Abstract
The majority of healthy individuals exposed to Mycobacterium tuberculosis will not develop tuberculosis (TB), though many may become latently infected. More precise measurement of the human immune response to M. tuberculosis infection may help us understand this difference and potentially identify those subjects most at risk of developing active disease. Gamma interferon (IFN-γ) production has been widely used as a proxy marker to study infection and to examine the human immune response to specific M. tuberculosis antigens. It has been suggested that genetically distinct M. tuberculosis strains may invoke different immune responses, although how these differences influence the immune responses and clinical outcome in human tuberculosis is still poorly understood. We therefore evaluated the antigen-specific IFN-γ production responses in peripheral blood mononuclear cells from two cohorts of subjects recruited in Antananarivo, Madagascar, from 2004 to 2006 and examined the influence of the infecting M. tuberculosis strains on this response. The cohorts were sputum-positive index cases and their household contacts. Clinical strains isolated from the TB patients were typed by spoligotyping. Comparison of the IFN-γ responses with the spoligotype of the infecting clinical strains showed that “modern” M. tuberculosis strains, like Beijing and Central Asian (CAS) strains, tended to induce lower IFN-γ responses than “ancient” strains, like East African-Indian (EAI) strains, in index cases and their household contacts. These results suggest that new strains may have evolved to induce a host response different from that of ancient strains. These findings could have important implications in the development of therapeutic and diagnostic strategies.
Tuberculosis (TB), caused by Mycobacterium tuberculosis, is a major cause of global morbidity and mortality throughout the world. It is estimated that there are in excess of new 8 million cases of TB each year, and this represents just the tip of the iceberg. Infection with M. tuberculosis leads to clinically active TB in about 5 to 10% of exposed individuals. A much higher proportion of exposed individuals apparently become latently infected, and these individuals may remain noninfectious and symptom free for years. Approximately one-third of the world population is thought to be latently infected with M. tuberculosis. However, under some circumstances (in about 5% of the latently infected people), the host immune response is perturbed and latent M. tuberculosis infection may develop into clinically active TB (52). This process is most prominent in individuals coinfected with human immunodeficiency virus (HIV), but it can also occur with impairment of the immune system associated with old age, malnutrition, anti-inflammatory drug treatment, etc. Reactivation of latent disease is thought to contribute roughly half of all TB cases, and thus, understanding the factors controlling the development of acute primary TB or latent infection is crucial to TB control (64).
Gamma interferon (IFN-γ) production has been widely used to study infection and to examine the human immune response to specific M. tuberculosis antigens. The 6-kDa early secreted antigenic target (ESAT-6) antigen, encoded by genes located within region of difference 1 (RD1) of the M. tuberculosis genome, is much more specific for M. tuberculosis than purified protein derivative (PPD), as these genes were deleted from M. bovis in the development of BCG substrains or are not found in most environmental mycobacteria (29, 53). Some studies showed that the level of IFN-γ release in response to ESAT-6 could identify TB contacts at risk of developing active disease after recent infection (3, 18, 30). CFP7 or TB10.4 is an immunodominant antigen recognized by TB patients and M. bovis BCG-vaccinated subjects, while ESAT-6 is specific to TB patients and induces a strong IFN-γ response (51). Moreover, since CFP7 induces strong protection against infection by M. tuberculosis, it was proposed to be a TB vaccine candidate (1, 19).
There is a growing number of observations indicating that TB cases resulting from infection with epidemic strains, such as the W-Beijing strains (22, 35, 39, 44), may display a more severe pathology or more severe symptoms. Beijing strains were also found to induce higher fevers in pulmonary TB patients than other strains (62). In addition, the Beijing genotype, which is responsible for more than 80% of TB cases in China, was associated with virulence and high transmissibility (7, 28). The same has been found more recently with the RD(Rio) strains belonging to the Latin America-Mediterranean (LAM) family (38). Despite the fact that other epidemiological and clinical studies have failed to confirm any association between the mycobacterial genotype and the clinical presentation (8, 41, 43), the immunological aspects of infection with these strains is still of interest and poorly described.
Epidemiological studies carried out in Madagascar showed no association between IS6110 patterns and clinical tuberculosis presentation (47), but they did reveal a heterologous population of M. tuberculosis strains, including the existence of a high frequency of unusual genotypes, such as the shared type 109 from the EAI8-MDG family (SpolDB4) (10) or strains with a single copy of IS6110 (24, 46). Since there are limited data on the correlation of the strain genotype with clinical features or the host immune response in patients and their contacts (57, 59), we investigated the IFN-γ response to the ESAT-6, CFP7, and PPD antigens in pulmonary TB patients and their household contacts (as this is commonly used as a biomarker to identify M. tuberculosis infection) and examined the influence of the M. tuberculosis genotype on this response.
MATERIALS AND METHODS
Study site and subjects.
Newly recruited sputum-positive TB patients (index cases [ICs]) over 15 years of age were recruited between June 2004 and December 2005 at the principal antituberculosis center of Antananarivo, the capital of Madagascar, where the annual incidence of newly diagnosed sputum smear-positive patients is about 80 cases per 100,000 inhabitants. Positivity was defined as two sputum samples positive by microscopy and confirmed by culture on Löwenstein-Jensen (LJ) medium. The patients were treated according to the National TB Control Programme (NTCP) strategy and were followed up by the clinical physicians; sputum samples were collected 2, 5, and 7 months after the start of treatment.
The household contacts (HCs) of the included index cases were visited at home by the study physicians and were invited to join the study. They were included if they were ≥1 year old and had been living in the same house as the case patient for at least 6 months.
The subjects were invited by the study physicians to give informed consent, interviewed, and examined. Only subjects who accepted an HIV test, after counseling, and who had given informed consent were included in the study. A venous blood sample was drawn into a Vacutainer tube containing heparin for enzyme-linked immunospot (ELISPOT) assay and into a dry Vacutainer tube for an HIV test. HIV-positive subjects were excluded from the study.
Household contacts and community controls underwent a PPD skin test (10 units; tuberculin purified protein derivative; Aventis Pasteur). Induration was recorded after 72 h. Subjects with a positive PPD test result of >14 mm of induration at the time of inclusion in the study or for which conversion of the PPD test occurred during follow-up were offered chest radiography, and those with symptoms underwent a clinical examination. Individuals with a diagnosis of TB disease were referred to the antituberculosis center for treatment.
For all subjects, epidemiological, clinical, and bacteriology data were recorded prospectively on individual record forms.
The study was approved by the National Ethics Committee of the Ministry of Health in Madagascar.
Mycobacteriology procedures.
Sputum was decontaminated using the sodium lauryl sulfate method (55). One drop of the decontaminated sputum was fixed on a slide, stained with auramine-phenol, and examined under a fluorescence microscope (objective, ×40) for acid-fast bacilli (AFB). The remaining decontaminated specimen was inoculated into two tubes of standard LJ medium (Diagnostics Pasteur, Paris, France) and on one tube of LJ medium without glycerol but supplemented with 0.5% pyruvate. The numbers of CFU growing in each LJ tube were counted. Mycobacterial isolates were identified according to growth on LJ medium, colony morphology, and biochemical tests for the following: niacin production, catalase, urease, and nitrate reductase (23). The M. tuberculosis isolates were tested for their susceptibility to streptomycin (4 μg/ml; catalog no. D-5155; Sigma), isoniazid (0.2 μg/ml; catalog no. I3377; Sigma), rifampin (40 μg/ml; catalog no. R-8626; Sigma), and ethambutol (2 μg/ml; catalog no. E-4630; Sigma) using the proportion method on Löwenstein-Jensen medium, as recommended by the Global Tuberculosis Programme of the World Health Organization and the International Union against Tuberculosis and Lung Disease (11). The critical proportion value was 1% for all drugs.
Spoligotyping.
Genomic DNA was extracted from the M. tuberculosis cultures as described by van Soolingen et al. (63). Spoligotyping of the M. tuberculosis isolates was performed as described by Kamerbeek et al. (34). A cluster of M. tuberculosis strains was defined as two or more isolates with identical spoligotype patterns. A cluster of patients was defined as two or more patients with identical strains. The spoligotype and spoligotype family designations were attributed by comparing the patterns with those from the SITVIT database (http://www.pasteur-guadeloupe:8081/SIIVITdemo) and from the SpolDB4 international spoligotype database (10).
ELISPOT assay.
Peripheral blood mononuclear cells (PBMCs) were separated from heparinized whole blood as described previously (20) within the 4 h following blood collection. Briefly, PBMCs were enriched by centrifugation over Ficoll-Hypaque (catalog no. H-8889; Sigma). The PBMCs were then washed with complete RPMI 1640 medium (catalog no. 51800035; Gibco) containing 1% l-glutamine and 1% penicillin-streptomycin. Viable cells were counted after staining of the PBMCs with trypan blue and diluted to 2 × 106 viable cells/ml in R10 medium (complete RPMI 1640 medium supplemented with 5% heat-inactivated fetal bovine serum [catalog no. 16000-044; Invitrogen, Gibco]), and 100 μl (2 × 105 cells) per well was used for the ELISPOT assay.
The ex vivo ELISPOT assay was performed with fresh samples, as described elsewhere (36), using mouse antibody-capture anti-human IFN-γ (1D1K; catalog no. 3420-3-1000; Mabtech). For this study, PPD (Statens Serum Institute, Copenhagen, Denmark) was used at 5 μg/ml. We tested each of the recombinant antigens ESAT-6 and CFP7 (Statens Serum Institute) at a concentration of 10 μg/ml, on the basis of the optimal responsiveness detected in prior titration studies. Phytohemagglutinin (5 μg/ml; catalog no. L-2769; Sigma) was used as a positive control, and RPMI 1640 medium was used as a negative control. Briefly, 96-well plates (catalog no. MAIP S4510; Millipore) were coated with 0.5 μg/well of the anti-human IFN-γ (α-hIFN-γ) 1D1K antibody in carbonate buffer (pH 9.6) overnight at 4 to 8°C. The plates were washed the next morning in phosphate-buffered saline (PBS) and blocked with R10 medium. The antigens at various concentrations were placed in the coated wells, together with 2 × 105 PBMCs per well. After approximately 16 h of incubation at 37°C in 5% CO2, the wells were washed in PBS-0.05% Tween 20, and 50 ng biotin-labeled α-hIFN-γ monoclonal antibody (7-B6-1; catalog no. 3420-6-250; Mabtech) in PBS-1% BSA was added to each well. The plates were incubated for 4 h at room temperature, washed, and incubated with streptavidin-alkaline phosphatase conjugate (catalog no. 17066432; Bio-Rad) diluted 1/1,000 in PBS-0.05% Tween 20 for 1 h at 37°C. Between steps, the plates were washed five times in PBS-0.05% Tween 20. The spots were developed by addition of alkaline phosphatase substrate (catalog no. 170-6432; Bio-Rad), and the reactions were stopped by extensively washing the plates with tap water. The spots in each wells were counted by two independent readers using a dissecting microscope. The mean number of spot-forming cells (SFCs) per well for each antigen was calculated. The difference between duplicate wells was consistently less than 30% of the mean. The mean number of SFCs of the negative control was subtracted and transformed to the number of SFCs per 2 × 105 cells. The cutoff point for positivity was taken as the mean response of unstimulated wells for the whole cohort plus 1.64 standard deviations.
Data management and statistical analysis.
Data were entered into Access or Excel databases and checked for errors. Analyses were performed using Statistica software or the EpiInfo (version 6) program. Comparisons were assessed by the Kruskal-Wallis test or the Mann-Whitney U test. A P value of <0.05 was considered statistically significant.
RESULTS
Study participants.
From 15 June 2004 to December 2005, 85 ICs and 293 HCs were recruited and were followed up until December 2006. All subjects were Malagasy and HIV negative.
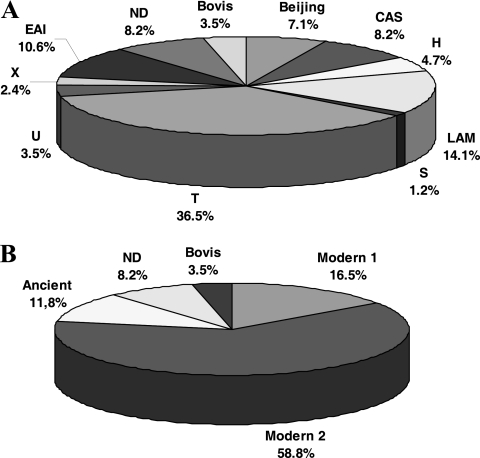
M. tuberculosis isolates from 81 ICs were typed by spoligotyping. On the basis of the SITVIT guidelines (http://www.pasteur-guadeloupe:8081/SITVITdemo) and the data in the SpolDB4 database (10), the spoligotypes could be classified into 10 families (Fig. 1 A). The strain distribution was similar to that described in a previous study (24). Then, for the analysis, we considered the phylogeny described elsewhere (5, 9), where the M. tuberculosis strains were divided into an “ancient” strain group (spoligotypes of the East African-Indian [EAI] family) and a “modern” strain group, with modern group 1 including the Beijing and Central Asian (CAS) families and modern group 2 containing the T (undefined family), Haarlem, LAM, U, X, and S families (Fig. 1B).
FIG. 1.
Distribution of the spoligotype families found among the M. tuberculosis strains isolated from the index cases. (A) Spoligotype family according to data in the SpolDB4 database (CAS, Central Asian; EAI, East African Indian; LAM, Latin American-Mediterranean; H, Haarlem; T, undefined family) (10); ND, not described in SpolDB4 database. (B) Groups according to Arnold (5).
Follow-up.
Of the ICs, three patients died: one during the course of treatment and two after relapse. Six patients interrupted TB treatment, one patient relapsed, and one patient failed after 5 months of therapy; all of them returned to the health center and completed the standardized retreatment therapy regimen.
The ICs who died during the course of treatment were infected by an M. tuberculosis strain of the LAM spoligotype; both of the other two patients who relapsed and died later were infected by a strain with the T spoligotype. One patient, who relapsed but who was cured after retreatment therapy, was infected by a strain with the CAS genotype. The patient who failed TB treatment was infected by a T-family strain.
Two HCs died during the study, one of a cerebral vascular accident and one of pleurisy, which was not proved to be linked to M. tuberculosis infection. Twelve HCs (4.1%) developed TB disease during the follow-up: one developed pleural TB, six had pulmonary AFB-negative TB with radiological symptoms, three patients from the same household developed clinical TB with erythema nodosum, and two contacts developed AFB-positive pulmonary TB.
Of the 12 HCs who developed clinical TB disease, 10 were contacts of IC patients infected with T-family strains, 1 was the contact subject of a LAM-family-strain-infected IC, and 1 was the contact of a M. bovis-family-strain-infected IC. However, the number of HCs who developed TB was so small that it was not possible to find any correlation between the genotype and the development of TB among the contacts.
Influence of spoligotype on bacillary load.
We examined whether there was any correlation between the spoligotype of the infecting strains and the M. tuberculosis sputum load in TB patients. The sputum bacterial load was measured as the number of AFB observed by microscopy and by the number of CFU obtained by culture (Table 1). Though the CAS and Beijing spoligotypes were more likely to give a lower bacillus load than the other spoligotypes, this difference was not statistically significant.
TABLE 1.
M. tuberculosis bacillus load in smear-positive patients, according to the infecting strain spoligotype
| Bacterial sputum load | No. (%) of patients infected by M. tuberculosis spoligotypea |
|||
|---|---|---|---|---|
| Bovis | CAS/Beijing | Modern group 2 | EAI | |
| No. of AFB | ||||
| ≥100 AFB per field | ND | 4 (28.6) | 23 (45.1) | 4 (40) |
| <100 AFB per field | ND | 10 (71.4) | 28 (54.9) | 6 (60) |
| No. of CFU on LJ medium | ||||
| ≥300 CFU/tube | 1 | 8 (57.1) | 35 (68.6) | 5 (50) |
| < 300 CFU/tube | 2 | 6 (42.9) | 16 (31.4) | 5 (50) |
None of the differences between groups was statistically significant by the χ2 test. ND, not determined.
All except two strains were drug susceptible. The exceptions were one strain with a LAM spoligotype which was resistant to isoniazid and one strain with a Haarlem spoligotype which was multiresistant to streptomycin, isoniazid, rifampin, and ethambutol. On analysis, we did not find any correlation between the drug resistance of the strains and the spoligotype of the strains (data not shown).
Influence of spoligotype on IFN-γ ELISPOT assay response.
Of the recruited individuals, 230 (55 ICs, 175 HCs) had adequate blood specimens available and ELISPOT assay results that met the inclusion criteria (Table 2). With all the antigens (PPD, CFP7, and ESAT-6), the proportion of cells positive by the IFN-γ ELISPOT assay was higher for the TB patient group than for the healthy household contact (HHC) group during the inclusion period. There was no significant difference in the ESAT-6-induced IFN-γ ELISPOT assay response according to the bacterial load (P > 0.05).
TABLE 2.
Characteristics of the individuals for whom IFN-γ ELISPOT assay results were obtained at the inclusion period
| Characteristica | Index cases | TBHC | HHCs | P valueb |
|---|---|---|---|---|
| No. of individuals | 55 | 6 | 169 | |
| Mean (range) age (yr) | 35.38 (17-70) | 21 (2-47) | 20.95 (1-79) | |
| Sex (no. of individuals) | ||||
| Male | 31 | 3 | 79 | |
| Female | 24 | 3 | 90 | |
| TST result at day 0 (no. [%] of individuals)c | ||||
| Negative | 4 (15.3) | 0 | 38 (22.5) | |
| 5-14 mm | 10 (38.5) | 0 | 46 (27.2) | |
| ≥15 mm | 12 (46.2) | 6 (100) | 84 (49.7) | |
| ND | 29 | 0 | 1 | |
| BCG vaccination (no. [%] of individuals) | ||||
| Yes | 40 (72.7) | 6 (100) | 150 (99.7) | |
| No | 6 | 0 | 9 | |
| IND | 9 | 0 | 10 | |
| IFN-γ ELISPOT assay response to antigensd | ||||
| CFP7 | 233 (65.5) | 276 (66.6) | 164 (51.5) | 0.16 |
| ESAT-6 | 225 (61.8) | 158 (50) | 105 (43.8) | 0.066 |
| PPD | 772 (94.5) | 396 (100) | 304 (74.6) | 0.0026 |
ND, not determined; IND, indeterminate.
χ2 test.
Percentages are the percent positive among those tested.
The responses are given as the median number of SFCs per 106 PBMCs (percent). The cutoff point for positivity was taken as the mean response of the unstimulated wells for the whole cohort plus 1.64 standard deviations.
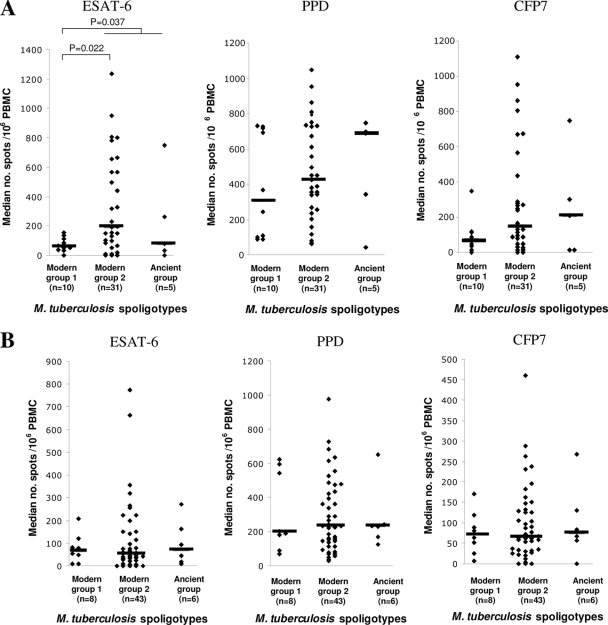
We then compared the genotype of the infecting strains in those subjects for which both ELISPOT assay results and spoligotypes were available. The median PPD- and CFP7-induced IFN-γ responses were higher in IC patients from whom we isolated ancient group M. tuberculosis strains than in IC patients from whom we isolated modern group 1 or modern group 2 strains; however, this difference was not statistically significant (Fig. 2 A). The ESAT-6-induced IFN-γ responses of IC patients from whom we isolated modern group 1 strains (Beijing, CAS), however, was significantly lower than the responses of IC patients from whom we isolated strains belonging to modern group 2 strains or to the ancient strain group (P = 0.037). The ELISPOT assay results for cured ICs, i.e., after the end of the TB treatment, showed no differences according to the spoligotype of the infecting strains (Fig. 2B).
FIG. 2.
In vitro IFN-γ responses of PBMCs from ICs to restimulation with M. tuberculosis ESAT-6, PPD, and CFP7 antigens at the inclusion period (A) and after treatment (B), according to the spoligotypes of the infecting M. tuberculosis strains. The levels of IFN-γ which were significantly different between groups (Mann-Whitney test) are indicated. The horizontal bars indicate the median number of spots per 106 PBMCs.
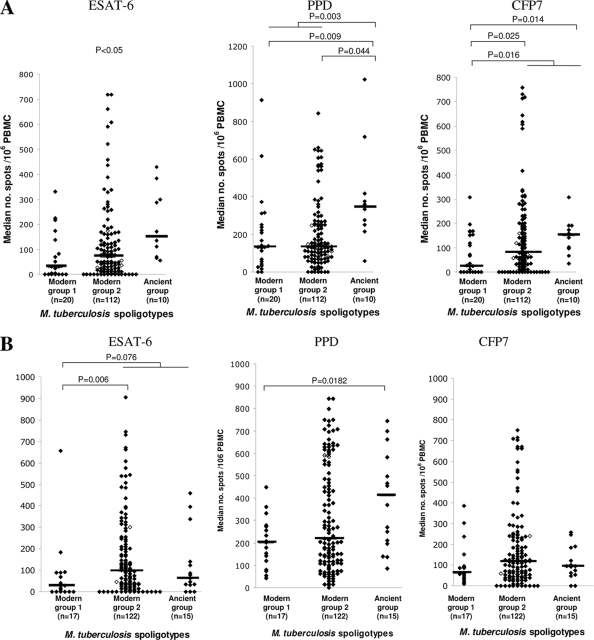
We assumed that HCs, all of whom had significant exposure times, would most likely be exposed to and thus react to the same strains as their respective ICs, even if this might involve superinfection on top of a previous, unidentified exposure, in some cases. Therefore, to determine whether some strains induced different IFN-γ responses among the contacts of TB patients, the IFN-γ response in the HC cohort was assessed according to the genotype of the strains isolated from their respective ICs (Fig. 3 A). Both the ESAT-6- and the PPD-induced IFN-γ responses were statistically higher in the HCs of patients infected by EAI strains than in the HCs of patients infected by modern group 1 or group 2 strains (P = 0.010 and P = 0.003, respectively). The CFP7-induced IFN-γ responses were significantly higher in the HCs of TB patients infected with EAI strains than in the HCs of patients infected with modern group 1 strains (P = 0.014). Even though the CFP7-induced IFN-γ responses were also higher in the HCs of EAI strain-infected patients than in the HCs of modern group 2 strain-infected patients, this difference was not statistically significant. Likewise, the ESAT-6-induced and CFP7-induced IFN-γ responses at the inclusion were significantly lower in the HCs of patients from whom CAS or Beijing strains were isolated than the responses in the contacts of modern group 2 or ancient strain-infected patients (P = 0.016).
FIG. 3.
In vitro IFN-γ responses of PBMCs from household contacts to restimulation with the ESAT-6, PPD, and CFP7 antigens at the inclusion period (A) and 3 months after the inclusion period (B), according to the spoligotypes of the infecting M. tuberculosis strains isolated from their respective IC patients. The levels of IFN-γ which were significantly different between groups (Mann-Whitney test) are indicated. The horizontal bars indicate the median number of spots per 106 PBMCs. ⧫, HHCs; ⋄, TBHCs.
With all the antigens tested here, there was no statistically significant difference between the IFN-γ responses induced by M. bovis strains and M. tuberculosis strains in patients and in contacts (P > 0.05) (data not shown).
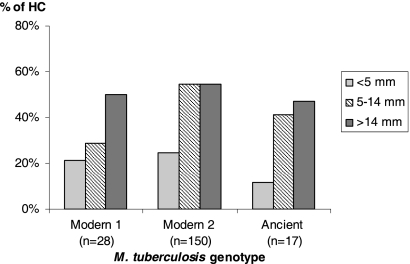
The IFN-γ responses were proportional to the tuberculin skin test (TST) response (Table 3). However, there was no significant correlation between the TST results for the HCs and the genotype of the strain isolated from their respective ICs (Fig. 4).
TABLE 3.
Correlation between ESAT-6-, PPD-, and CFP7-induced IFN-γ responses and TST results in household contacts
| Antigen | Median no. of spots/106 cells for individuals with the following TST result: |
P valueb | ||
|---|---|---|---|---|
| <5 mm (na = 34) | 5-14 mm (n = 40) | >14 mm (n = 75) | ||
| ESAT-6 | 17.5 | 37.5 | 115 | 0.000006 |
| PPD | 92.5 | 130 | 175 | 0.0034 |
| CFP7 | 22.5 | 45 | 135 | 0.00012 |
n, number of subjects.
Kruskal-Wallis test.
FIG. 4.
TST results for household contacts, according to the genotype of the M. tuberculosis isolates from the index case patients.
Three months following recruitment (and the initiation of treatment of the ICs) the PPD-induced IFN-γ responses remained higher in the contacts of patients infected with EAI strains than in the contacts of patients infected with modern group 1 strains (Fig. 3B), but no difference was observed between the contacts of patients infected with modern group 1 and modern group 2 strains. The ESAT-6-induced IFN-γ responses were lower in the contacts of patients infected with modern group 1 strains than in the contacts of patients infected with modern group 2 or ancient strains (P = 0.0076). With the CFP7 antigen, no difference was observed between the different groups of household contacts.
DISCUSSION
A number of studies have used the magnitude of the IFN-γ response to ESAT-6 for the diagnosis of M. tuberculosis infection in different populations (4, 16, 32), but this is the first study addressing the issue of strain-specific differences in IFN-γ responses in humans, although strain-specific variability has been observed in animal models (44).
In Madagascar, TB is still a serious public health problem, and in common with the practice in many countries where TB is endemic, the NTCP focuses on the diagnosis and treatment of smear-positive patients to stop the transmission of bacilli. However, currently, there is no actual policy regarding the detection of M. tuberculosis infection among the family and the contacts of contagious cases. It should be noted that BCG vaccination is common, and whether it is due to this or to exposure to atypical mycobacteria, the tuberculin test is positive for a high proportion of the healthy population (13). ESAT-6 is encoded by the RD1 genomic region, which is present in M. tuberculosis and pathogenic M. bovis but which is lacking in all BCG vaccine strains. However, it is an important virulence factor and is highly conserved in clinical TB strains, and therefore, this antigen is expected to be recognized by M. tuberculosis-infected subjects but not by healthy vaccinated subjects (6), and the magnitude of the response to ESAT-6 has been used as a proxy marker for both the bacterial load and exposure to M. tuberculosis (3, 32). We thus used PPD, ESAT-6, and the ESAT-6-related antigen CFP7 (also called TB10.4; this antigen is an ESAT-6 family member, but unlike ESAT-6, it is also present in BCG) to stimulate PBMCs from the recruited cohorts to determine whether the genotype of the infecting strain might have an influence on the IFN-γ response induced by these antigens.
While there is some evidence of strain-specific virulence, for example, from animal models, an association between molecular epidemiology and experimental model systems showed the improved growth of clustered strains in THP-1 cells compared with that of unique strains and the more efficient infection of human monocyte-derived macrophages with extrarespiratory strains than pulmonary strains (26, 58). Furthermore, a cell culture study in Uganda separated isolates on the basis of their frequency of transmission to household contacts, and those which transmitted efficiently showed more rapid growth in THP-1 cells than nontransmitted isolates (57). However, so far there are few data on the effects of M. tuberculosis isolates with different genotypes on clinical presentation or outcome in human TB. In a previous study, we did not find any obvious association between the IS6110-based restriction fragment length polymorphism (IS6110-RFLP) patterns and the clinical presentation of tuberculosis (47). However, there was some association of specific IS6110-RFLP patterns with disease presentation, suggesting that some strains may have the ability to disseminate more easily than others. Similarly, studies performed in Vietnam reported that the Euro-American lineage (corresponding to the modern group 2 strains [5]) associated with radiographically detected lung consolidation in pulmonary TB patients was more likely to result in pulmonary TB than meningeal TB (59) and less likely to cause extrapulmonary disease (12). In The Gambia, contacts exposed to Beijing strains were most likely to develop TB disease (14). Another study conducted in China associated the Beijing strains with less fever and night sweats as well as less cavitary disease in patients (54), consistent with the hypothesis that these strains induce the production of fewer proinflammatory mediators. Ulrichs et al. (61) reported that the number of IFN-γ-producing cells was higher in TB patients after treatment than in untreated TB patients. Controversially, however, others found lower numbers of IFN-γ-producing cells after treatment and that patients with cavitary disease had higher counts of ESAT-6-secreting cells than those without cavitary disease, suggesting that there may be a relationship between IFN-γ release and bacterial load (49). Since Beijing strains were found to be associated with less cavitary disease in Chinese patients (54), we expected that the IFN-γ response to ESAT-6 induced by Beijing strains will be lower than that induced by non-Beijing strains. Our results are concordant with this hypothesis. However, we did not find any significant difference in the ESAT-6-induced IFN-γ response in index cases according to the bacterial load. Furthermore, even though modern group 1 strains tend to give a lower bacterial load than the other strains, there was no statistically significant influence of the infecting strains' spoligotype on the bacterial load in the sputum of TB patients. It is probable that other factors, such as individual variability (49), may influence disease importance. However, in the present study, we found a weaker ESAT-6-induced-IFN-γ responses in patients infected with modern group 1 strains (CAS and Beijing) than in patients infected with strains of other genotypes, consistent with the hypothesis that these strains induce lower inflammatory responses.
These results are compatible with the finding that Beijing strains induced the poorer expression of IFN-γ in the mouse M. tuberculosis infection model (39). The authors suggested that, consequently, the Th1 cells were inefficiently activated, leading to the progression of TB disease. Our results are also compatible with those of an assay with a THP-1 monocytic cell line model, where the Beijing and CAS strains induced low levels of tumor necrosis factor alpha secretion and IFN-γ production in the whole-blood assays among healthy BCG-vaccinated volunteers (56). IFN-γ is one of the major components of the Th1 immune response against M. tuberculosis. However, the Th1 response also leads to the inflammation and pathology associated with TB (granulomatous inflammation and necrosis). Therefore, a low IFN-γ rate could explain the low histopathological score, the disseminated infection, and the higher virulence observed with Beijing strains in some studies (22, 28, 35, 39, 62). Some experimental models suggest that Beijing strains induced the downregulation of proinflammatory cytokines (44, 48, 50) associated with the production of the phenolic glycolipid (PGL) (33), a cell wall component important in the pathogenic process and in strain dissemination and survival (60).
Since, in contrast to TST, the ESAT-6 ELISPOT assay results had a strong positive relation with increasing exposure and were not correlated with BCG vaccination status, this test could allow the more accurate identification of symptom-free infected individuals recently exposed to M. tuberculosis (37). In countries with a low TB incidence, the ESAT-6-induced IFN-γ response was found to be sensitive and specific in diagnosing latent TB infection (18, 31), and exposed contacts with high levels of IFN-γ production have a greater possibility of developing active TB than those with lower IFN-γ levels (30). However, in high-TB-incidence countries, different data were obtained. For example, a study in Ethiopia reported that the high IFN-γ response to ESAT-6 by healthy household contacts of TB patients correlates with the subsequent development of active TB during a 2-year follow-up period (21). However, other data suggested that the level of IFN-γ response to ESAT-6 alone is not indicative of disease progression and that the response to another specific antigen, Rv2031, may be important (16). Prospective studies are still required to determine if the IFN-γ response is predictive of a high risk of active TB progression in high-burden settings (17). In the present study, the positive reactivity of household contacts to ESAT-6 antigen at the inclusion period did not correlate with disease progression during follow-up, since there was no significant difference in the ELISPOT assay response between HHCs and household contacts who developed TB disease (TBHCs) (Table 2). Even if most of the index cases of contacts who developed TB disease during follow-up were infected by modern group 2 strains, no significant association was found, since the TBHC sample size was too small. It was then interesting to determine whether the ESAT-6-induced IFN-γ response, i.e., latent infection among contacts, was associated with the genotype of the strains isolated from index cases.
The EAI family spoligotype was previously found to be relatively frequent (23.5%) in Madagascar (10, 23), suggesting that this spoligotype is widely transmitted in the Malagasy population. We did not see an IFN-γ response in EAI-infected index cases significantly different from that in index cases infected with any of the other strains of M. tuberculosis. However, in response to stimulation with ESAT-6, the ELISPOT assay count was significantly higher in the household contacts of EAI-infected patients than in the household contacts of CAS/Beijing-infected patients. These results therefore seemed to confirm the observation of others regarding the fact that modern group 1 strains seem to induce a weaker immune response than either the ancient EAI lineage or the strains of the modern group 2 lineage.
These results can be interpreted in several different ways. It is possible that the lower ESAT-6-specific IFN-γ responses observed among the HCs of modern group 1-infected patients may reflect a lower capacity of these strains to be transmitted, perhaps because the longer history of EAI strains in the region may have allowed them to better infect Malagasy individuals. However, this interpretation is hard to reconcile with the rapid spread of modern group 1 lineages in the region. Another explanation could be that the transmission rate is similar for all strains but that the IFN-γ response resulting from the infection is higher in the contacts of EAI-infected patients than in those of modern group 1 strain-infected patients. If (as we believe) this is true, it may affect the relative virulence of EAI and modern-lineage strains, explaining their relative prevalence in Madagascar (10).
Regarding the results obtained with PPD and CFP7, the responses to PPD were higher than the responses to CFP7, since PPD contains several antigens, including CFP7. However, similar responses to both antigens were observed in TB patients and in contacts independently of the infecting strains. The identical results obtained with the PPD and CFP7 antigens could be explained by the use of the BCG vaccine in the country, since both antigens are also present in BCG vaccine strains.
This is the first study analyzing the ESAT-6- and PPD-induced IFN-γ responses and genotypes of infecting M. tuberculosis strains in humans. While the study provides some key findings, it should be noted that there are certain limits to the results obtained. First, in order to standardize the tests, only fresh PBMCs were used in the IFN-γ ELISPOT assay (20), limiting the number of available ELISPOT assay results. Second, the sample size of some clusters of strains was small, so these results, while they are statistically robust, must still be considered preliminary. Finally, this study relied on analysis of the IFN-γ response observed with circulating cells (PBMCs). Therefore, we must be prudent when interpreting those results, since they do not necessarily reflect what happens at the site of the infection.
For this analysis, we have focused on ESAT-6-induced IFN-γ, since this has been suggested to be a proxy for the bacterial load (42) and, possibly, for the risk of subsequent progression to disease (3, 21). It would, of course, be interesting to determine if these effects could be explained by the induction of other cytokines, for example, interleukin-4, which has been associated with a poor prognosis in TB patients (15, 25, 45) and which is thought to be preferentially induced by PGL from at least some strains of the Beijing lineage (40). Since the current data were gathered as part of a longitudinal cohort study, we are addressing these questions.
Some studies have described the global genetic population structure of M. tuberculosis and the geographical distribution of M. tuberculosis genotypes (10, 26), and the findings of those studies support the hypothesis of a human host-specific adaptation of the pathogen. It is known that strain genetic variation is important for vaccine development. For instance, geographic variation in the protective efficacy of BCG has been observed (2); that can be due to environmental factors, but it can also be due to differences in the vaccine strain. These observations may have implications for TB control and the development of new vaccines. Thus, our observations on the variations in the IFN-γ responses of the human host according to the genotype of the mycobacteria could be useful in performing more accurate IFN-γ response-based TB diagnostic tests or using other tools to monitor for a protective host immune response in clinical trials for new vaccines or therapies.
Ultimately, our findings suggest that genotyping of M. tuberculosis strains and immune responses to these strains may help identify markers of virulence that could be involved in the development of TB disease or, conversely, in the correlates of protection of the human host.
Acknowledgments
We thank Elie Jeanne Vololonirina, Antra Andriamiadamahatratra, and Rina Ralaiarijaona for technical assistance; the Centre de Biologie Clinique of the Institut Pasteur de Madagascar; the clinical physicians of the Dispensaire Anti-Tuberculeux d'Antananarivo; the Radiology Department of the Institut d'Hygiène Sociale in Antananarivo; the staff of the National Mycobacteria Laboratory of the Ministry of Health; and the National TB Control Programme of the Ministry of Health for their contribution to the study. We thank Christophe Sola for quality control of spoligotyping and for helping with determining the ST and family of spoligotypes.
This work was supported by the European Commission through EU contract ICA4-CT-2002-10052.
The VACSEL/VACSIS study group also includes Helen Fletcher (until 2003) and Louise Kim, University College London; Chifumbe Chintu, Gina Mulundu, and Peter Mwaba, University of Zambia School of Medicine, Lusaka, Zambia; K. P. W. J. McAdam (until 2003), Patrick Owiafe, David Warndorff (until 2001), Christian Lienhardt (until 2001), R. Brookes, and Phillip Hill (from 2001), MRC, The Gambia; and Howard Engers, Abraham Aseffa, Abebech Demissie, Markos Abebe, and Liya Wassie, AHRI.
Footnotes
Published ahead of print on 12 May 2010.
REFERENCES
- 1.Aagaard, C., T. T. Hoang, A. Izzo, R. Billeskov, J. Troudt, K. Arnett, A. Keyser, T. Elvang, P. Andersen, and J. Dietrich. 2009. Protection and polyfunctional T cells induced by Ag85B-TB10.4/IC31 against Mycobacterium tuberculosis is highly dependent on the antigen dose. PLoS One 4:e5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, P., and T. M. Doherty. 2005. The success and failure of BCG:implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 3:656-662. [DOI] [PubMed] [Google Scholar]
- 3.Andersen, P., T. M. Doherty, M. Pai, and K. Weldingh. 2007. The prognosis of latent tuberculosis: can disease be predicted? Trends Mol. Med. 13:175-182. [DOI] [PubMed] [Google Scholar]
- 4.Arend, S. M., P. Andersen, K. E. van Meijgaarden, R. L. Skjot, Y. W. Subronto, J. T. van Dissel, and T. H. Ottenhoff. 2000. Detection of active tuberculosis infection by T cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J. Infect. Dis. 181:1850-1854. [DOI] [PubMed] [Google Scholar]
- 5.Arnold, C. 2007. Molecular evolution of Mycobacterium tuberculosis. Clin. Microbiol. Infect. 13:120-128. [DOI] [PubMed] [Google Scholar]
- 6.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. [DOI] [PubMed] [Google Scholar]
- 7.Bifani, P. J., B. Mathema, N. E. Kurepina, and B. N. Kreiswirth. 2002. Global dissemination of the Mycobacterium tuberculosis W-Beijing family strains. Trends Microbiol. 10:45-52. [DOI] [PubMed] [Google Scholar]
- 8.Borgdorff, M. W., H. Van Deutekom, P. E. De Haas, K. Kremer, and D. Van Soolingen. 2004. Mycobacterium tuberculosis, Beijing genotype strains not associated with radiological presentation of pulmonary tuberculosis. Tuberculosis (Edinb.) 84:337-340. [DOI] [PubMed] [Google Scholar]
- 9.Brosch, R., S. V. Gordon, M. Marmiesse, P. Brodin, C. Buchrieser, K. Eiglmeier, T. Garnier, C. Gutierrez, G. Hewinson, K. Kremer, L. M. Parsons, A. S. Pym, S. Samper, D. van Soolingen, and S. T. Cole. 2002. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc. Natl. Acad. Sci. U. S. A. 99:3684-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brudey, K., J. R. Driscoll, L. Rigouts, W. M. Prodinger, A. Gori, S. A. Al-Hajoj, C. Allix, L. Aristimuno, J. Arora, V. Baumanis, L. Binder, P. Cafrune, A. Cataldi, S. Cheong, R. Diel, C. Ellermeier, J. T. Evans, M. Fauville-Dufaux, S. Ferdinand, D. Garcia de Viedma, C. Garzelli, L. Gazzola, H. M. Gomes, M. C. Guttierez, P. M. Hawkey, P. D. van Helden, G. V. Kadival, B. N. Kreiswirth, K. Kremer, M. Kubin, S. P. Kulkarni, B. Liens, T. Lillebaek, M. L. Ho, C. Martin, C. Martin, I. Mokrousov, O. Narvskaia, Y. F. Ngeow, L. Naumann, S. Niemann, I. Parwati, Z. Rahim, V. Rasolofo-Razanamparany, T. Rasolonavalona, M. L. Rossetti, S. Rusch-Gerdes, A. Sajduda, S. Samper, I. G. Shemyakin, U. B. Singh, A. Somoskovi, R. A. Skuce, D. van Soolingen, E. M. Streicher, P. N. Suffys, E. Tortoli, T. Tracevska, V. Vincent, T. C. Victor, R. M. Warren, S. F. Yap, K. Zaman, F. Portaels, N. Rastogi, and C. Sola. 2006. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol. 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canetti, G., N. Rist, and J. Grosset. 1963. Measurement of sensitivity of the tuberculous bacillus to antibacillary drugs by the method of proportions. Methodology, resistance criteria, results and interpretation. Rev. Tuberc. Pneumol. (Paris) 27:217-272. (In French.) [PubMed] [Google Scholar]
- 12.Caws, M., G. Thwaites, S. Dunstan, T. R. Hawn, N. T. Lan, N. T. Thuong, K. Stepniewska, M. N. Huyen, N. D. Bang, T. H. Loc, S. Gagneux, D. van Soolingen, K. Kremer, M. van der Sande, P. Small, P. T. Anh, N. T. Chinh, H. T. Quy, N. T. Duyen, D. Q. Tho, N. T. Hieu, E. Torok, T. T. Hien, N. H. Dung, N. T. Nhu, P. M. Duy, N. van Vinh Chau, and J. Farrar. 2008. The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog. 4:e1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Champetier de Ribes, G., G. Ranaivoson, E. Rakotoherisoa, B. Andriamahefazafy, and S. Blanchy. 1997. Annual risk of tuberculosis infection in Madagascar: study from 1991 to 1994. Bull. Soc Pathol. Exot. 90:349-352. (In French.) [PubMed] [Google Scholar]
- 14.de Jong, B. C., P. C. Hill, A. Aiken, T. Awine, M. Antonio, I. M. Adetifa, D. J. Jackson-Sillah, A. Fox, K. Deriemer, S. Gagneux, M. W. Borgdorff, K. P. McAdam, T. Corrah, P. M. Small, and R. A. Adegbola. 2008. Progression to active tuberculosis, but not transmission, varies by Mycobacterium tuberculosis lineage in The Gambia. J. Infect. Dis. 198:1037-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demissie, A., M. Abebe, A. Aseffa, G. Rook, H. Fletcher, A. Zumla, K. Weldingh, I. Brock, P. Andersen, and T. M. Doherty. 2004. Healthy individuals that control a latent infection with Mycobacterium tuberculosis express high levels of Th1 cytokines and the IL-4 antagonist IL-4delta2. J. Immunol. 172:6938-6943. [DOI] [PubMed] [Google Scholar]
- 16.Demissie, A., E. M. Leyten, M. Abebe, L. Wassie, A. Aseffa, G. Abate, H. Fletcher, P. Owiafe, P. C. Hill, R. Brookes, G. Rook, A. Zumla, S. M. Arend, M. Klein, T. H. Ottenhoff, P. Andersen, and T. M. Doherty. 2006. Recognition of stage-specific mycobacterial antigens differentiates between acute and latent infections with Mycobacterium tuberculosis. Clin. Vaccine Immunol. 13:179-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dheda, K., R. van Zyl Smit, M. Badri, and M. Pai. 2009. T-cell interferon-gamma release assays for the rapid immunodiagnosis of tuberculosis: clinical utility in high-burden vs. low-burden settings. Curr. Opin. Pulm. Med. 15:188-200. [DOI] [PubMed] [Google Scholar]
- 18.Diel, R., R. Loddenkemper, K. Meywald-Walter, S. Niemann, and A. Nienhaus. 2008. Predictive value of a whole blood IFN-gamma assay for the development of active tuberculosis disease after recent infection with Mycobacterium tuberculosis. Am. J. Respir. Crit. Care Med. 177:1164-1170. [DOI] [PubMed] [Google Scholar]
- 19.Dietrich, J., C. Aagaard, R. Leah, A. W. Olsen, A. Stryhn, T. M. Doherty, and P. Andersen. 2005. Exchanging ESAT6 with TB10.4 in an Ag85B fusion molecule-based tuberculosis subunit vaccine: efficient protection and ESAT6-based sensitive monitoring of vaccine efficacy. J. Immunol. 174:6332-6339. [DOI] [PubMed] [Google Scholar]
- 20.Doherty, T. M., A. Demissie, D. Menzies, P. Andersen, G. Rook, and A. Zumla. 2005. Effect of sample handling on analysis of cytokine responses to Mycobacterium tuberculosis in clinical samples using ELISA, ELISPOT and quantitative PCR. J. Immunol. Methods 298:129-141. [DOI] [PubMed] [Google Scholar]
- 21.Doherty, T. M., A. Demissie, J. Olobo, D. Wolday, S. Britton, T. Eguale, P. Ravn, and P. Andersen. 2002. Immune responses to the Mycobacterium tuberculosis-specific antigen ESAT-6 signal subclinical infection among contacts of tuberculosis patients. J. Clin. Microbiol. 40:704-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dormans, J., M. Burger, D. Aguilar, R. Hernandez-Pando, K. Kremer, P. Roholl, S. M. Arend, and D. van Soolingen. 2004. Correlation of virulence, lung pathology, bacterial load and delayed type hypersensitivity responses after infection with different Mycobacterium tuberculosis genotypes in a BALB/c mouse model. Clin. Exp. Immunol. 137:460-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Helali, N., and P. Vergez. 1993. Identification des mycobactéries. Feuillets Biol. 190:5-18. [Google Scholar]
- 24.Ferdinand, S., C. Sola, S. Chanteau, H. Ramarokoto, T. Rasolonavalona, V. Rasolofo-Razanamparany, and N. Rastogi. 2005. A study of spoligotyping-defined Mycobacterium tuberculosis clades in relation to the origin of peopling and the demographic history in Madagascar. Infect. Genet. Evol. 5:340-348. [DOI] [PubMed] [Google Scholar]
- 25.Fletcher, H. A., P. Owiafe, D. Jeffries, P. Hill, G. A. Rook, A. Zumla, T. M. Doherty, and R. H. Brookes. 2004. Increased expression of mRNA encoding interleukin (IL)-4 and its splice variant IL-4delta2 in cells from contacts of Mycobacterium tuberculosis, in the absence of in vitro stimulation. Immunology 112:669-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gagneux, S., K. DeRiemer, T. Van, M. Kato-Maeda, B. C. de Jong, S. Narayanan, M. Nicol, S. Niemann, K. Kremer, M. C. Gutierrez, M. Hilty, P. C. Hopewell, and P. M. Small. 2006. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 103:2869-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia de Viedma, D., G. Lorenzo, P. J. Cardona, N. A. Rodriguez, S. Gordillo, M. J. Serrano, and E. Bouza. 2005. Association between the infectivity of Mycobacterium tuberculosis strains and their efficiency for extrarespiratory infection. J. Infect. Dis. 192:2059-2065. [DOI] [PubMed] [Google Scholar]
- 28.Hanekom, M., G. D. van der Spuy, E. Streicher, S. L. Ndabambi, C. R. McEvoy, M. Kidd, N. Beyers, T. C. Victor, P. D. van Helden, and R. M. Warren. 2007. A recently evolved sublineage of the Mycobacterium tuberculosis Beijing strain family is associated with an increased ability to spread and cause disease. J. Clin. Microbiol. 45:1483-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harboe, M., T. Oettinger, H. G. Wiker, I. Rosenkrands, and P. Andersen. 1996. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect. Immun. 64:16-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higuchi, K., N. Harada, K. Fukazawa, and T. Mori. 2008. Relationship between whole-blood interferon-gamma responses and the risk of active tuberculosis. Tuberculosis (Edinb.) 88:244-248. [DOI] [PubMed] [Google Scholar]
- 31.Higuchi, K., Y. Kawabe, S. Mitarai, T. Yoshiyama, N. Harada, and T. Mori. 2009. Comparison of performance in two diagnostic methods for tuberculosis infection. Med. Microbiol. Immunol. 198:33-37. [DOI] [PubMed] [Google Scholar]
- 32.Hill, P. C., D. Jackson-Sillah, A. Fox, K. L. Franken, M. D. Lugos, D. J. Jeffries, S. A. Donkor, A. S. Hammond, R. A. Adegbola, T. H. Ottenhoff, M. R. Klein, and R. H. Brookes. 2005. ESAT-6/CFP-10 fusion protein and peptides for optimal diagnosis of Mycobacterium tuberculosis infection by ex vivo enzyme-linked immunospot assay in The Gambia. J. Clin. Microbiol. 43:2070-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huet, G., P. Constant, W. Malaga, M. A. Laneelle, K. Kremer, D. van Soolingen, M. Daffe, and C. Guilhot. 2009. A lipid profile typifies the Beijing strains of Mycobacterium tuberculosis: identification of a mutation responsible for a modification of the structures of phthiocerol dimycocerosates and phenolic glycolipids. J. Biol. Chem. 284:27101-27113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamerbeek, J., L. Schouls, A. Kolk, M. van Agterveld, D. van Soolingen, S. Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, M. Goyal, and J. van Embden. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong, Y., M. D. Cave, L. Zhang, B. Foxman, C. F. Marrs, J. H. Bates, and Z. H. Yang. 2007. Association between Mycobacterium tuberculosis Beijing/W lineage strain infection and extrathoracic tuberculosis: insights from epidemiologic and clinical characterization of the three principal genetic groups of M. tuberculosis clinical isolates. J. Clin. Microbiol. 45:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lalvani, A., R. Brookes, S. Hambleton, W. J. Britton, A. V. Hill, and A. J. McMichael. 1997. Rapid effector function in CD8+ memory T cells. J. Exp. Med. 186:859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lalvani, A., A. A. Pathan, H. Durkan, K. A. Wilkinson, A. Whelan, J. J. Deeks, W. H. Reece, M. Latif, G. Pasvol, and A. V. Hill. 2001. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet 357:2017-2021. [DOI] [PubMed] [Google Scholar]
- 38.Lazzarini, L. C., R. C. Huard, N. L. Boechat, H. M. Gomes, M. C. Oelemann, N. Kurepina, E. Shashkina, F. C. Mello, A. L. Gibson, M. J. Virginio, A. G. Marsico, W. R. Butler, B. N. Kreiswirth, P. N. Suffys, E. S. J. R. Lapa, and J. L. Ho. 2007. Discovery of a novel Mycobacterium tuberculosis lineage that is a major cause of tuberculosis in Rio de Janeiro, Brazil. J. Clin. Microbiol. 45:3891-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez, B., D. Aguilar, H. Orozco, M. Burger, C. Espitia, V. Ritacco, L. Barrera, K. Kremer, R. Hernandez-Pando, K. Huygen, and D. van Soolingen. 2003. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin. Exp. Immunol. 133:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manca, C., M. B. Reed, S. Freeman, B. Mathema, B. Kreiswirth, C. E. Barry III, and G. Kaplan. 2004. Differential monocyte activation underlies strain-specific Mycobacterium tuberculosis pathogenesis. Infect. Immun. 72:5511-5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maree, F., A. C. Hesseling, H. S. Schaaf, B. J. Marais, N. Beyers, P. van Helden, R. M. Warren, and J. F. Schoeman. 2007. Absence of an association between Mycobacterium tuberculosis genotype and clinical features in children with tuberculous meningitis. Pediatr. Infect. Dis. J. 26:13-18. [DOI] [PubMed] [Google Scholar]
- 42.Millington, K. A., J. A. Innes, S. Hackforth, T. S. Hinks, J. J. Deeks, D. P. Dosanjh, V. Guyot-Revol, R. Gunatheesan, P. Klenerman, and A. Lalvani. 2007. Dynamic relationship between IFN-gamma and IL-2 profile of Mycobacterium tuberculosis-specific T cells and antigen load. J. Immunol. 178:5217-5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicol, M. P., C. Sola, B. February, N. Rastogi, L. Steyn, and R. J. Wilkinson. 2005. Distribution of strain families of Mycobacterium tuberculosis causing pulmonary and extrapulmonary disease in hospitalized children in Cape Town, South Africa. J. Clin. Microbiol. 43:5779-5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ordway, D., M. Henao-Tamayo, M. Harton, G. Palanisamy, J. Troudt, C. Shanley, R. J. Basaraba, and I. M. Orme. 2007. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J. Immunol. 179:522-531. [DOI] [PubMed] [Google Scholar]
- 45.Ordway, D. J., L. Costa, M. Martins, H. Silveira, L. Amaral, M. J. Arroz, F. A. Ventura, and H. M. Dockrell. 2004. Increased interleukin-4 production by CD8 and gammadelta T cells in health-care workers is associated with the subsequent development of active tuberculosis. J. Infect. Dis. 190:756-766. [DOI] [PubMed] [Google Scholar]
- 46.Rasolofo-Razanamparany, V., H. Ramarokoto, G. Auregan, B. Gicquel, and S. Chanteau. 2001. A combination of two genetic markers is sufficient for restriction fragment length polymorphism typing of Mycobacterium tuberculosis complex in areas with a high incidence of tuberculosis. J. Clin. Microbiol. 39:1530-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rasolofo Razanamparany, V., D. Menard, G. Auregan, B. Gicquel, and S. Chanteau. 2002. Extrapulmonary and pulmonary tuberculosis in Antananarivo (Madagascar): high clustering rate in female patients. J. Clin. Microbiol. 40:3964-3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reed, M. B., P. Domenech, C. Manca, H. Su, A. K. Barczak, B. N. Kreiswirth, G. Kaplan, and C. E. Barry III. 2004. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 431:84-87. [DOI] [PubMed] [Google Scholar]
- 49.Ribeiro, S., K. Dooley, J. Hackman, C. Loredo, A. Efron, R. E. Chaisson, M. B. Conde, N. Boechat, and S. E. Dorman. 2009. T-SPOT.TB responses during treatment of pulmonary tuberculosis. BMC Infect. Dis. 9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sinsimer, D., G. Huet, C. Manca, L. Tsenova, M. S. Koo, N. Kurepina, B. Kana, B. Mathema, S. A. Marras, B. N. Kreiswirth, C. Guilhot, and G. Kaplan. 2008. The phenolic glycolipid of Mycobacterium tuberculosis differentially modulates the early host cytokine response but does not in itself confer hypervirulence. Infect. Immun. 76:3027-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skjot, R. L., I. Brock, S. M. Arend, M. E. Munk, M. Theisen, T. H. Ottenhoff, and P. Andersen. 2002. Epitope mapping of the immunodominant antigen TB10.4 and the two homologous proteins TB10.3 and TB12.9, which constitute a subfamily of the esat-6 gene family. Infect. Immun. 70:5446-5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith, P. G., and A. R. Moss. 1994. Epidemiology of tuberculosis, p. 47-59. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. American Society for Microbiology, Washington, DC.
- 53.Sorensen, A. L., S. Nagai, G. Houen, P. Andersen, and A. B. Andersen. 1995. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 63:1710-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun, Y. J., T. K. Lim, A. K. Ong, B. C. Ho, G. T. Seah, and N. I. Paton. 2006. Tuberculosis associated with Mycobacterium tuberculosis Beijing and non-Beijing genotypes: a clinical and immunological comparison. BMC Infect. Dis. 6:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tacquet, A., and F. Tison. 1961. Nouvelle technique d'isolement des mycobactéries par le lauryl sulfate de sodium. Ann. Inst. Pasteur (Paris) 100:676-680. [PubMed] [Google Scholar]
- 56.Tanveer, M., Z. Hasan, A. Kanji, R. Hussain, and R. Hasan. 2009. Reduced TNF-alpha and IFN-gamma responses to Central Asian strain 1 and Beijing isolates of Mycobacterium tuberculosis in comparison with H37Rv strain. Trans. R. Soc. Trop. Med. Hyg. 103:581-587. [DOI] [PubMed] [Google Scholar]
- 57.Theus, S. A., M. D. Cave, K. Eisenach, J. Walrath, H. Lee, W. Mackay, C. Whalen, and R. F. Silver. 2006. Differences in the growth of paired Ugandan isolates of Mycobacterium tuberculosis within human mononuclear phagocytes correlate with epidemiological evidence of strain virulence. Infect. Immun. 74:6865-6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Theus, S. A., M. D. Cave, and K. D. Eisenach. 2005. Intracellular macrophage growth rates and cytokine profiles of Mycobacterium tuberculosis strains with different transmission dynamics. J. Infect. Dis. 191:453-460. [DOI] [PubMed] [Google Scholar]
- 59.Thwaites, G., M. Caws, T. T. Chau, A. D'Sa, N. T. Lan, M. N. Huyen, S. Gagneux, P. T. Anh, D. Q. Tho, E. Torok, N. T. Nhu, N. T. Duyen, P. M. Duy, J. Richenberg, C. Simmons, T. T. Hien, and J. Farrar. 2008. Relationship between Mycobacterium tuberculosis genotype and the clinical phenotype of pulmonary and meningeal tuberculosis. J. Clin. Microbiol. 46:1363-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsenova, L., E. Ellison, R. Harbacheuski, A. L. Moreira, N. Kurepina, M. B. Reed, B. Mathema, C. E. Barry III, and G. Kaplan. 2005. Virulence of selected Mycobacterium tuberculosis clinical isolates in the rabbit model of meningitis is dependent on phenolic glycolipid produced by the bacilli. J. Infect. Dis. 192:98-106. [DOI] [PubMed] [Google Scholar]
- 61.Ulrichs, T., R. Anding, S. H. Kaufmann, and M. E. Munk. 2000. Numbers of IFN-gamma-producing cells against ESAT-6 increase in tuberculosis patients during chemotherapy. Int. J. Tuber. Lung Dis. 4:1181-1183. [PubMed] [Google Scholar]
- 62.van Crevel, R., R. H. Nelwan, W. de Lenne, Y. Veeraragu, A. G. van der Zanden, Z. Amin, J. W. van der Meer, and D. van Soolingen. 2001. Mycobacterium tuberculosis Beijing genotype strains associated with febrile response to treatment. Emerg. Infect. Dis. 7:880-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Soolingen, D., P. W. Hermans, P. E. de Haas, D. R. Soll, and J. D. van Embden. 1991. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J. Clin. Microbiol. 29:2578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.WHO. 2009. Global tuberculosis control 2009: epidemiology, strategy, financing, vol. WHO/HTM/TB/2009.411. World Health Organization, Geneva, Switzerland.