Abstract
Tuberculosis (TB) remains a threat to global health. While advances in diagnostics and treatment are crucial to the containment of the epidemic, it is likely that elimination of the disease can only be achieved through vaccination. Vaccine-induced protection from Mycobacterium tuberculosis is dependent, at least in part, on a robust Th1 response, yet little is known of the ability of TB vaccines to induce other T-cell subsets which may influence vaccine efficacy. Interleukin-17A (IL-17A) is a proinflammatory cytokine produced by Th17 cells which has been associated with both immune pathology and protection against infectious disease. Following vaccination with MVA85A, a viral vector vaccine aimed at enhancing immune responses to M. tuberculosis, antigen-specific IL-17A-producing T cells were induced in the peripheral blood of healthy volunteers. These T cells are detected later than gamma interferon (IFN-γ)-secreting T cells and are of a low magnitude. Preexisting immune responses to mycobacterial antigens were associated with higher CD4+ CD25hi CD39+ T-cell levels in the periphery and a reduced capacity to produce IL-17A following immunization. These data highlight the intricate balance of effector and regulatory immune responses induced by vaccination and that preexisting immunity to mycobacterial antigens may affect the composition of vaccine-induced T-cell subsets.
Tuberculosis (TB) remains a global health problem due to the emergence of drug-resistant strains of Mycobacterium tuberculosis, HIV-TB coinfection, and failure of the BCG vaccine to control adult pulmonary TB (9, 13). There is evidence that protection from M. tuberculosis is, at least in part, dependent on a robust Th1 response and the secretion of gamma interferon (IFN-γ) by antigen-specific T cells (1, 15, 22). Although IFN-γ alone is not sufficient for protection, in the absence of a better biomarker, early clinical trials of new TB vaccines use antigen-specific IFN-γ production as the primary gauge of vaccine-induced immune responses (16). In early clinical trials, modified vaccinia virus Ankara expressing antigen 85A from M. tuberculosis (MVA85A), a subunit vaccine designed to increase immune protection conferred by BCG, has been found to induce high levels of antigen-specific IFN-γ-secreting CD4+ T cells in individuals previously vaccinated with BCG (28, 29, 31).
CD4+ T cells can differentiate into diverse effector cell subsets upon antigenic stimulation and the classical Th1/Th2 paradigm has now been expanded to include Th17 and T-regulatory (Treg) cells. Th17 cells are potent inflammatory cells which produce interleukin-17A (IL-17A) as their hallmark cytokine (17, 30). Th17 cells are mainly known for their role in mediating autoimmune pathology (19, 35) but are also thought to be involved in mediating protection against certain extracellular pathogens and fungi which are not effectively cleared by Th1- and Th2-type responses (12, 20). In contrast to Th17 cells, Treg cells comprise a regulatory cell subset of CD4+ T cells which act to suppress T-cell responses and are thereby thought to prevent pathology from chronic or excessive immune responses (21, 32, 33).
Although a role has been defined for Th17 cells and Treg cells in other diseases, their role in TB remains unclear and even less is known about the effect of vaccination on these T-cell subsets.
Clinical trials evaluating the safety and immunogenicity of MVA85A in BCG-vaccinated adults provide an opportunity to further investigate the induction and dynamics of vaccine-induced Th17 cells and Treg cells. Determining the effect of vaccination with MVA85A on these T-cell subsets is important, as protection from TB is likely to be dependent not only upon a Th1 response but also upon the balance between this effector response and the Th17 and Treg responses.
MATERIALS AND METHODS
Vaccine study participants.
Subjects were recruited for clinical trials approved by the Medicines and Healthcare products Regulatory Agency and the Gene Therapy Advisory Committee. All of the trials were registered on a clinical trials database (ClinicalTrials.gov ID NCT00465465, NCT00456183, NCT00427830, and NCT00653770). Samples from four clinical trials were investigated in this study, and the dose of MVA85A administered in each of these is shown in Table 1. All participants in the high-, medium-, and low-dose trials were BCG-vaccinated adults (maximum Heaf test reaction grade II, negative by ex vivo enzyme-linked immunospot [ELISPOT] assay for ESAT-6 and CFP-10) 18 to 50 years of age who tested seronegative for HIV, as well as hepatitis B and C viruses (5, 29). Participants in the cohort latently infected with M. tuberculosis were healthy adults, most of whom had been BCG vaccinated (Heaf test grade II-IV, positive on ex vivo ELISPOT for ESAT-6 and CFP-10), between 18 to 50 years of age who tested seronegative for HIV as well as hepatitis B and C viruses (34). Eight subjects were selected from each trial. Samples were investigated at week 0 (the day of MVA85A vaccination) and at 1, 2, 4, and 24 weeks after vaccination for the high-dose group. Samples from week 0 (day of MVA85A vaccination) and week 4 were investigated for all of the other trials.
TABLE 1.
Doses of MVA85A received by subjects in the trials investigated in this study
| Trial | Dose (PFU) of MVA85A |
|---|---|
| High dose | 1 × 108 |
| Medium dose | 5 × 107 |
| Low dose | 1 × 107 |
| Latently infected cohort | 5 × 107 |
PBMC preparation.
Peripheral blood mononuclear cells (PBMCs) obtained from vaccinated subjects were frozen in freezing mix (50% fetal bovine serum [FBS; BioSera Ltd.], 40% RPMI 1640 with 10% dimethyl sulfoxide [both from Sigma-Aldrich]) at the time of sample acquisition, in aliquots containing approximately 5 × 106 PBMC/ml. Samples selected for investigation were removed from long-term storage in liquid nitrogen and placed on dry ice in preparation for thawing. Vials were flash thawed at 37°C and then thawed into 9 ml of R10 medium (RPMI 1640 from Sigma-Aldrich, 10% FBS [Biosera Ltd.], 2 mM l-glutamine; 100 U/ml penicillin, 100 μg/ml streptomycin sulfate, 1.7 mM sodium glutamate [all from Invitrogen]). After transfer into R10 medium, cells were washed twice and left to rest with Benzonase (Novagen) for 2 h at 37°C in 5% CO2. Cells were washed and resuspended at approximately 1 × 106 PBMCs/ml and counted in duplicate using a CASY cell counter (Schärfe System GmbH).
Ex vivo IL-17A ELISPOT assay.
The ex vivo IL-17A ELISPOT assay (eBioscience) was performed with PBMCs according to the manufacturer's protocol. Two hundred fifty thousand PBMCs per well in 100 μl R10 medium were plated onto precoated ELISPOT assay plates (Millipore UK Ltd.) in the presence of antigen. The cells were stimulated with recombinant antigen 85A (r85A; 10 μg/ml; Leiden University), purified protein derivative (PPD; 20 μg/ml; Statens Serum Institute), and the total peptide pool from antigen 85A (one pool of 66 15-mer peptides overlapping by 10 amino acids; 2-μg/ml final concentration of each peptide per ELISPOT assay well [29]) for 18 h at 37°C in 5% CO2. PMA (phorbol 12-myristate 13-acetate; 1 μg/ml; Sigma-Aldrich) and ionomycin (0.01 μg/ml; Sigma-Aldrich) were used in combination as a positive control. Cells and medium alone were used as a negative control to assess background levels of IL-17A secretion. Assays were developed using color-developing agents (Bio-Rad AP conjugate substrate kit, Bio-Rad AP developing buffer), and ELISPOT assay plates were counted using the AID plate reader software (AID; Cadama Medical). Assays were performed in duplicate, and the results from duplicate wells were averaged. The ELISPOT assay data were analyzed by subtracting the mean number of spots in the negative-control wells from the mean number of spots in the antigen and peptide pool wells. Responses at least double the background were considered positive.
Intracellular cytokine staining (ICS) assay for IL-17A.
To detect IL-17A-producing cells, PBMCs from subjects in the high-dose group were stimulated with PMA (1 μg/ml) and ionomycin (0.01 μg/ml) or r85A (10 μg/ml) in the presence of anti-CD28 (0.5 μg/ml) and anti-CD49d (0.5 μg/ml) antibodies (both from BD Biosciences) and incubated at 37°C in 5% CO2. After 6 h, brefeldin A (10 μg/ml; Sigma-Aldrich) was added to the stimulated cells. Following stimulation, cells were washed and stained with vivid LIVE/DEAD (Invitrogen), CD14-Pacific blue (PB) (Invitrogen), CD19-PB, and CD4-allophycocyanin (APC) (both from eBioscience). After washing, the cells were permeabilized with fixation/permeabilization buffer (BD Biosciences) and stained intracellularly with CD3 phycoerythrin (PE)-Cy5, IFN-γ-fluorescein isothiocyanate (FITC), IL-17A-PE (all from eBioscience), and CD8-APC-AlexaFlour750 (Invitrogen). Cells were acquired on an LSR II (BD Biosciences) using FACSDiva software (BD Biosciences) and analyzed using FlowJo, Version 8.3 (Tree Star, Inc.). Unstained cells and singly stained anti-mouse Ig/κ compensation beads (BD Biosciences) were used as controls and to automatically calculate compensations. All antibodies were titrated for optimal staining.
Treg cell detection.
Treg cells were detected using a human regulatory T-cell kit (eBioscience). Cells were stained with vivid LIVE/DEAD, CD19-PB (eBioscience), CD14-PB (Invitrogen), CD4-APC, CD25-FITC, and CD39-PE (all from eBioscience). After washing, cells were permeabilized with fixation/permeabilization buffer and stained intracellularly with FoxP3 AF700. Cells were resuspended in fluorescence-activated cell sorter (FACS) buffer for sample acquisition. Cells were acquired on an LSR II, and the data were analyzed using FlowJo. Unstained cells and singly stained anti-mouse Ig/κ compensation beads (BD Biosciences) were used as controls and to automatically calculate compensations. All antibodies were titrated for optimal staining.
Statistical analyses.
Wilcoxon signed-rank tests, Mann-Whitney tests, Kruskal-Wallis tests, and Spearman rank correlations were performed using SPSS statistical software (SPSS, Inc.).
RESULTS
Peak IL17A secretion occurs later than the peak IFN-γ response in individuals receiving a high dose of MVA85A.
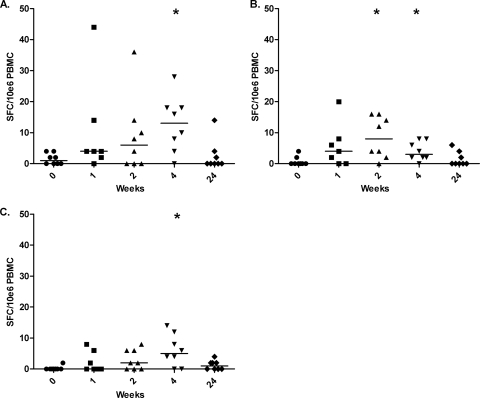
The kinetics of IL-17A secretion were investigated in BCG-vaccinated individuals receiving a high dose of 1 × 108 PFU of MVA85A (5). Low levels of antigen-specific IL-17A secretion were detectable in all of the subjects at all of the time points investigated. IL-17A secretion in response to r85A and PPD was found to peak at 4 weeks postvaccination, with levels of IL-17A being significantly higher than the levels observed on the day of vaccination (r85A, P = 0.016; PPD, P = 0.031) (Fig. 1 A and C). IL-17A secretion in response to the peptide pool of antigen 85A peaked at week 2 and was significantly higher than the baseline response at both week 2 (P = 0.016) and week 4 (P = 0.031) postvaccination. The kinetics and magnitude of the IL-17A response differed from those of the IFN-γ response seen with this vaccine. Antigen 85A-specific IFN-γ responses were found to peak 1 week after vaccination and were a median of 1,645 spot-forming cells (SFC)/million at this time point, approximately 100-fold higher than the peak IL-17A response seen in our study in response to r85A following the same dose of MVA85A (5). The highest antigen-specific IL-17A responses observed were to r85A and the peptide pool of antigen 85A, which is not surprising, as antigen 85A is the antigen in MVA85A.
FIG. 1.
Antigen-specific IL-17A is significantly increased following vaccination with MVA85A. Shown are responses to r85A (A), responses to the total peptide pool of antigen 85A (B), and responses to PPD (C). IL-17A ELISPOT assay responses observed across all of the subjects over the time course investigated are expressed as numbers of SFC per 1 million PBMCs. The median is represented by a solid line. *, P < 0.05 versus week 0 (Wilcoxon signed-rank test).
The dose of MVA85A affects the induction of antigen-specific IL-17A-secreting T cells.
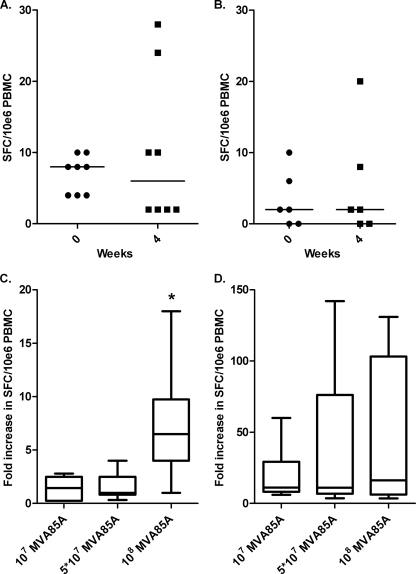
As IL-17A responses peaked at week 4 in the high-dose cohort, samples collected at weeks 0 and 4 from subjects in other clinical trials with MVA85A were investigated. These subjects were BCG immunized and received either a low dose of MVA85A (1 × 107 PFU) or a medium dose of MVA85A (5 × 107 PFU) (29). There was no significant induction of IL-17A-producing cells following either low-dose or medium-dose MVA85A immunization (P > 0.05) (Fig. 2 A and B). The fold increase in IL-17A prevaccination and 4 weeks postvaccination in response to r85A was determined, and a significant increase in the fold change in IL-17A secretion was seen only in subjects receiving the higher dose of MVA85A (P < 0.05) (Fig. 2C). There was no difference in the fold change in IFN-γ responses across the different doses of MVA85A in these volunteers prevaccination and 4 weeks postvaccination (Fig. 2D).
FIG. 2.
The dose of MVA85A administered affects the fold change in IL-17A secretion in response to r85A. IL-17A responses in the medium-dose trial (A) and IL-17A responses in the low-dose trial (B) are not significantly increased following immunization with MVA85A. Also shown are the fold increase in IL-17A responses observed across different vaccine doses (C) and the fold increase in IFN-γ responses observed across different vaccine doses (D). IL-17A and IFN-γ responses to r85A are shown as numbers of SFC per 1 million PBMCs. The median is represented by a solid line. *, P < 0.05 versus dose of MVA85A (Mann-Whitney test).
Individuals latently infected with M. tuberculosis do not have increased numbers of IL-17A-producing T cells following immunization with MVA85A.
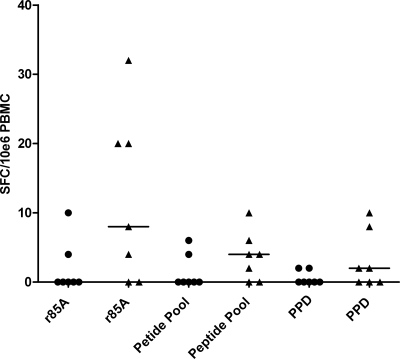
We then investigated the induction of IL-17A secretion in latently M. tuberculosis-infected subjects vaccinated with a medium dose of MVA85A (5 × 107 PFU), as adults exposed to mycobacteria have been shown to have high levels of IL-17A-producing T cells (36). There was no significant increase in the number of IL-17A-producing T cells in latently infected subjects following immunization with a medium dose of MVA85A (Fig. 3). This indicates that the induction of IL-17A-producing cells is similar in subjects with and without latent disease who receive 5 × 107 PFU of MVA85A. It would be interesting to determine the effects of 1 × 108 PFU of MVA85A on the induction of IL-17A-producing T cells in individuals latently infected with M. tuberculosis. There has, however, been no clinical trial of the 1 × 108-PFU dose of MVA85A in HIV-negative adults latently infected with M. tuberculosis, so we were unable to make that comparison in this study.
FIG. 3.
Individuals latently infected with M. tuberculosis do not have increased numbers of IL-17A-producing T cells following immunization with MVA85A. IL-17A responses preimmunization (•) and postimmunization (▴) are shown as numbers of SFC per 1 million PBMCs in response to r85A, the peptide pool of 85A and PPD. The median is represented by a solid line.
Treg cells are affected by immunization with MVA85A.
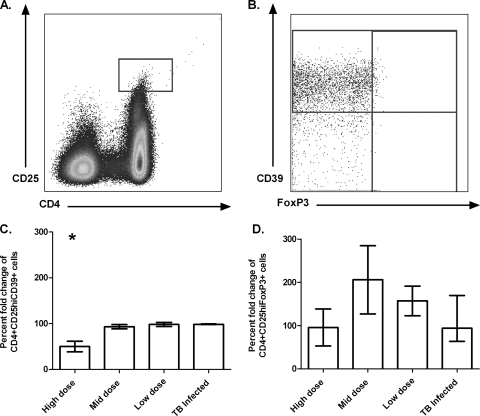
To determine the impact of immunization with MVA85A on circulating Treg cells, PBMCs from subjects across all of the trials were stained for Treg markers pre- and postimmunization. In addition to the classical Treg markers CD4, CD25, and FoxP3, cells were also stained for CD39. The CD39 protein is an ATP diphosphohydrolase which hydrolyzes extracellular ATP and ADP to AMP and has recently emerged as a functional marker for Treg cells (7, 10, 27). To identify Treg cells, CD4+ CD25hi events were selected by gating any events that were above the CD4− CD25+ events (2), and then individual function gates were drawn for FoxP3- and CD39-positive events (Fig. 4 A and B). The Boolean gate platform in FlowJo was then used to create all of the possible combinations of response patterns (37). CD4+ CD25hi CD39+ cells and CD4+ CD25hi FoxP3+ cells were compared preimmunization and at 4 weeks postimmunization (Fig. 4C and D). Investigating these two cell populations, a small but statistically significant decrease in CD4+ CD25hi CD39+ cells was found following immunization in the high-dose trial (P < 0.05) (Fig. 4C). This effect was not seen at other vaccine doses (Fig. 4C). There was no change in the circulating numbers of CD4+ CD25hi FoxP3+ cells following immunization (P > 0.05) (Fig. 4D).
FIG. 4.
Gating strategy for the identification of Treg cells in PBMCs and effect of immunization on Treg cells. CD4+ CD25hi cells were gated according to Baecher-Allan et al. (2) (A). Individual function gates were drawn for CD39- and FoxP3-positive cells (B). CD4+ CD25hi CD39+ cells are reduced following immunization with a high dose of MVA85A (C). CD4+ CD25hi FoxP3+ cells are not affected by immunization (D). The mean fold change (plus the standard error of the mean) in the responses of eight or nine individuals at week 0 compared to 4 weeks following immunization with MVA85A is shown. The M. tuberculosis-infected cohort received a medium dose of MVA85A. *, P < 0.05 versus dose of MVA85A (Kruskal-Wallis test).
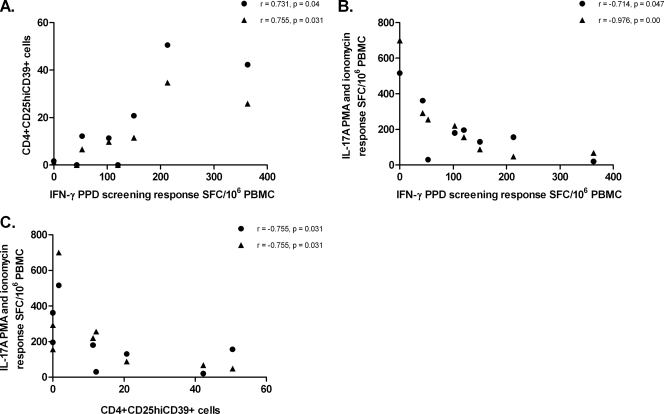
IFN-γ responses preimmunization affect circulating Treg cells and IL-17 responses.
As circulating CD4+ CD25hi CD39+ cells were decreased postimmunization in the high-dose trial, Treg responses were correlated with IFN-γ and IL-17A responses to MVA85A, as determined by ELISPOT assay. A weak positive correlation was found between the IFN-γ response to PPD preimmunization and circulating CD4+ CD25hi CD39+ cells pre- and postimmunization (P = 0.04, r = 0.731 and P = 0.031, r = 0.755, respectively) (Fig. 5 A). This suggests that prior exposure to mycobacterial antigens may be associated with higher levels of circulating Treg cells following subsequent immunization. In addition to this, a negative correlation was found between the IFN-γ response to PPD preimmunization and the IL-17A response to PMA and ionomycin pre- and postimmunization (P = 0.047, r = −0.714 and P < 0.01, r = −0.976) (Fig. 5B). The Treg response prior to immunization was also found to negatively correlate with the capacity of cells to produce IL-17A pre- and postimmunization (P = 0.031, r = −0.755) (Fig. 5C). In contrast, the specific IL-17A response to antigen 85A pre- and postimmunization did not correlate with the Treg responses prior to immunization.
FIG. 5.
Previous exposure to mycobacterial antigens affects IL-17A and Treg responses. IFN-γ responses to PPD preimmunization positively correlate with CD4+ CD25hi CD39+ cells preimmunization (•) and postimmunization (▴) (A). IFN-γ responses to PPD preimmunization negatively correlate with IL-17A responses to PMA and ionomycin preimmunization (•) and postimmunization (▴) (B). The Treg response prior to immunization negatively correlates with the capacity of cells to produce IL-17A preimmunization (•) and postimmunization (▴) (C). Spearman rank correlations are shown. IL-17A and IFN-γ responses are expressed as numbers of SFC per 1 million PBMCs. CD4+ CD25hi CD39+ T-cell responses are expressed as frequency of parent.
DISCUSSION
Immunization of BCG-vaccinated subjects with MVA85A has elicited high levels of antigen-specific IFN-γ-secreting T cells in recent clinical trials (5, 29, 31). The study reported here demonstrates that in addition to the induction of a strong Th1 response there is also a clear induction of antigen-specific IL-17A-secreting cells and a decrease in CD4+ CD25hi CD39+ Treg cells in circulation following immunization with a high dose of MVA85A. These two cell types, which may play a role in protection against TB, have so far not been investigated comparatively following immunization with various doses of MVA85A. Here the magnitude and dynamics of IL-17A production and Treg responses were investigated in a series of phase I clinical trials assessing the safety and immunogenicity of different doses of MVA85A.
The recently identified Th17 cell subset has been shown to be associated with increased protection from disease following mycobacterial challenge in the murine model (23). However, the role of Th17 cells in protection against TB remains controversial, as Th17 cells are also implicated in TB immune pathology (24). Determining the effect of vaccination upon the Th17 cell subset is important, as protection from TB is likely to be dependent not only upon a Th1 effector response but also upon the balance between a Th17 and a regulatory T-cell response. Antigen-specific increases in IL-17A were seen in response to vaccination with a high dose of MVA85A, although the magnitude of the IL-17A response was relatively modest, at approximately 100-fold lower than that seen for IFN-γ in these same volunteers at the peak time point (5). A Th17 response 5- to 10-fold lower than the Th1 response has previously been reported in mice infected with M. tuberculosis (23, 25). Khader et al. found that the low-frequency Th17 cells were important for immune protection from M. tuberculosis, as they recruited IFN-γ-producing T cells to the lung (23). In challenge studies with cattle, MVA85A-induced IFN-γ and IL-17 responses have been associated with protection against Mycobacterium bovis challenge, although the relative levels of Th1 and Th17 could not be assessed in this experiment (39). In the experiments performed by Khader et al., the kinetics of the Th17 and Th1 responses were the same although the response magnitudes differed. In our experiments, we found that the Th17 response was delayed compared to the Th1 response and peaked at 4 weeks rather than 1 week following vaccination with MVA85A. BCG vaccination has been shown to rapidly suppress a Th17 response, which is thought to be due to a strong induction of IL-12p70 and a reduction in IL-23 (11). It is possible that a strong and rapid induction of a Th1 response by MVA85A leads to a delay in the generation of a Th17 response.
A significant induction of IL-17A was only detected in the high-dose trial. Though the data are not shown, flow cytometry was used to investigate the phenotype of cells producing the IL-17A detected by ELISPOT assay. In the literature, PMA and ionomycin are often used to nonspecifically stimulate the induction of IL-17A responses to determine the capacity of cells to produce IL-17A, as antigen-specific IL-17A responses are generally of a low magnitude (23, 38). Stimulating PBMCs from subjects in the high-dose trial with PMA and ionomycin induced IL-17A- and IFN-γ-producing cells in all of the subjects. Stimulating cells with r85A only induced low levels of antigen-specific responses. Results above the baseline were only obtained for the highest IL-17A responder in the ELISPOT assay, where IL-17A-producing cells were subsequently identified to be CD4+ T cells, as previously identified in other studies (17, 36). That IL-17A responses above the baseline in the ICS assay were only detected for the highest responder in the ELISPOT assay suggests that the ELISPOT assay is more sensitive than the ICS assay in detecting antigen-specific responses of low magnitude, as investigated here. In contrast to our findings, using a whole-blood ICS assay, healthy, mycobacterium-exposed adults in South Africa have been found to have high levels of CD4+ IL-17+ T cells and more than 20% of the antigen-specific cytokine-producing CD4+ T cells were shown to express IL-17 or IL-22 (36), another Th17 cell effector cytokine (4). Using the same whole-blood ICS assay, CD4+ IL-17+ T cells were also identified in South African subjects vaccinated with MVA85A (18). We have previously compared the whole-blood ICS assay with our PBMC-based ICS assay and have found differences in the abilities of these assays to detect IFN-γ-producing CD8+ T-cell responses, which are easily detected in the 12-h whole-blood assay and undetectable in the ex vivo ELISPOT and PBMC flow assays (5). It is possible that IL-17A responses are also differentially detected in whole blood and PBMCs, and this phenomenon is currently under further investigation in our laboratory. United Kingdom adults latently infected with M. tuberculosis and immunized with MVA85A were investigated for IL-17A responses in this study. No difference in the induction of IL-17A-secreting cells was seen in latently infected compared to noninfected individuals. However, these individuals received a medium dose of MVA85A and it is possible that at a higher dose of MVA85A, IL-17A-producing T cells could have been induced.
A reciprocal relationship has been suggested to exist between Th17 and Treg cell types (3). FoxP3, the master regulator of Treg development, and ROR-γt, the key regulator of Th17 differentiation, have been shown to antagonize each other through direct binding (40). A constant interplay among several factors that determine whether the immune response is skewed toward Th17 cells or Treg cells has been suggested, though some functional plasticity is thought to exist regarding CD4+ T cells (26). In our study, the markers CD4, CD25, FoxP3, and CD39 were used to identify Treg cells. CD39 is thought to be a better marker of human regulatory T-cell activity than either FoxP3 or CD127 and has been found to be increased in patients with active TB (10). Previously, Fletcher et al. have investigated Treg cells in subjects receiving a low dose of MVA85A. In these subjects, there was no difference pre- and postvaccination in the CD4+ CD25hi FoxP3+ cell population (14). No change in this cell population was seen at the different doses of MVA85A investigated in this study either. Interestingly, we did see a small but significant decrease in CD4+ CD25hi CD39+ cells 4 weeks postvaccination with MVA85A, which was the time point at which antigen-specific IL-17A-producing cells became detectable. In addition to this, we saw an inverse correlation between CD4+ CD25hi CD39+ cells and the capacity of cells to produce IL-17A, indicating that CD4+ CD25hi CD39+ cells may have a role in the regulation of a Th17 response.
IL-17A responses were found be affected by prior exposure to mycobacterial antigens, as IFN-γ immune responses to PPD before immunization inversely correlated with the capacity of cells to produce IL-17A following immunization. Prior exposure to mycobacterial antigens was also associated with increased numbers of CD4+ CD25hi CD39+ cells postimmunization. This indicates that preexisting immune responses to mycobacteria may be associated with higher numbers of regulatory T cells and lower numbers of IL-17A cells in response to MVA85A vaccination. Several hypotheses regarding the failure of the BCG vaccine are attributed to the effects of previous exposure to environmental mycobacteria, which are thought to block or mask BCG-induced immune protection (6, 8). Although small, our study indicates that prior exposure to mycobacterial antigens may result in increased vaccine-induced regulatory T cells and a lowered Th17 immune response, factors which could influence vaccine efficacy.
In summary, we have found that a high dose of viral vector vaccine can induce antigen-specific IL-17A-producing T cells, although these appear later than IFN-γ-producing T cells and are of a lower magnitude. Preexisting immune responses to mycobacterial antigens are associated with a higher CD4+ CD25hi CD39+ T-cell response and a reduced capacity to produce IL-17A following immunization. Further comparative studies of IL-17A and CD4+ CD25hi CD39+ T-cell responses in United Kingdom and African cohorts are needed to examine the impact of prior mycobacterial exposure on the vaccine-induced immune response and the role of these T-cell subsets in vaccine efficacy.
Acknowledgments
We thank all of the subjects who took part in the trials reported here.
A.H. is a Wellcome Trust Principal Research Fellow, and H.M. is a Wellcome Trust Senior Clinical Fellow. A.H. and H.M. are Jenner Institute investigators.
A.H., A.A.P., and H.M. are named inventors in a patent filing related to MVA85A and are shareholders in a joint venture, OETC, formed for the future development of this vaccine. There are no other conflicts of interest.
Footnotes
Published ahead of print on 19 May 2010.
REFERENCES
- 1.Altare, F., A. Durandy, D. Lammas, J. F. Emile, S. Lamhamedi, F. Le Deist, P. Drysdale, E. Jouanguy, R. Döffinger, F. Bernaudin, O. Jeppsson, J. A. Gollob, E. Meinl, A. W. Segal, A. Fischer, D. Kumararatne, and J. L. Casanova. 1998. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 280:1432-1435. [DOI] [PubMed] [Google Scholar]
- 2.Baecher-Allan, C., J. A. Brown, G. J. Freeman, and D. A. Hafler. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167:1245-1253. [DOI] [PubMed] [Google Scholar]
- 3.Bettelli, E., Y. Carrier, W. Gao, T. Korn, T. B. Strom, M. Oukka, H. L. Weiner, and V. K. Kuchroo. 2006. Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory cells. Nature 441:235-238. [DOI] [PubMed] [Google Scholar]
- 4.Bettelli, E., T. Korn, M. Oukka, and V. K. Kuchroo. 2008. Induction and effector functions of T(H)17 cells. Nature 453:1051-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beveridge, N. E., H. A. Fletcher, J. Hughes, A. A. Pathan, T. J. Scriba, A. Minassian, C. R. Sander, K. T. Whelan, H. M. Dockrell, A. V. Hill, W. A. Hanekom, and H. McShane. 2008. A comparison of IFNgamma detection methods used in tuberculosis vaccine trials. Tuberculosis 88:631-640. [DOI] [PubMed] [Google Scholar]
- 6.Black, G. F., R. E. Weir, S. Floyd, L. Bliss, D. K. Warndorff, A. C. Crampin, B. Ngwira, L. Sichali, B. Nazareth, J. M. Blackwell, K. Branson, S. D. Chaguluka, L. Donovan, E. Jarman, E. King, P. E. Fine, and H. M. Dockrell. 2002. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet 359:1393-1401. [DOI] [PubMed] [Google Scholar]
- 7.Borsellino, G., M. Kleinewietfeld, D. Di Mitri, A. Sternjak, A. Diamantini, R. Giometto, S. Höpner, D. Centonze, G. Bernardi, M. L. Dell'Acqua, P. M. Rossini, L. Battistini, O. Rötzschke, and K. Falk. 2007. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 110:1225-1232. [DOI] [PubMed] [Google Scholar]
- 8.Brandt, L., J. Feino Cunha, A. Weinreich Olsen, B. Chilima, P. Hirsch, R. Appelberg, and P. Andersen. 2002. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect. Immun. 70:672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brewer, T. F. 2000. Preventing tuberculosis with bacillus Calmette-Guerin vaccine: a meta-analysis of the literature. Clin. Infect. Dis. 31 (Suppl. 3):S64-S67. [DOI] [PubMed] [Google Scholar]
- 10.Chiacchio, T., R. Casetti, O. Butera, V. Vanini, S. Carrara, E. Girardi, D. Di Mitri, L. Battistini, F. Martini, G. Borsellino, and D. Goletti. 2009. Characterization of regulatory T cells identified as CD4+CD25highCD39+ in patients with active tuberculosis. Clin. Exp. Immunol. 156:463-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz, A., S. A. Khader, E. Torrado, A. Fraga, J. E. Pearl, J. Pedrosa, A. M. Cooper, and A. G. Castro. 2006. Cutting edge: IFN-gamma regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J. Immunol. 177:1416. [DOI] [PubMed] [Google Scholar]
- 12.Curtis, M. M., and S. S. Way. 2009. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology 126:177-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dye, C., K. Floyd, and M. Uplekar. 2008. Global tuberculosis control: surveillance, planning, financing. World Health Organization, Geneva, Switzerland.
- 14.Fletcher, H. A., A. A. Pathan, T. K. Berthoud, S. J. Dunachie, K. T. Whelan, N. C. Alder, C. R. Sander, A. V. Hill, and H. McShane. 2008. Boosting BCG vaccination with MVA85A down-regulates the immunoregulatory cytokine TGF-<beta>1. Vaccine 26:5269-5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanekom, W. A., H. M. Dockrell, T. H. Ottenhoff, T. M. Doherty, H. Fletcher, H. McShane, F. F. Weichold, D. F. Hoft, S. K. Parida, and U. J. Fruth. 2008. Immunological outcomes of new tuberculosis vaccine trials: WHO panel recommendations. PLoS Med. 5:e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrington, L. E., R. D. Hatton, P. R. Mangan, H. Turner, T. L. Murphy, K. M. Murphy, and C. T. Weaver. 2005. Interleukin 17 producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123-1132. [DOI] [PubMed] [Google Scholar]
- 18.Hawkridge, T., T. J. Scriba, S. Gelderbloem, E. Smit, M. Tameris, S. Moyo, T. Lang, A. Veldsman, M. Hatherill, L. V. Merwe, H. A. Fletcher, H. Mahomed, A. V. Hill, W. A. Hanekom, G. D. Hussey, and H. McShane. 2008. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in healthy adults in South Africa. J. Infect. Dis. 198:544-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofstetter, H. H., S. M. Ibrahim, D. Koczan, N. Kruse, A. Weishaupt, K. V. Toyka, and R. Gold. 2005. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell. Immunol. 237:123-130. [DOI] [PubMed] [Google Scholar]
- 20.Huang, W., L. Na, P. L. Fidel, and P. Schwarzenberger. 2004. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J. Infect. Dis. 190:624-631. [DOI] [PubMed] [Google Scholar]
- 21.Itoh, M., T. Takahashi, N. Sakaguchi, Y. Kuniyasu, J. Shimizu, F. Otsuka, and S. Sakaguchi. 1999. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 162:5317-5326. [PubMed] [Google Scholar]
- 22.Jouanguy, E., F. Altare, S. Lamhamedi, P. Revy, J. F. Emile, M. Newport, M. Levin, S. Blanche, E. Seboun, A. Fischer, and J. L. Casanova. 1996. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N. Engl. J. Med. 335:1956-1961. [DOI] [PubMed] [Google Scholar]
- 23.Khader, S. A., G. K. Bell, J. E. Pearl, J. J. Fountain, J. Rangel-Moreno, G. E. Cilley, F. Shen, S. M. Eaton, S. L. Gaffen, S. L. Swain, R. M. Locksley, L. Haynes, T. D. Randall, and A. M. Cooper. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4 T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8:369-377. [DOI] [PubMed] [Google Scholar]
- 24.Khader, S. A., and A. M. Cooper. 2008. IL-23 and IL-17 in tuberculosis. Cytokine 41:79-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khader, S. A., J. E. Pearl, K. Sakamoto, L. Gilmartin, G. K. Bell, D. M. Jelley-Gibbs, N. Ghilardi, F. deSauvage, and A. M. Cooper. 2005. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J. Immunol. 175:788-795. [DOI] [PubMed] [Google Scholar]
- 26.Koenen, H. J., R. L. Smeets, P. M. Vink, E. V. Rijssen, A. M. Boots, and I. Joosten. 2008. Human CD25highFoxp3pos regulatory T-cells differentiate into IL-17 producing cells. Blood 112:2340-2352. [DOI] [PubMed] [Google Scholar]
- 27.Mandapathil, M., S. Lang, E. Gorelik, and T. L. Whiteside. 2009. Isolation of functional human regulatory T cells (Treg) from the peripheral blood based on the CD39 expression. J. Immunol. Methods 346:55-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McShane, H., A. A. Pathan, C. R. Sander, N. P. Goonetilleke, H. A. Fletcher, and A. V. S. Hill. 2005. Boosting BCG with MVA85A: the first candidate subunit vaccine for tuberculosis in clinical trials. Tuberculosis 85:47-52. [DOI] [PubMed] [Google Scholar]
- 29.McShane, H., A. A. Pathan, C. R. Sander, S. M. Keating, S. C. Gilbert, K. Huygen, H. A. Fletcher, and A. V. Hill. 2004. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat. Med. 10:1240-1244. [DOI] [PubMed] [Google Scholar]
- 30.Park, H., Z. Li, X. O. Yang, S. H. Chang, R. Nurieva, Y.-H. Wang, Y. Wang, L. Hood, Z. Zhu, Q. Tian, and C. Dong. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6:1133-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pathan, A. A., C. R. Sander, H. A. Fletcher, I. Poulton, N. C. Alder, N. E. Beveridge, K. T. Whelan, A. V. Hill, and H. McShane. 2007. Boosting BCG with recombinant modified vaccinia Ankara expressing antigen 85A: different boosting intervals and implications for efficacy trials. PLoS One 2:e1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakaguchi, S. 2000. Regulatory T cells: key controllers of immunologic self-tolerance. Cell 101:455-458. [DOI] [PubMed] [Google Scholar]
- 33.Sakaguchi, S., T. Yamaguchi, T. Nomura, and M. Ono. 2008. Regulatory T cells and immune tolerance. Cell 133:775-787. [DOI] [PubMed] [Google Scholar]
- 34.Sander, C. R., A. A. Pathan, N. E. Beveridge, I. Poulton, A. Minassian, N. Alder, J. Van Wijgerden, A. V. Hill, F. V. Gleeson, R. J. Davies, G. Pasvol, and H. McShane. 2009. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in Mycobacterium tuberculosis-infected individuals. Am. J. Respir. Crit. Care Med. 179:724-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato, K., A. Suematsu, K. Okamoto, A. Yamaguchi, Y. Morishita, Y. Kadono, S. Tanaka, T. Kodama, S. Akira, Y. Iwakura, D. J. Cua, and H. Takayanagi. 2006. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 203:2673-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scriba, T. J., B. Kalsdorf, D. A. Abrahams, F. Isaacs, J. Hofmeister, G. Black, H. Y. Hassan, R. J. Wilkinson, G. Walzl, S. J. Gelderbloem, H. Mahomed, G. D. Hussey, and W. A. Hanekom. 2008. Distinct, specific IL-17-and IL-22-producing CD4 T cell subsets contribute to the human anti-mycobacterial immune response. J. Immunol. 180:1962-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seder, R. A., P. A. Darrah, and M. Roederer. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8:247-258. [DOI] [PubMed] [Google Scholar]
- 38.Umemura, M., A. Yahagi, S. Hamada, M. D. Begum, H. Watanabe, K. Kawakami, T. Suda, K. Sudo, S. Nakae, Y. Iwakura, and G. Matsuzaki. 2007. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis Bacille Calmette-Guerin infection. J. Immunol. 178:3786-3796. [DOI] [PubMed] [Google Scholar]
- 39.Vordermeier, H. M., B. Villarreal-Ramos, P. J. Cockle, M. McAulay, S. G. Rhodes, T. Thacker, S. C. Gilbert, H. McShane, A. V. Hill, Z. Xing, and R. G. Hewinson. 2009. Viral booster vaccines improve Mycobacterium bovis BCG-induced protection against bovine tuberculosis. Infect. Immun. 77:3364-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou, L., J. E. Lopes, M. M. W. Chong, I. I. Ivanov, R. Min, G. D. Victora, Y. Shen, J. Du, Y. P. Rubstov, A. Y. Rudensky, S. F. Ziegler, and D. R. Littman. 2008. TGFbeta-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORgammat function. Nature 453:236-240. [DOI] [PMC free article] [PubMed] [Google Scholar]