cultured hepatocytes express low levels of cytoplasmic and membrane bound B7-H1, which IFN-gamma can up-regulate (Figure 6A, Supplemental Figure 2). Hepatocyte-expressed B7-H1 can potentially signal to PD-1 on T cells, as all three types of hepatocytes express MHC Class I constitutively and inducibly (Figure 6). Hepatocyte expression of B7-DC is noted occasionally in biopsies from patients with chronic liver diseases, as shown in Figure 5 (not quantified), particularly in the livers from HCV and AIH patients. Primary, cultured hepatocytes express B7-DC basally and strongly up-regulate expression following IFN-gamma stimulation; however, Huh 7.5 and HepG2 cell lines do not express B7-DC constitutively or following IFN-gamma stimulation (data not shown). The physiologic relevance and viability of primary hepatocytes was confirmed by staining for albumin.
Liver Damage Correlates with Increased PD-1, B7-H1, and B7-DC Expression
Induction of PD family members in the context of either acute or chronic inflammation is thought to provide the immune system a means of negative-regulation. The biopsies provided an opportunity to examine the effects of chronic liver inflammation on expression levels. We compared liver damage, as measured by HAI, to CD3, PD-1, MHC Class I, B7-H1, and B7-DC expression frequencies/intensities in the entire liver (combined portal/periportal and lobular scores). Frequency of lymphocytes and HAI are established measures of liver inflammation and liver damage, respectively. ALT, a less sensitive and specific measure of hepatocyte damage was also compared to marker expression profiles. HAI scores, but not ALT levels, significantly, positively correlate with CD3, PD-1, B7-H1, and B7-DC expression (Figure 7). Importantly, B7-H3 expression, a molecule related to B7-H1 and B7-DC, did not show a correlation with HAI. This suggests that the association of PD-1, B7-H1 and B7-DC with HAI is specific, rather than an overall increase in this family of proteins.
Discussion
The liver is an immune tolerant organ; despite exposure to large concentrations of food antigens and microbes, inflammatory responses are infrequently generated to antigens that first encounter the immune system in the liver. This anergic response is protective in the case of harmless food antigens, yet potentially detrimental with respect to hepatic infections. Failure to generate robust CD8+ T cell mediated immune responses during acute infection by HBV or HCV contributes to the development of persistent infections. Introduction to the immune system via the liver does not guarantee sustained immune privilege as the pathogenicity of HBV and HCV result from delayed host immune responses. While sufficient to induce hepatocyte cell death, these responses fail to clear the infections. Our work sought to understand how the liver balances immunogenic and amnestic responses.
In this report, we demonstrated that chronic liver disease induced by HBV, HCV, AIH but not NAFLD enhance expression of PD-1 and its ligands. Our study included patients with chronic hepatitis resulting from a variety of causes, as well as normal controls. This enabled differentiating steady state liver immune function from that modulated during chronic liver disease. HBV, HCV, and AIH livers contain significant increases in CD3+ and PD-1+ lymphocytes, compared to normal livers. Notably, chronic liver inflammation due to HBV, HCV, and AIH strongly, positively correlates with inhibitory B7 family expression. These findings lead us to speculate that ligation of PD-1 potentially provides the already inflamed liver means to dampen the immune response. PD-1 ligand expression by Kupffer cells, LSECs, and leukocytes may modulate leukocyte activity; these cell types contact PD-1 expressing lymphocytes as well as express MHC Class I. Additionally, hepatocytes, which also express MHC Class I and B7-H1 could limit CD8+ T cell effector functions. The finding that biopsies from AIH patients express B7-H1 and B7-DC also supports the idea that the underlying mechanism of hepatic damage during AIH is from highly active leukocytes and not from some decreased expression or inability to express anti-inflammatory proteins. However, we need to identify AIH patients (prior to becoming symptomatic) to properly evaluate this theory.
It is important to stress that CD8+ T cells play a pivotal role in initiating immune-mediated liver disease. CD8+ T cells induce hepatic damage in HBV, HCV, AIH, and NAFLD. Hepatocyte death in necroinflammatory liver diseases largely results from CD8+ T cell mediated killing. In addition, CD8+ T cells modulate the progression of liver disease by inducing B7-H1 expression (7). Our data support a model in which PD-1 up-regulation on virus specific CD8+ T cells inhibits IFN-gamma release by a small subset of antigen specific T cells during acute infection. This potentially contributes to impaired acute viral clearance. There is, however, a tipping point at which the virus has not been cleared, but a weak CD8+ T cell response develops, resulting in liver inflammation. This inflammation, as well as gut antigens, may induce NK and CD8+ T cell release of IFN-gamma, further contributing to chronic liver inflammation. IFN-gamma simultaneously induces expression of the B7 inhibitory ligands in an antigen-independent manner. This provides a feedback mechanism to control the immune response. Over-expression of B7 family members potentially provides both beneficial and injurious effects on hosts of liver pathogens. It could halt the path that leads from fibrosis to cirrhosis to HCC, by inhibiting cycles of cell death and proliferation that provide material for oncogenic transformation. On the other hand, dampening the immune response impairs immune surveillance and provides the opportunity for chronic infections and cancer. Correlating disease progression with expression levels of PD-1 family members may provide insight into which of these mechanisms occurs in vivo. As such, PD-1-PD-1 ligand expression levels potentially serve as markers of disease progression. This contrasts with the lack of correlation between HBV/HCV viral loads and liver damage. Finally, PD-1 family member expression profiles may serve as predictors of response to currently available treatments. In the case of AIH, longitudinal studies would assist in determining if up-regulation is a cause or result of disease flares; impacts of corticosteroid therapy could also be ascertained.
In summary expression of PD-1 as well as its ligands increase during chronic liver diseases caused by HBV, HCV, and AIH but not by NAFLD. Immune inhibition via the PD-1 inhibitory pathway during the chronic phases of HBV, HCV, and AIH may be protective by limiting excessive inflammatory responses in the liver. These inflammatory responses, while perhaps aiming to protect the liver from infectious insults and regulate aberrant cell division, often mediate necroinflammatory liver damage. The optimal immune response in chronically infected livers may not be maximal effector activation; rather, dampening the immune response decreases both liver damage and cancer potential. Our studies suggest means to modulate chronic liver disease by harnessing the PD-1 pathway. PD-1 immunotherapy recently entered human clinical trials. In a stage 1 clinical trial anti-PD-1 monoclonal antibody therapy demonstrated a lack of significant side effects (39). Such therapy could improve clearance of HBV and HCV and regulate autoimmune responses in AIH. At the same time, our study suggests that blocking PD-1 may be detrimental during chronic inflammation. Rather, PD-1 agonists may prove beneficial.
Supplementary Material
Acknowledgments
Caroline Hall and Christine Rudy assisted with culture of hepatocytes. Caroline also assisted with manuscript preparation. Computer support provided by Daniel Williams and John Tran.
Grant Support:
NIH Grants (DK066754, U19AI066328 to YSH) and Training Fellowships (MSTP 5-T32-GM007267, Infectious Disease 5-T32-AI007046 to RK)
References
- 1.Bennett F, Luxenberg D, Ling V, Wang IM, Marquette K, Lowe D, Khan N, et al. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J Immunol. 2003;170:711–718. doi: 10.4049/jimmunol.170.2.711. [DOI] [PubMed] [Google Scholar]
- 2.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 3.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 4.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 5.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 7.Muhlbauer M, Fleck M, Schutz C, Weiss T, Froh M, Blank C, Scholmerich J, et al. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J Hepatol. 2006;45:520–528. doi: 10.1016/j.jhep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Chen L. Co-signaling molecules of the B7-CD28 family in positive and negative regulation of T lymphocyte responses. Microbes Infect. 2004;6:759–766. doi: 10.1016/j.micinf.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int Immunol. 1998;10:1563–1572. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 13.Martin-Orozco N, Wang YH, Yagita H, Dong C. Cutting Edge: Programmed death (PD) ligand-1/PD-1 interaction is required for CD8+ T cell tolerance to tissue antigens. J Immunol. 2006;177:8291–8295. doi: 10.4049/jimmunol.177.12.8291. [DOI] [PubMed] [Google Scholar]
- 14.Rehermann B. Chronic infections with hepatotropic viruses: mechanisms of impairment of cellular immune responses. Semin Liver Dis. 2007;27:152–160. doi: 10.1055/s-2007-979468. [DOI] [PubMed] [Google Scholar]
- 15.Vergani D, Mieli-Vergani G. The impact of autoimmunity on hepatocytes. Semin Liver Dis. 2007;27:140–151. doi: 10.1055/s-2007-979467. [DOI] [PubMed] [Google Scholar]
- 16.Washington MK. Autoimmune liver disease: overlap and outliers. Mod Pathol. 2007;20(Suppl 1):S15–30. doi: 10.1038/modpathol.3800684. [DOI] [PubMed] [Google Scholar]
- 17.Czaja AJ. Autoimmune liver disease. Curr Opin Gastroenterol. 2008;24:298–305. doi: 10.1097/MOG.0b013e3282f57268. [DOI] [PubMed] [Google Scholar]
- 18.Lalor PF, Faint J, Aarbodem Y, Hubscher SG, Adams DH. The role of cytokines and chemokines in the development of steatohepatitis. Semin Liver Dis. 2007;27:173–193. doi: 10.1055/s-2007-979470. [DOI] [PubMed] [Google Scholar]
- 19.Gehring AJ, Sun D, Kennedy PT, Nolte-'t Hoen E, Lim SG, Wasser S, Selden C, et al. The level of viral antigen presented by hepatocytes influences CD8 T-cell function. J Virol. 2007;81:2940–2949. doi: 10.1128/JVI.02415-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mataki N, Kikuchi K, Kawai T, Higashiyama M, Okada Y, Kurihara C, Hokari R, et al. Expression of PD-1, PD-L1, and PD-L2 in the liver in autoimmune liver diseases. Am J Gastroenterol. 2007;102:302–312. doi: 10.1111/j.1572-0241.2006.00948.x. [DOI] [PubMed] [Google Scholar]
- 21.Muhlbauer M, Ringel S, Hartmann A, Lallinger G, Weiss TS, Gabele E, Wunsch PH, et al. Lack of association between the functional CX3CR1 polymorphism V249I and hepatocellular carcinoma. Oncol Rep. 2005;13:957–963. [PubMed] [Google Scholar]
- 22.Oikawa T, Takahashi H, Ishikawa T, Hokari A, Otsuki N, Azuma M, Zeniya M, et al. Intrahepatic expression of the co-stimulatory molecules programmed death-1, and its ligands in autoimmune liver disease. Pathol Int. 2007;57:485–492. doi: 10.1111/j.1440-1827.2007.02129.x. [DOI] [PubMed] [Google Scholar]
- 23.Yu MC, Chen CH, Liang X, Wang L, Gandhi CR, Fung JJ, Lu L, et al. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40:1312–1321. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Zhang JY, Wherry EJ, Jin B, Xu B, Zou ZS, Zhang SY, et al. Dynamic Programmed Death 1 Expression by Virus-Specific CD8 T Cells Correlates With the Outcome of Acute Hepatitis B. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 25.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang JY, Zhang Z, Wang X, Fu JL, Yao J, Jiao Y, Chen L, et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109:4671–4678. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- 27.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–9258. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasprowicz V, Schulze Zur Wiesch J, Kuntzen T, Nolan BE, Longworth S, Berical A, Blum J, et al. High PD-1 expression on HCV-specific CD8+ and CD4+ T cells during acute Hepatitis C irrespective of clinical outcome. J Virol. 2007 doi: 10.1128/JVI.02474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol. 2008;45:963–970. doi: 10.1016/j.molimm.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 32.Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, Shaked A, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927–1937. 1937, e1921–1922. doi: 10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rutebemberwa A, Ray SC, Astemborski J, Levine J, Liu L, Dowd KA, Clute S, et al. High-programmed death-1 levels on hepatitis C virus-specific T cells during acute infection are associated with viral persistence and require preservation of cognate antigen during chronic infection. J Immunol. 2008;181:8215–8225. doi: 10.4049/jimmunol.181.12.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeong HY, Lee YJ, Seo SK, Lee SW, Park SJ, Lee JN, Sohn HS, et al. Blocking of monocyte-associated B7-H1 (CD274) enhances HCV-specific T cell immunity in chronic hepatitis C infection. J Leukoc Biol. 2008;83:755–764. doi: 10.1189/jlb.0307168. [DOI] [PubMed] [Google Scholar]
- 35.Brunt EM. Grading and Staging the Histopathological Lesions of Chronic Hepatitis: The Knodell Histology Activity Index and Beyond. Hepatology. 2000;31:241–246. doi: 10.1002/hep.510310136. [DOI] [PubMed] [Google Scholar]
- 36.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 37.Blight KJ, McKeating JA, Marcotrigiano J, Rice CM. Efficient replication of hepatitis C virus genotype 1a RNAs in cell culture. J Virol. 2003;77:3181–3190. doi: 10.1128/JVI.77.5.3181-3190.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herzer K, Falk CS, Encke J, Eichhorst ST, Ulsenheimer A, Seliger B, Krammer PH. Upregulation of major histocompatibility complex class I on liver cells by hepatitis C virus core protein via p53 and TAP1 impairs natural killer cell cytotoxicity. J Virol. 2003;77:8299–8309. doi: 10.1128/JVI.77.15.8299-8309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, Koren-Michowitz M, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.