Abstract
Acquired immunodeficiency due to autoantibody against gamma interferon has recently been associated with opportunistic nontuberculous mycobacteriosis, especially among Southeast Asians. We report another 8 cases, all except one apparently immunocompetent hosts who suffered from concomitant or sequential infections by other intracellular pathogens causing penicilliosis, extraintestinal nontyphoidal salmonellosis, and burkholderiosis. The only case with an underlying immunodeficiency syndrome had systemic lupus erythematosus that was quiescent throughout the multiple infective episodes. Eight out of 10 (80.0%) patients with serological evidence of penicilliosis, 5 out of 7 (71.4%) with culture-positive extraintestinal nontyphoidal salmonellosis, 5 out of 28 (17.9%) with serological evidence of melioidosis, and 7 out of 13 (53.8%) with culture-positive nontuberculous mycobacteriosis possessed autoantibody against gamma interferon, whereas only 1 out of 100 patients with systemic lupus erythematosus did. Our study represents the first and largest case series linking this emerging immunodeficiency syndrome with these atypical infections in apparently immunocompetent hosts. Thus, we advocate that any patient with unexplained recurrent or polymicrobial infections due to these intracellular pathogens should be screened for acquired immunodeficiency due to autoantibody against gamma interferon.
Since Wheelock's first report in 1965 on the antiviral activity of gamma interferon (IFN-γ) in the supernatant fluid of cultures of fresh human leukocytes after incubation with phytohemagglutinin, a wide range of other biological activities, including antimicrobial, anti-inflammatory, and immunomodulating effects, have been attributed to this unique cytokine (1). Recently, autoantibody against IFN-γ has been linked with severe disseminated nontuberculous mycobacteriosis in patients without classical cell-mediated immune defects, such as transplantations (hematopoietic stem cells and solid organs), malignancies (especially hematological), systemic immunosuppressive therapies, and AIDS due to human immunodeficiency virus (HIV) infection (4, 5, 8, 10, 11, 14, 20). Patients with these risk factors are also prone to infections by other intracellular pathogens, such as Penicillium marneffei, nontyphoid Salmonella enterica, and Burkholderia spp. (6, 13, 22). While sporadic cases of penicilliosis have been reported among non-HIV-infected Southeast Asians and travelers returning from the region (3, 9, 15, 23-25), concomitant or sequential infections with these intracellular pathogens in both apparently immunocompetent hosts and those with systemic lupus erythematosus (SLE) are extremely rare in the literature. In this study, we first describe in detail the clinical courses of 3 cases with autoantibody against IFN-γ who suffered from recurrent culture-positive nontuberculous mycobacteriosis, penicilliosis, and nontyphoidal salmonellosis and then further elaborate on the findings of the immunological workup of these 3 patients and 5 others who also exhibited clinically significant opportunistic infections with the background of autoantibody against IFN-γ.
CASE REPORTS
Case 1: recurrent nontuberculous mycobacteriosis.
A 42-year-old woman presented with pyrexia of unknown origin and bilateral cervical lymphadenopathy in 1998. The lymph node culture yielded Mycobacterium chelonae (sensitive only to imipenem), and histology showed granulomatous inflammation (Table 1). She responded to a 6-month course of intravenous imipenem, but her symptoms recurred once the antibiotic was stopped, and therefore, further treatment was given. However, her symptoms recurred soon after the antibiotic was stopped each time. In 2004, when she had been free from antibiotics for 6 months, she presented with fever, left elbow joint swelling, and cervical and left axillary lymphadenitis. A computerized tomography scan of the thorax and abdomen showed multiple lymphadenopathy and splenic microabscesses. The left elbow synovium and the left axillary lymph node biopsies both yielded Mycobacterium kansasii. In view of the splenic abscesses, serology of other opportunistic infections was checked and revealed high titers of antibody against P. marneffei and Burkholderia pseudomallei. Complete resolution of fever and joint swelling and partial reduction of lymphadenopathy and antibody titers were attained by the use of itraconazole, meropenem, amikacin, and tigecycline in combination. However, her symptoms recurred again once the antimicrobials were stopped. Extensive immunological workup, including HIV antibody, human T-lymphotropic virus type 1 antibody, nitroblue tetrazolium (NBT) reduction assay, bone marrow examination, lymphocyte subset, mitogen-stimulated cytokine profile, and immunoglobulin pattern, was unremarkable (Table 2). Mutations of genes encoding type I IFN pathways (e.g., interleukin 12 [IL-12] and IFN-γ receptors) were not found.
TABLE 1.
Epidemiological, clinical, and microbiological details of our case seriesa
| Case no. | Sex | Age (at onset) (yr) | Test results |
||||
|---|---|---|---|---|---|---|---|
| Penicilliosis | Non-tuberculous mycobacteriosis | Non-typhoidal salmonellosis | Melioidosis | Other | |||
| 1 | F | 42 | Serology >1:5,120 | LN, M. chelonae (1999, 2000, 2004); joint, M. kansasii (2005, 2007) | X | IgM, IgG | X |
| 2 | F | 45 | LN culture (2001, 2006); serology, >1:2,560 | LN, MAI (1998); BMA, MAI (2001); LN, M. fortuitum (2007) | X | X | HBV carrier; lung granuloma 2004 |
| 3 | F | 39 | Serology, 1:320 | LN and alveolar fluid, M. kansasii (2001) | Recurrent bacteremia and tubo-ovarian abscess | X | SLE on steroid |
| 4 | F | 67 | Serology, 1:640 | Blood and bone marrow, MAI (2008) | X | IgG | Paraproteinemia |
| 5 | M | 53 | Serology, 1:320 | LN, M. chelonae (2009) | Recurrent bacteremia, stool and alveolar fluid culture positive | IgG | X |
| 6 | M | 49 | Serology, 1:1,280 | X | Recurrent bacteremia, stool culture positive | IgG | Multiple LN, Bell's palsy, pericardial and pleural effusion |
| 7 | M | 87 | Serology, 1:320 | Sternal wound and sputum, MAI | Bacteremia once, stool culture positive | IgG | Multiple LN; HBV carrier; IGT and IHD |
| 8 | M | 54 | LN culture (2007); serology, 1:2,560 | LN, M. chelonae | Buttock abscess | X | HBV carrier; DM; pericardial and pleural effusion |
BMA, bone marrow aspirate; CSF, cerebrospinal fluid; DM, diabetes mellitus; F, female; HBV, hepatitis B virus; IGT, impaired glucose tolerance; IHD, ischemic heart disease; M, male; X, negative.
TABLE 2.
Immunological workup
| Case | Resultsa |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-stimulated cytokine profileb |
IgG (819-1,725) | IgA (70-386) | IgM (55-307) | B cells (160-708) | CD3 (716-2,011) | CD4 (415-1,418) | CD8 (292-1,258) | NK cells (158-1,156) | ANA (highest) | ||
| IFN-γ | IL-12 | ||||||||||
| 1 | Unstimulated, 0 (N); PHA induced, 2,480 (N); ConA induced, 10,440 (N) | Unstimulated, 15 (N); PHA induced, 95 (L); SAC induced, 100 (N) | 1,930 | 382 | 351 | 123 | 1,189 | 534 | 626 | 287 | 1:80 |
| 2 | Unstimulated, 0 (N); PHA induced, 4,440 (H); ConA induced, 13,950 (H) | Unstimulated, 0 (N); PHA induced, 75 (L); SAC induced, 35 (L) | 4,950 | 243 | 86 | 81 | 822 | 3,997 | 363 | 769 | NA |
| 3 | NA | NA | 2,060 | 61 | 67 | 62 | 1,349 | 671 | 712 | 415 | 1:160 |
| 4 | NA | NA | 2,480 | 252 | 150 | 45 | 933 | 506 | 347 | 495 | Neg |
| 5 | NA | NA | 1,110 | 228 | 113 | 263 | 1,048 | 444 | 627 | 140 | Neg |
| 6 | Unstimulated, 0 (N); PHA induced, 12,240 (H); ConA induced, 26,280 (H) | Unstimulated, 0 (N); PHA induced, 280 (N); SAC induced, 160 (N) | 1,660 | 330 | 170 | 70 | 2,530 | 1,170 | 1,340 | 40 | 1:80 |
| 7 | NA | NA | 4,440 | 440 | 98 | NA | NA | NA | NA | NA | 1:80 |
| 8 | NA | NA | 5,480 | 294 | 113 | NA | NA | NA | NA | NA | Neg |
ANA, anti-nuclear antibody; CD, cluster of differentiation; ConA, concanavalin A; H, high; L, low; N, normal; NA, not available; Neg, negative; NK, natural killer; PHA, phytohemagglutinin; SAC, Staphylococcus aureus Cowan-I. Abnormal results are in italics.
IFN-γ- and IL-12-producing cells were measured by enzyme-linked immunospot (ELISPOT) assays. Patients' peripheral blood mononuclear cells were unstimulated or stimulated with PHA, ConA, and SAC in plates with nitrocellulose membranes coated with anti-cytokine capture antibody. Cells with intracellular IL-12 or IFN-γ were detected by a biotinylated anti-cytokine detection antibody, streptavidin-alkaline phosphatase, and 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium chloride. The ELISPOTs were counted, and the numbers of cytokine-secreting cells per 106 peripheral blood mononuclear cells were calculated.
Case 2: recurrent penicilliosis and nontuberculous mycobacteriosis.
A 45-year-old female hepatitis B carrier was treated with standard 6-month antituberculous therapy based on clinical presentation of pyrexia of unknown origin and radiological evidence of tuberculosis in 1996. She developed a second episode of pyrexia of unknown origin in 1998, with a computerized tomography scan of the thorax and abdomen showing multiple hilar and intra-abdominal lymphadenopathies and numerous lytic lesions over the thoracic and lumbar spine. Culture of intra-abdominal lymph nodes was positive for Mycobacterium avium-M. intracellulare (MAI), and she was therefore treated with a combination of isoniazid, ethambutol, clarithromycin, ofloxacin, and amikacin from January 1999 to October 2000 (Table 1). Her fever recurred again in March 2001, with a bone marrow biopsy specimen culture positive for MAI and submandibular lymphadenopathy culture positive for P. marneffei. In view of the undetermined significance of the isolation of P. marneffei and the clinical response to antimycobacterial combination antibiotics consisting of ethambutol, clarithromycin, amikacin, and ofloxacin, she was kept on the regimen until 2004, during which time she underwent segmental lung resection for diagnosis and therapy of a left upper lobe granuloma that was found on computerized tomography. However, despite surgery, her fever recurred soon after cessation of antibiotics, and she therefore resumed combination antimycobacterial therapy in February 2005. She developed cervical lymphadenopathy while on treatment in 2006, and a biopsy was performed. The culture showed P. marneffei, which responded to a 12-week course of antifungal therapy consisting of amphotericin B for 2 weeks, followed by 10 weeks of itraconazole. The cervical lymphadenopathy recurred in May 2007, and the biopsy specimen was culture positive for Mycobacterium fortuitum. The antibiotic regimen was consequently changed to clarithromycin, levofloxacin, and amikacin. Immunological workup revealed IL-12 deficiency, while the HIV antibody, NBT reduction, and dihydrorhodamine reduction assays were unremarkable. Her lymphocyte subset and immunoglobulin pattern are shown in Table 2.
Case 3: recurrent nontyphoidal salmonellosis and nontuberculous mycobacteriosis.
A 39-year-old woman with SLE diagnosed in 2000 developed bacteremia due to highly susceptible Salmonella enterica serotype Enteritidis in 2001 while on hydroxychloroquine and prednisolone (10 mg daily). She responded to cefoperazone/sulbactam followed by ampicillin. However, she developed another episode of bacteremia with S. enterica serotype Typhimurium 4 months later, which responded to levofloxacin. Three months later, she developed an acute left tubo-ovarian abscess, which was culture positive for group B S. enterica and responded to treatment with ciprofloxacin. A chest radiograph showed a left upper zone shadow, and a computerized tomography scan of the thorax showed a soft tissue mass in the left upper lobe, together with multiple enlarged lymph nodes in the supraclavicular area, mediastinal space, pretracheal space, and subcarinal space. Histology of the left supraclavicular lymph node showed acid-fast bacilli, and bronchoalveolar lavage yielded M. kansasii (Table 1). The P. marneffei serology was 1:320. She was given azithromycin, rifampin, and levofloxacin for a total of 18 months. Her immunological workup, including HIV antibody, NBT reduction, and dihydrorhodamine reduction assays, was unremarkable. Her autoimmune markers were quiescent during the various infective episodes, and her immunosuppressive therapy had not been escalated. Her lymphocyte subset and immunoglobulin pattern are shown in Table 2.
MATERIALS AND METHODS
The study was approved by the Institutional Review Board of the Hospital Authority in Hong Kong.
Collection of patient sera.
Screening for autoantibody against IFN-γ was performed on archived sera collected between January 2003 and October 2008 from adult HIV-negative patients who were positive by P. marneffei indirect immunofluorescence (with any titer at or above 1:40), Widal test (any titer), or B. pseudomallei enzyme immunoassay (EIA) (either IgG or IgM positive) and from those with an unusually severe or atypical presentation of common intracellular infections, including extrapulmonary tuberculosis, nontuberculous mycobacteriosis, and extraintestinal nontyphoidal salmonellosis. Patients with SLE were also tested, while those with HIV infection or below age 18 were excluded.
Screening EIA for detection of autoantibody against IFN-γ.
One thousand nanograms of IFN-γ (R&D Systems, Inc.) was used to coat each well of a Nunc immunoplate (Nalge Nunc International, Denmark). After 24 h of incubation, 300 μl of blocking solution (5% normal goat serum in phosphate-buffered solution) was added to each well and incubated for 1 h before a washing step. Patients' sera were diluted 1,000 times with 5% normal goat serum in phosphate-buffered solution, and 100 μl was then added to each well for 1 h of incubation at 37°C. Goat anti-human total Ig horseradish peroxidase conjugate (Zymed; for primary screening prior to a spiking assay) and/or goat anti-human IgG (Biosource; for secondary isotyping of Ig in confirmed cases after the spiking assay) were then added, with tetramethylbenzidine as a substrate. Optical density (OD) values were read at 450 nm. All assays were performed in duplicate.
Plasma spiking with IFN-γ.
A spiking assay with total Ig conjugate was performed for samples with an OD value higher than 0.5 to confirm the presence of autoantibody. Diluted patient serum samples (6 different dilutions) were spiked with 1,000 pg of IFN-γ (R&D systems, Inc.) and incubated for 1 h at 37°C in order to bind with the autoantibody against IFN-γ. Unbound IFN-γ was then detected with a human IFN-γ enzyme-linked immunosorbent assay (ELISA) kit (BD OptEIA; BD Diagnostics) according to the manufacturer's instructions. All assays were performed in duplicate.
RESULTS
Between January 2003 and October 2008, a total of 240 serum samples were tested. They included 33 samples with a P. marneffei indirect-immunofluorescence titer of 1:40 or above, 37 samples with positive titers in the Widal test, 28 samples with antibodies (IgG, IgM, or both) against B. pseudomallei, 35 samples from patients with disseminated mycobacteriosis, 7 samples from patients with extraintestinal nontyphoidal salmonellosis, and 100 samples from patients with SLE (Table 3).
TABLE 3.
Mean OD values of 8 cases with autoantibody against IFN-γ and controls
| Category | Description | No. of cases | Mean OD valuea (range) |
|---|---|---|---|
| A | Cases with autoantibody against IFN-γ | 2.175 | |
| Case 1 | 2.649 | ||
| Case 2 | 2.814 | ||
| Case 3 | 1.319 | ||
| Case 4 | 2.809 | ||
| Case 5 | 2.593 | ||
| Case 6 | 2.9 | ||
| Case 7 | 0.959 | ||
| Case 8 | 1.358 | ||
| B | Penicilliosis | 33 | 0.061 (0.029-0.093) |
| Indirect immunofluorescence at or above 1:160 but without autoantibody against IFN-γ | 2/33 | ||
| C | Salmonellosis | 44 | 0.080 (0.021-1.143) |
| Widal test positive but without autoantibody against IFN-γ | 37/37 | ||
| Extraintestinal nontyphoidal salmonellosis but without autoantibody against IFN-γ | 2/7 | ||
| D | Burkholderiosis | 28 | 0.169 (0.022-1.026) |
| B. pseudomallei IgG and/or IgM positive but without autoantibody against IFN-γ | 23/28 | ||
| E | Mycobacteriosis | 35 | 0.043 (0.022-0.144) |
| Mycobacteriosis but without autoantibody against IFN-γ | 28/35 | ||
| F | Systemic lupus erythematosus | 100 | 0.048 (0.003-0.306) |
| Systemic lupus erythematosus but without autoantibody against IFN-γ | 99/100 |
From the screening assay for autoantibody against IFN-γ.
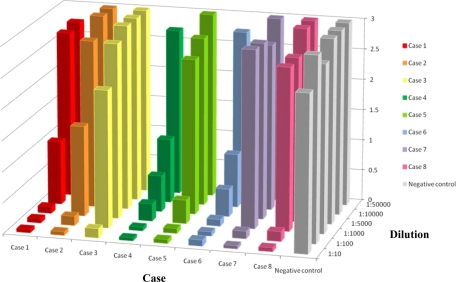
Altogether, autoantibody against IFN-γ was detected in the sera of 8 patients by EIA and confirmed by spiking assay. Their mean age was 54.5 years. The male-to-female ratio was 1:1, and all of them were of Chinese origin. All 8 (100%) patients had serological evidence of penicilliosis, with 2 of them having positive cultures from clinical specimens (Table 1). Seven (87.5%) of them also had culture-positive nontuberculous mycobacteriosis, 5 (62.5%) had culture-positive nontyphoidal salmonellosis, and 5 (62.5%) had serological evidence of melioidosis. All of the demonstrated autoantibodies were positive for IgG in the secondary isotyping of Ig. Their mean OD value for total Ig conjugate was 2.175, which was much higher than those of the controls (Table 3). Two of the 8 patients had detectable spiked IFN-γ in the plasma spiking assay only when their sera were diluted 10,000 to 50,000 times (Fig. 1).
FIG. 1.
Levels of unbound IFN-γ, expressed as OD values (vertical scale), from serial dilutions of the serum samples of the 8 patients and negative control after being spiked with IFN-γ. A lower OD value indicates a smaller fraction of unbound IFN-γ and thus a larger fraction of IFN-γ bound by the autoantibody in the patients' sera.
Among the 33 samples with a P. marneffei indirect-immunofluorescence titer of 1:40 or above, 10 had a titer of 1:160 or above, which was considered diagnostic of penicilliosis (26). Eight out of these 10 samples had an OD value above 0.5 in the screening EIA and showed autoantibody against IFN-γ in the spiking assay (cases 1 to 8). The mean OD value of the 2 cases (2/33) with a P. marneffei titer at or above 1:160 but without autoantibody against IFN-γ for total Ig conjugate was 0.061.
Among the 37 samples with a positive Widal test, 1 had an OD value above 0.5 in the screening EIA, but it did not show any autoantibody against IFN-γ in the spiking assay. Five of the 7 samples from patients with extraintestinal nontyphoidal salmonellosis demonstrated autoantibody against IFN-γ in the spiking assay (cases 3 and 5 to 8). The mean OD value of those with a positive Widal test (37/37) or extraintestinal nontyphoidal salmonellosis (2/7) but without autoantibody against IFN-γ for total Ig conjugate was 0.080.
Among the 28 samples with antibodies against B. pseudomallei, 6 had an OD value above 0.5 in the screening EIA, and 5 of them demonstrated autoantibody against IFN-γ in the spiking assay (cases 1 and 4 to 7). The mean OD value for total Ig conjugate of those with positive B. pseudomallei IgG and/or IgM but without autoantibody against IFN-γ (23/28) was 0.169.
Among the 35 patients with disseminated mycobacteriosis, 13 had nontuberculous mycobacteriosis and 22 had tuberculosis. Seven out of the 13 patients with disseminated nontuberculous mycobacteriosis (cases 1 to 5, 7, and 8) and none of those with tuberculosis had an OD value above 0.5 in the screening EIA and demonstrated autoantibody against IFN-γ in the spiking assay. The mean OD value for total Ig conjugate of those with disseminated mycobacteriosis but without autoantibody against IFN-γ (28/35) was 0.043.
One out of 100 randomly selected patients with SLE had an OD value above 0.5 in the screening EIA and demonstrated autoantibody against IFN-γ in the spiking assay (case 3). The mean OD value for total Ig conjugate of those with SLE but without autoantibody against IFN-γ (99/100) was 0.048.
DISCUSSION
We have described the first case series linking autoantibody against IFN-γ with penicilliosis, nontyphoidal salmonellosis, and melioidosis, in addition to disseminated nontuberculous mycobacteriosis, which has been previously reported (4, 5, 8, 10, 11, 14, 19). There is one recent study describing 5 out of 129 patients with disseminated nontuberculous mycobacteriosis who had concomitant penicilliosis, salmonellosis, or melioidosis, but the clinical information for these patients was not described (4).
Similar to the cases described in the literature (Table 4), the outcome of our cases has remained poor up to this stage. All of them suffered from recurrent infections requiring prolonged courses of antimicrobials, severely affecting the quality of their lives, and one succumbed because of comorbidities during hospitalization (case 7). For the other reported cases, similar outcomes, with two fatal cases, were described. One of our cases (case 1) and one reported case (11) had been given intravenous immunoglobulin for neutralization of the autoantibody and for immunomodulation. Our case had also undergone plasmapheresis for removal of autoantibody. However, the condition of neither patient improved.
TABLE 4.
Summary of all reported cases with autoantibody against IFN-γ in the literaturea
| Sexb | Age (yr) | Ethnicity | NTMc | Site | Other infectious diseasesd | AAbe | Outcomef | Reference |
|---|---|---|---|---|---|---|---|---|
| M | 47 | Filipino | M. chelonae | Pericardium; skin; pleura; LN; bone marrow; liver | Pulmonary and LN Mycobacterium tuberculos is; CMV viremia; oral candidiasis | IgG | Died | 5 |
| F | 25 | Thai | M. chelonae | Bone marrow | B. cocovenenans disseminated infection | IgG4 | Died | 8 |
| F | 46 | British | MAI | Bone; neck mass | None | IgG3 | Healthy on long-term IFN-γ | 10 |
| M | 32 | South African | MAI | Spine | None | IgM | Improved after antibiotics | 10 |
| F | 59 | British | M. fortuitum | Lung | Pulmonary aspergillosis | IgM | Improved after antibiotics | 10 |
| F | 44 | Japanese | MAI | Bone and muscle abscesses | None | IgG | Improved after drainage; antibiotics and IVIg | 11 |
| F | 35 | Taiwanese | MAI | Bone; skin and soft tissue | Pneumonia | IgG | Complete resolution | 14 |
| F | 40 | Filipino | MAI; M. chelonae | LN; lung; skin | Meningoencephalitis | IgG | Persistent infection | 14 |
| F | 39 | Chinese-Vietnamese | Mycobacterium szulgai; M. kansasii; M. scrofulaceum | Bone; LN; lung; skin | M. tuberculosis | IgG | Persistent infection | 14 |
| F | 34 | Filipino | MAI; Mycobacterium abscessus; M. fortuitum | LN; lung | None | IgG | Persistent infection | 14 |
| F | 30 | Filipino | MAI | Appendix; bone; skin and soft tissue; pharyngeal space | VZV (multiple dermatomes) | IgG | Persistent infection | 14 |
| F | 61 | Filipino | M. abscessus; MAI | LN; lung | HCV; Pseudomonas aeruginosa bacteremia; Enterobacter cloacae pneumonia; Achromobacter xylosoxidans bacteremia | IgG | Persistent infection | 14 |
| M | 54 | Japanese | MAI | Lung; pleura; bone marrow; LN | Streptococcus pyogenes bacteremia/osteomyelitis | IgG | Improved after antibiotics | 19 |
Five other cases of autoantibody against IFN-γ were reported without detailed information and therefore were not included in the table.
M, male; F, female.
NTM, nontuberculous mycobacteria.
CMV, cytomegalovirus; HCV, hepatitis C virus; VZV, varicella-zoster virus.
AAb, autoantibody.
IVIg, intravenous immunoglobulin.
Although we have not verified the biological function of the autoantibody against IFN-γ, previous reports have shown its blocking and neutralizing effects (8, 10, 11, 14, 20). In infections with nontuberculous mycobacteria, P. marneffei, nontyphoidal salmonella, and Burkholderia spp., it is highly likely that the autoantibody neutralizes the normal IFN-γ and impairs their intracellular killing by macrophages, leading to dissemination and recurrence. Furthermore, the blocking of the action of IFN-γ may result in a lower level of IL-12, as observed in case 2, due to the loss of positive feedback between IFN-γ and IL-12 (16).
P. marneffei is endemic in Southeast Asia and is the third most common AIDS-defining illness in that region. A patient with cell-mediated immunodeficiency who is susceptible to infection by environmental intracellular pathogens may be simultaneously infected by both nontuberculous mycobacteria and P. marneffei, as both are ubiquitous in the environment. Dimorphic fungal infections, like histoplasmosis (27) and paracoccidioidomycosis (12), have been reported in patients with Mendelian susceptibility to mycobacterial disease (MSMD) due to inborn errors of the IFN-γ pathway and IL-12/IL-23 pathways, respectively. Furthermore, it has been shown that IFN-γ is obligatory for host survival in mouse models of P. marneffei infection (18). Therefore, it is plausible that the autoantibody against IFN-γ predisposes these patients to penicilliosis.
Compared to nontuberculous mycobacteriosis and penicilliosis, fewer of our patients developed nontyphoidal salmonellosis as a result of limited exposure to the organism due to improved food hygiene. However, those with the infection tended to have recurrent bacteremia without an identifiable intravascular focus. It should be noted that patients with MSMD also developed nontyphoidal salmonellosis (2, 6, 13, 22).
Similar to nontuberculous mycobacteria and P. marneffei, B. pseudomallei is also endemic in Southeast Asia. A local seroprevalence survey done more than 20 years ago showed that up to 14% of patients in a tuberculosis sanatorium were positive for B. pseudomallei (19). There was one reported case of autoantibody against IFN-γ in a patient suffering from both M. chelonae and Burkholderia cocovenenans infection (8) in which Burkholderia was isolated from the patient's lymph nodes (LN). Phylogenetically, B. cocovenenans is closely related to B. pseudomallei (7). Although the immune response to burkholderiosis in humans is incompletely understood, IFN-γ may be important, as suggested by its therapeutic application in patients with chronic granulomatous disease who suffer from Burkholderia cepacia infection (21). Furthermore, IFN-γ plays an extremely vital role in protecting mice from acute lethal B. pseudomallei infection (17). The 50% lethal dose for the mice that received neutralizing monoclonal antibody against IFN-γ was 5 log units lower than that for the controls, and their bacterial burdens in the liver (8,500-fold) and spleen (4,400-fold) were markedly higher. By the same token, a much lower infective dose would suffice to cause infection in patients with autoantibody against IFN-γ.
In the present study, 1 out of 100 patients with SLE tested showed autoantibody against IFN-γ (case 3). Clinically, the patient had serological evidence of penicilliosis, culture-proven nontuberculous mycobacteriosis, and recurrent nontyphoidal salmonellosis despite having quiescent lupus activity all along. Although patients with SLE are susceptible to opportunistic infections due to cell-mediated immunodeficiency, the occurrence of concomitant infections due to P. marneffei, nontyphoidal salmonella, and B. pseudomallei has never been reported. Thus, the coexisting autoantibody against IFN-γ, rather than the underlying lupus activity alone, provides the likely explanation for the patient's strong predisposition to infections. Therefore, it would be prudent to perform screening for autoantibody against IFN-γ in patients with SLE who have unusually frequent or sequential infections by these pathogens, especially when the underlying disease activity remains stable.
It is also noteworthy that the majority of the cases in the literature are female (10/13 [77%]). This is not unexpected if autoantibody against gamma interferon is an autoimmune disease, which generally has a female predisposition. However, in our series, there was no gender predisposition, with a male-to-female ratio of 1 to 1. Further study of the genetic predisposition is warranted, in view of the high proportion of Asian ethnicity.
In conclusion, this is the first and largest case series linking penicilliosis, nontyphoidal salmonellosis, and burkholderiosis with acquired immunodeficiency due to autoantibody against IFN-γ. As individuals with this immunodeficiency syndrome may otherwise appear to be immunocompetent, any patient with unexplained recurrent or polymicrobial infections due to these intracellular pathogens should be tested. Furthermore, patients with underlying SLE who present with unusually frequent or concomitant infections due to these pathogens despite having quiescent lupus activity should also be tested, as the two conditions may coexist in a minority of them. Studies on the pathogenesis, as well as the treatment options, especially those that target lymphocytes and hence antibody production (e.g., monoclonal antibody against lymphocytes, such as rituximab), for these patients are urgently needed.
Acknowledgments
We thank Cindy W. S. Tse and the frontline medical staff for retrieval of patients' sera from Princess Margaret Hospital and Kwong Wah Hospital.
We have no conflicting interests.
This work was partly funded by the HKSAR Research Fund-commissioned Block Grant for the Control of Infectious Diseases and by the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Disease for the Department of Health, Health, Welfare, and Food Bureau, of the Hong Kong Special Administrative Region, China.
Footnotes
Published ahead of print on 5 May 2010.
REFERENCES
- 1.Billiau, M., and P. Matthys. 2009. Interferon-gamma: a historical perspective. Cytokine Growth Factor Rev. 20:97-113. [DOI] [PubMed] [Google Scholar]
- 2.Bustamante, J., S. Boisson-Dupuis, E. Jouanguy, C. Picard, A. Puel, L. Abel, and J. L. Casanova. 2008. Novel primary immunodeficiencies revealed by the investigation of paediatric infectious diseases. Curr. Opin. Immunol. 20:39-48. [DOI] [PubMed] [Google Scholar]
- 3.Chan, Y. H., K. M. Wong, K. C. Lee, P. C. Kwok, W. L. Chak, K. S. Choi, K. F. Chau, and C. S. Li. 2004. Pneumonia and mesenteric lymphadenopathy caused by disseminated Penicillium marneffei infection in a cadaveric renal transplant recipient. Transpl. Infect. Dis. 6:28-32. [DOI] [PubMed] [Google Scholar]
- 4.Chetchotisakd, P., S. Kiertiburanakul, P. Mootsikapun, S. Assanasen, R. Chaiwarith, and S. Anunnatsiri. 2007. Disseminated nontuberculous mycobacterial infection in patients who are not infected with HIV in Thailand. Clin. Infect. Dis. 45:421-427. [DOI] [PubMed] [Google Scholar]
- 5.Döffinger, R., M. R. Helbert, G. Barcenas-Morales, K. Yang, S. Dupuis, L. Ceron-Gutierrez, C. Espitia-Pinzon, N. Barnes, G. Bothamley, J. L. Casanova, H. J. Longhurst, and D. S. Kumararatne. 2004. Autoantibodies to interferon-gamma in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin. Infect. Dis. 38:e10-e14. [DOI] [PubMed] [Google Scholar]
- 6.Döffinger, R., S. Patel, and D. S. Kumararatne. 2005. Human immunodeficiencies that predispose to intracellular bacterial infections. Curr. Opin. Rheumatol. 17:440-446. [DOI] [PubMed] [Google Scholar]
- 7.Gee, J. E., C. T. Sacchi, M. B. Glass, B. K. De, R. S. Weyant, P. N. Levett, A. M. Whitney, A. R. Hoffmaster, and T. Popovic. 2003. Use of 16S rRNA gene sequencing for rapid identification and differentiation of Burkholderia pseudomallei and B. mallei. J. Clin. Microbiol. 41:4647-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Höflich, C., R. Sabat, S. Rosseau, B. Temmesfeld, H. Slevogt, W. D. Döcke, G. Grütz, C. Meisel, E. Halle, U. B. Göbel, H. D. Volk, and N. Suttorp. 2004. Naturally occurring anti-IFN-gamma autoantibody and severe infections with Mycobacterium cheloneae and Burkholderia cocovenenans. Blood 103:673-675. [DOI] [PubMed] [Google Scholar]
- 9.Hung, C. C., P. R. Hsueh, M. Y. Chen, C. H. Hsiao, S. C. Chang, and K. T. Luh. 1998. Invasive infection caused by Penicillium marneffei: an emerging pathogen in Taiwan. Clin. Infect. Dis. 26:202-203. [DOI] [PubMed] [Google Scholar]
- 10.Kampmann, B., C. Hemingway, A. Stephens, R. Davidson, A. Goodsall, S. Anderson, M. Nicol, E. Schölvinck, D. Relman, S. Waddell, P. Langford, B. Sheehan, L. Semple, K. A. Wilkinson, R. J. Wilkinson, S. Ress, M. Hibberd, and M. Levin. 2005. Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-gamma. J. Clin. Invest. 115:2480-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koya, T., C. Tsubata, H. Kagamu, K. Koyama, M. Hayashi, K. Kuwabara, T. Itoh, Y. Tanabe, T. Takada, and F. Gejyo. 2009. Anti-interferon-gamma autoantibody in a patient with disseminated Mycobacterium avium complex. J. Infect. Chemother. 15:118-122. [DOI] [PubMed] [Google Scholar]
- 12.Moraes-Vasconcelos, D., A. S. Grumach, A. Yamaguti, M. E. Andrade, C. Fieschi, L. de Beaucoudrey, J. L. Casanova, and A. J. Duarte. 2005. Paracoccidioides brasiliensis disseminated disease in a patient with inherited deficiency in the beta1 subunit of the interleukin (IL)-12/IL-23 receptor. Clin. Infect. Dis. 41:e31-e37. [DOI] [PubMed] [Google Scholar]
- 13.Ottenhoff, T. H., F. A. Verreck, E. G. Lichtenauer-Kaligis, M. A. Hoeve, O. Sanal, and J. T. van Dissel. 2002. Genetics, cytokines and human infectious disease: lessons from weakly pathogenic mycobacteria and salmonellae. Nat. Genet. 32:97-105. [DOI] [PubMed] [Google Scholar]
- 14.Patel, S. Y., L. Ding, M. R. Brown, L. Lantz, T. Gay, S. Cohen, L. A. Martyak, B. Kubak, and S. M. Holland. 2005. Anti-IFN-gamma autoantibodies in disseminated nontuberculous mycobacterial infections. J. Immunol. 175:4769-4776. [DOI] [PubMed] [Google Scholar]
- 15.Pun, T. S., and D. Fang. 2000. A case of Penicillium marneffei osteomyelitis involving the axial skeleton. Hong Kong Med. J. 6:231-233. [PubMed] [Google Scholar]
- 16.Rosenzweig, S. D., and S. M. Holland. 2005. Defects in the interferon-gamma and interleukin-12 pathways. Immunol. Rev. 203:38-47. [DOI] [PubMed] [Google Scholar]
- 17.Santanirand, P., V. S. Harley, D. A. Dance, B. S. Drasar, and G. J. Bancroft. 1999. Obligatory role of gamma interferon for host survival in a murine model of infection with Burkholderia pseudomallei. Infect. Immun. 67:3593-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sisto, F., A. Miluzio, O. Leopardi, M. Mirra, J. R. Boelaert, and D. Taramelli. 2003. Differential cytokine pattern in the spleens and livers of BALB/c mice infected with Penicillium marneffei: protective role of gamma interferon. Infect. Immun. 71:465-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.So, S. Y., P. Y. Chau, M. Aquinas, M. Gabriel, and W. K. Lam. 1987. Melioidosis: a serological survey in a tuberculosis sanatorium in Hong Kong. Trans. R. Soc. Trop. Med. Hyg. 81:1017-1019. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka, Y., T. Hori, K. Ito, T. Fujita, T. Ishikawa, and T. Uchiyama. 2007. Disseminated Mycobacterium avium complex infection in a patient with autoantibody to interferon-gamma. Intern. Med. 46:1005-1009. [DOI] [PubMed] [Google Scholar]
- 21.van den Berg, J. M., E. van Koppen, A. Ahlin, B. H. Belohradsky, E. Bernatowska, L. Corbeel, T. Español, A. Fischer, M. Kurenko-Deptuch, R. Mouy, T. Petropoulou, J. Roesler, R. Seger, M. J. Stasia, N. H. Valerius, R. S. Weening, B. Wolach, D. Roos, and T. W. Kuijpers. 2009. Chronic granulomatous disease: the European experience. PLoS One 4:e5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van de Vosse, E., M. A. Hoeve, and T. H. Ottenhoff. 2004. Human genetics of intracellular infectious diseases: molecular and cellular immunity against mycobacteria and salmonellae. Lancet Infect. Dis. 4:739-749. [DOI] [PubMed] [Google Scholar]
- 23.Wong, S. S., K. H. Wong, W. T. Hui, S. S. Lee, J. Y. Lo, L. Cao, and K. Y. Yuen. 2001. Differences in clinical and laboratory diagnostic characteristics of penicilliosis marneffei in human immunodeficiency virus (HIV)- and non-HIV-infected patients. J. Clin. Microbiol. 39:4535-4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong, S. S., P. C. Woo, and K. Y. Yuen. 2001. Candida tropicalis and Penicillium marneffei mixed fungaemia in a patient with Waldenström's macroglobulinaemia. Eur. J. Clin. Microbiol. Infect. Dis. 20:132-135. [DOI] [PubMed] [Google Scholar]
- 25.Woo, P. C., S. K. Lau, C. C. Lau, K. T. Chong, W. T. Hui, S. S. Wong, and K. Y. Yuen. 2005. Penicillium marneffei fungaemia in an allogeneic bone marrow transplant recipient. Bone Marrow Transplant. 35:831-833. [DOI] [PubMed] [Google Scholar]
- 26.Yuen, K. Y., S. S. Wong, D. N. Tsang, and P. Y. Chau. 1994. Serodiagnosis of Penicillium marneffei infection. Lancet 344:444-445. [DOI] [PubMed] [Google Scholar]
- 27.Zerbe, C. S., and S. M. Holland. 2005. Disseminated histoplasmosis in persons with interferon-gamma receptor 1 deficiency. Clin. Infect. Dis. 41:e38-e41. [DOI] [PubMed] [Google Scholar]