Abstract
Some individuals have experienced meningococcal disease despite receiving the meningococcal serogroup C conjugate (MCC) vaccine in adolescence. We sought to determine whether this is due to subclinical functional B- or T-cell immunodeficiency. Of 53 vaccine failures identified by enhanced surveillance of England and Wales from 1999 to 2004, 15 received MCC vaccine in adolescence, 9 of whom were recruited 2 to 6 years following convalescence from meningococcal disease. Their peripheral blood mononuclear cells (PBMCs) were incubated with polyclonal activators designed to mimic T-cell-independent B-cell stimulation by bacterial polysaccharides and the T-cell stimulation provided by the protein component of the conjugate vaccine. Subsequent proliferation and activation of T and B lymphocytes were measured, along with T-cell help to B cells. Compared to age-, sex-, geographically, and ethnicity-matched controls, CD4 T-cell proliferation rates in response to both anti-CD3 (T-cell receptor [TCR]) stimulation and anti-CD3 in the presence of B cells activated through anti-IgD conjugated to dextran (α-δ-dex) were lower in PBMCs derived from vaccine failures (P = 0.044 and P = 0.029, respectively). There was reduced CD4 cell activation of the patient cells compared to controls following stimulation by CD3 (P = 0.048). B-cell activation during incubation of PBMCs with the T-cell stimuli, anti-CD3 (P = 0.044), or anti-CD3 plus anti-CD28 (P = 0.018) was relatively impaired in patients. Anti-tetanus toxoid IgG concentrations were lower in the vaccine failure group (P = 0.0385). There was a relative defect of T-cell responsiveness to T-cell-dependent antigen stimulation in MCC vaccine failures, which was manifested in reduced T-cell help to B cells.
The meningococcal serogroup C conjugate (MCC) vaccine has markedly reduced the incidence of serogroup C meningococcal disease (MD) where it has been used (21), but the initial campaign did not protect all vaccinated people. By 2004, 53 vaccine failures (defined as a case of serogroup C MD occurring more than 10 days after vaccination with MCC vaccine) had been identified in the United Kingdom (1).
Acute- and convalescent-phase sera from these patients suggested there had been some initial protection induced by MCC vaccine that was boosted by disease, but the serum bactericidal antibody (SBA) titer at the time of subsequent contact with meningococci was not protective, and the memory response was too slow to confer protection (1). Most vaccine failures were infants. Since the majority of infants have a rapid wane of SBA titer (20), a booster dose of MCC vaccine at 1 year has now been added to the infant vaccine schedule in the United Kingdom. However, some vaccine failures were vaccinated in adolescence, a group that received a single dose of vaccine but in whom mature immune responses should occur (2).
Capsular polysaccharide (PS) is a T-cell-independent (TI-2) antigen. Polysaccharide-protein conjugate vaccines comprise the appropriate capsular polysaccharide covalently attached to a carrier protein, such as tetanus toxoid (TT), diphtheria toxoid (DT), or cross-reacting material (CRM) mutant toxin. Their superior efficacy compared to plain polysaccharide vaccines is due to the presentation of carrier epitopes by polysaccharide-specific B cells to carrier-specific T cells, followed by the provision of T-cell help to the PS-specific B cells. This T-cell help enhances B-cell proliferation, class switching, and memory B-cell formation (10). The levels of memory B cells induced correlate with both the initial antibody response and the persistence of the antibody response (3). The induction of a strong immune response against the polysaccharide component of the MCC vaccine therefore requires effective B-cell-T-cell cross talk in both directions.
As the immunological response to this vaccine is complex, it is possible that relative inefficiency of one or more components of the response might underlie variation in immunogenicity between individuals, the extreme manifestation of which is serogroup C disease in adolescents despite vaccination. Therefore, we tested the hypothesis that adolescent vaccine failures have an unidentified intrinsic defect in T- or B-cell function, or in cross talk between these cells, expressed as reduced activation and proliferation in response to appropriate stimuli.
MATERIALS AND METHODS
Patients.
Patients were recruited from the enhanced surveillance database of the Health Protection Agency (United Kingdom) and were residents of dispersed regions of England and Wales. They had microbiologically proven serogroup C meningococcal disease as determined by culture or PCR within a blood or cerebrospinal fluid sample at least 10 days following MCC vaccination. All of those over the age of 14 years at the time of the study were approached via their general practitioner. Those willing to participate were asked to nominate a local friend of the same sex, age, and ethnicity who had been vaccinated but had no history of meningitis and who would be willing to participate as a control, and information was then sent to their families. Patients and controls were seen together in their own community, where blood was taken and a health questionnaire was completed. Samples were then transported back to the laboratory within 12 h. The study was approved by the National Research Ethics Office for England and Wales.
Design of the cellular assay.
Peripheral blood mononuclear cells (PBMCs) were isolated from patients and controls and exposed to polyclonal activators that mimicked the components of conjugate vaccine using a previously described experimental system (9). To mimic B-cell responses to capsular polysaccharide component, PBMCs were exposed to anti-IgD conjugated to dextran (α-σ-dex). B-cell proliferation was assessed by carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution combined with CD19 staining to determine B-cell division. B-cell activation was assessed by flow-cytometric measurement of CD19 and CD86 expression. To measure T-cell responses to B cells, PBMCs were activated by α-σ-dex, together with suboptimal concentrations of anti-CD3 antibody, and T-cell proliferation and activation were assessed by CFSE dilution and flow-cytometric expression of CD4 and CD25. To assess subsequent T-cell help to B cells, PBMCs were activated using purified anti-CD3 and -CD28 antibodies, together with stimulation through the B-cell receptor using α-σ-dex. B-cell proliferation and activation and T-cell responses to the polyclonal T-cell activators were assessed as described above.
Cell culture.
PBMCs were prepared, and half of them were labeled with CFSE as previously described (9). Both labeled and unlabeled cells were then cultured with the following stimuli for 96 h in 48-well plates (1 × 106 cells/well): 500 μl medium alone (RPMI 1640 plus l-glutamine [Gibco, Invitrogen]) plus 10% autologous serum), 1 μg/ml α-δ-dex, wells precoated with 0.1 μg/ml purified anti-CD3, wells precoated with 0.1 μg/ml anti-CD3 plus 1 μg/ml α-δ-dex, wells precoated with 0.5 μg/ml purified anti-CD3 and 0.5 μg/ml purified anti-CD28, and wells precoated with 0.5 μg/ml anti-CD3 and anti-CD28 plus 1 μg/ml α-δ-dex.
Antibodies used for stimulation and flow-cytometric analysis.
All fluorescently labeled and unlabeled antibodies for stimulation (anti-CD28 and purified anti-CD3) were obtained from Caltag Laboratories (Buckingham, United Kingdom). α-δ-dex was prepared as described previously (17).
Phenotypic markers.
Following initial separation, the relative proportions of different mononuclear cells were estimated by flow cytometry. A sample of PBMCs was washed with fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline containing 0.1% bovine serum albumin [First Link {UK} Ltd., Birmingham, United Kingdom]) and labeled with cell markers: CD19-fluorescein isothiocyanate (FITC), CD14-phycoerythrin (PE), CD3-tricolor (TC), and CD4-allophycocyanin (APC). They were resuspended in FACS buffer and processed immediately using a FACSCalibur (BD Biosciences, CA), running CellQuest software (BD Biosciences). Data were analyzed using FlowJo software version 7.2 (Tree Star, Inc., Ashland, OR).
Cell activation and proliferation assay.
Cells for assessment of activation were labeled with CD19-FITC, CD86-TC, CD4-APC, and CD25-PE and analyzed as described above. Cells labeled with CFSE before culture, for assessment of proliferation, were further labeled with CD19-APC or CD4-APC prior to flow-cytometric analysis. The proliferation index was calculated by dividing the total number of cells at 0 to 8 divisions by the parent population. A proliferation index of 1 thus represents no division (personal communication from B. Lyons). CD86 and CD25 were chosen as activation markers of B and T cells, respectively, following preliminary studies, which showed good signal/noise ratios between activated and resting cells and lower natural variation in expression between control individuals than other potential activation markers studied (R. A. Foster, unpublished data).
Serology.
Meningococcal serogroup C SBA titers and IgG responses to diphtheria and tetanus toxoids and Haemophilus influenzae type B (Hib) polyribosylribitol phosphate (PRP) were measured at the Vaccine Evaluation Unit of the Health Protection Agency, Manchester, United Kingdom, using the standardized SBA assay and multiplex bead array assays as described previously (12, 13, 16, 18).
Statistical analysis.
Data were analyzed using Microsoft Excel, GraphPad Prism 5, and Microsoft Access. The fold increase in cell activation marker expression and proliferation following stimulation of cells was measured. As a reduction of activation and proliferation indices of cells from patients versus controls was anticipated, significance was assessed in each case using a paired one-tailed Student's t test. Likewise, anticipated reductions of serological responses to vaccines were analyzed using a one-tailed nonparametric test (Mann-Whitney U test).
RESULTS
Patient recruitment.
Of the 15 individuals classified as vaccine failures who were 14 years old or older at the time of the study, 9 agreed to participate and could be matched with a control. Their demographic information and time since illness and vaccination are shown in Table 1. None of the patients had a detectable deficiency in complement components or in total serum IgG, IgA, or IgM levels (Table 2). Of the nine patients included in the analysis, SBA titers were obtained in four cases from sera obtained during hospital admission (0 to 30 days after onset of disease). The SBA titers of these patients were 2, 2, 2, and 128. As the accepted “protective” SBA titer is considered to be ≥8 (4), 3 of these 4 patients did not have protective SBA titers at the onset of disease (Table 3) despite having received MCC vaccine.
TABLE 1.
Demographic details and clinical time intervals for vaccine failures and controls
| Patient no. | Sexa | Age at time of illness (yr) | Ethnicity | Time between illness and study (yr) | Time between vaccination and illness (mo) |
|---|---|---|---|---|---|
| 1 | M | 18 | Asian, Pakistani | 6 | 3 |
| 2 | M | 12 | White, Welsh | 5 | 10 |
| 3 | F | 18 | White, English | 5 | 17 |
| 4 | M | 12 | White, English | 5 | 11 |
| 5 | M | 18 | White, English | 4 | 26 |
| 6 | M | 13 | White, English | 4 | 8 |
| 7 | F | 19 | White, English | 3 | 38 |
| 8 | F | 19 | White, British | 3 | 41 |
| 9 | F | 12 | White, English | 2 | 40 |
M, male; F, female.
TABLE 2.
Complement and immunogolobulin levels in patients and controls
| Complement or immunogolobulin | Geometric mean titer |
P value of difference between patients and controls (Student's t test) | |
|---|---|---|---|
| Patients | Controls | ||
| CH50 (U/ml) | 27.66 | 23.62 | 0.533 |
| IgG (g/liter) | 11.55 | 11.33 | 0.796 |
| IgA (g/liter) | 2.043 | 2.516 | 0.265 |
| IgM (g/liter) | 1.296 | 1.277 | 0.927 |
TABLE 3.
Acute- and convalescent-phase and study SBA titers in vaccine failures
| Patient no. | SBA titer at hospital admission | Timing of acute-phase samplea | Convalescent-phase SBA titer | Timing of convalescent-phase samplea | SBA titer at time of study |
|---|---|---|---|---|---|
| 1 | 2,048 | 70 | 8,192 | 180 | 256 |
| 2 | 2 | 29 | 128 | 120 | 512 |
| 3 | NAb | NA | NA | NA | 32 |
| 4 | NA | NA | 65,536 | 14 | 512 |
| 5 | 2 | 0 | 8,192 | 32 | 128 |
| 6 | 128 | 0 | 8,192 | 88 | 256 |
| 7 | 2 | 3 | 514,288 | 10 | 1,024 |
| 8 | NA | NA | NA | NA | 512 |
| 9 | NA | NA | NA | NA | 512 |
Days after hospital admission.
NA, not applicable.
In these four patients, convalescent-phase titers, and even SBA titers at the time of the study (titers of 128, 512, 256, and 1,024), were significantly higher than acute-phase titers, and all were in the “protective” range, revealing that they were all capable of responding with high and sustained antibody titers after natural infection with serogroup C Neisseria meningitidis (Table 3).
Patient and control blood complement and immunoglobulin levels.
The CH50 assay (the CH50 measures the total hemolytic activity of a test sample and is the reciprocal of the dilution of serum complement needed to lyse 50% of a standardized suspension of sheep erythrocytes coated with antierythrocyte antibody) was performed on stored serum to screen for those with possible complement deficiency, followed by an assay of complement components C5 to C9 in those with a low CH50 result. None of the patients or controls was found to be complement deficient—even those with low initial CH50 (which could have been a consequence of sample handling and storage) had no deficiency of complement components C5 to C9. Immunoelectrophoresis was also performed on stored serum to assay for immunoglobulin deficiency. Again, none of the patients or controls was found to be immunoglobulin deficient, and the patients' immunoglobulin levels in the IgG, IgA, and IgM classes did not differ significantly from those of the controls. The geometric mean titers of these tests are shown in Table 2. Mannose binding lectin levels were also normal in the patients.
B-cell responses to polyclonal TI-2 mimics.
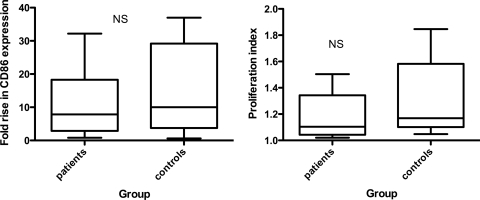
There were no detectable differences between patient and control cells in B-cell responsiveness to the polyclonal TI-2 mimic α-δ-dex, either in terms of B-cell activation (CD86 expression) or in terms of B-cell proliferation (Fig. 1).
FIG. 1.
Patient and control B-cell activation (left) and proliferation (right) in response to stimulation with α-δ-dex. The whiskers show minimum and maximum values. NS, no significant difference by Student's t test.
T-cell responses.
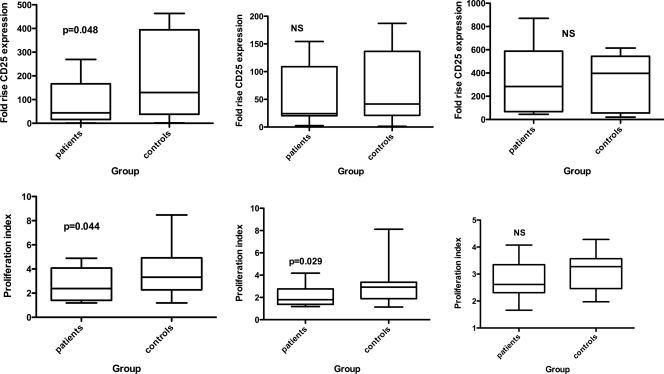
CD4 T-cell proliferation in response to both anti-CD3 (T-cell receptor [TCR]) stimulation and anti-CD3 in the presence of B cells activated through α-δ-dex was significantly lower in the patient than in the paired control samples (P = 0.044 and P = 0.029, respectively; Students t test). In both cases, control T cells underwent, on average, around one additional division over the 3-day incubation period (Fig. 2). There remained a trend toward lower proliferation of patients' cells even in the presence of anti-CD28, but the difference between patient and control cells was not statistically significant (P = 0.08).
FIG. 2.
Activation (top) and proliferation (bottom) of CD4 T cells of patients and controls in response to anti-CD3 alone (left), anti-CD3 and activated B cells (middle), and anti-CD3 plus anti-CD28 (right). The whiskers show maximum and minimum values; statistical analysis was by Student's t test.
CD25 expression was used as a marker for CD4 T-cell activation. There was a lower level of CD4 cell activation of the patient cells than of controls following stimulation by CD3 (P = 0.048), although in the presence of α-δ-dex or anti-CD28 stimulation, the difference between patient and control cells was not significant (Fig. 2).
T-cell help to B cells.
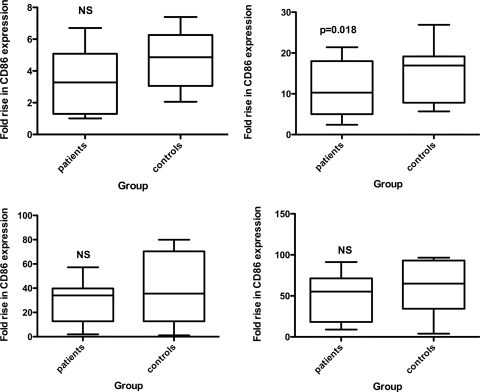
T-cell provision of bystander help to B cells was assessed by measuring B-cell activation in response to the T-cell stimuli, anti-CD3, or anti-CD3 plus anti-CD28. B cells of patients exhibited lower responses to both sets of stimuli. The mean fold rises in B-cell CD86 expression in response to anti-CD3 were 3.4 in the patient group and 4.73 in the control group (Fig. 3, NS). In response to anti-CD3 and anti-CD28, the mean fold rises were 11.5 in the patient group and 15.2 in the control group (P = 0.018), while the presence of α-δ-dex, in addition to either of these two sets of stimuli, was able to overcome these differences (Fig. 3).
FIG. 3.
Responsiveness of patient and control B cells to T-cell activation by anti-CD3 alone (left) or anti-CD3 and anti-CD28 (right) in the absence (top) or presence (bottom) of α-δ-dex stimulation. The whiskers show maximum and minimum values; statistical analysis was by Student's t test.
Responses of MCC vaccine failures to other antigens.
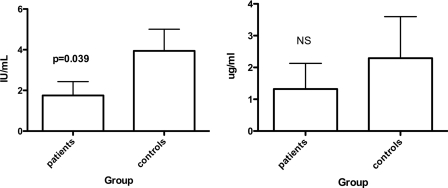
A subtle defect of T-cell responsiveness and the ability of T cells to deliver help in response to thymus-dependent (TD) antigens should affect immune responses against other TD antigens. In the United Kingdom, TT vaccine is routinely administered, along with DT, four times before the age of 5 and then again between ages 13 and 18. There was no significant difference in antibody levels against DT (not shown), but anti-TT IgG levels were significantly lower in the MCC vaccine failure group, with mean concentrations of 1.6 IU/ml in the MCC vaccine failures and 3.6 IU/ml in controls (P = 0.0385; Mann-Whitney U test) (Fig. 4). Vaccine failures had nonsignificantly lower anti-H. influenzae type b PRP antibody concentrations (Fig. 4). However, most patients (and controls) were born too early to receive routine Hib vaccinations in infancy, which began in the United Kingdom in 1992. By deduction, their immunity resulted from natural exposure to PRP, which in its purified form is a TI-2 antigen and, when delivered on intact bacteria, requires T-cell help for optimal responses (11).
FIG. 4.
Mean anti-tetanus toxoid (left) and anti-Hib (right) polysaccharide IgG titers at the time of the study. The error bars show standard errors of the mean. Statistical analysis was by Mann-Whitney U test.
DISCUSSION
Although, of necessity, this is a small case series, our findings suggest that adolescents who get MD despite having received MCC vaccine have relatively impaired in vitro T-cell responses. Thus, vaccine failures have a subtle defect in provision or receipt of T-cell help to B cells, reflected in lower expression of the B-cell activation marker CD86 following stimulation by activated T cells. The B cells of these individuals appear to have normal responses to the TI-2 model antigen α-δ-dex. The vaccine failures also appear to have inferior T-cell proliferation and activation in response to anti-CD3 (a mimic of TCR ligation by peptide-major histocompatibility complex [MHC] complexes). Taking these results together, there is evidence of a relative defect in T-cell responsiveness to TD antigens in MCC vaccine failures, which is manifested in lower T-cell help to B cells.
We measured the in vitro lymphocyte response to polyclonal mimics of TI-2 and TD antigens and the potential of activated T cells to convey “bystander” help to B cells to determine whether any of these responses were defective in the vaccine failure group. We did this because antigen-specific responses are difficult to measure in vitro due to the low numbers of antigen-specific cells. In addition, among vaccinees who have subsequently suffered meningococcal disease, antigen-specific responses would almost certainly be affected by the large bacterial load expressed by patients during disease. Indeed, we, like Auckland et al. (1), found convalescent SBA titers were very much higher than acute-phase titers in the vaccine failure group, showing that the experience of disease has a powerful boosting effect on antimeningococcal serogroup C responses.
In healthy hosts, generation of natural immunity to meningococcal disease is thought to be the result of recurrent oronasopharyngeal colonization by meningococci or other commensal Neisseria species. Most colonizing events are transient and result in expulsion of the organism, along with natural generation of protective IgG antibody against the colonizing strain (19). In adults in the United Kingdom, the prevalence of carriage of N. meningitidis is 25 to 37% (7), and therefore, the probability that an adult has never been exposed to the organism is low, and cumulative immunizing exposure to the meningococcus should be significant by the time a person reaches adulthood. We previously showed that PBMCs from unvaccinated adults with a history of serogroup C disease have lower B-cell proliferation in response to α-δ-dex stimulation than those of healthy controls but no defect in T-cell help (9). This suggests that protection against N. meningitidis infection in unvaccinated people relies at least partly on effective B-cell responses. In the majority of individuals, MCC vaccination protects against disease, so the population investigated in the current study probably represents a small fraction of the adult population who have shown themselves to be susceptible to serogroup C meningococcal disease and who were the population investigated in the previous study (9). B-cell responses directly to α-δ-dex were not significantly lower in the vaccine failures (Fig. 1), but T-cell help to B cells was significantly impaired (Fig. 3), which mirrors, in effect, the defect we observed in unvaccinated susceptible individuals (9). We presume, in other words, that there is perhaps a relatively large population with the subtle B-cell defect described previously (9), rendering them susceptible to meningococcal serogroup C disease as adults, in the absence of vaccination, due to poor immune responses to colonization. These individuals would be protected by MCC vaccination. There is also a smaller population with a subtle defect in T-cell help for whom conjugate vaccination is ineffective, and these are the people studied here. Of course, these individuals were adolescents whose colonization history would be much shorter than those of the adult nonvaccinated survivors studied previously. We predict that with time, colonization would have a protective effect in these individuals.
These vaccine failures are not obviously susceptible to infections typical of immunodeficiency, yet T-cell help is required for antibody responses to all protein antigens. The answer to this paradox is likely to be related to the scale of this deficiency, which appears in vitro to be a qualitative defect rather than complete absence of T-cell proliferation or the delivery of T-cell help. Although MCC vaccine failures did exhibit lower anti-TT responses than controls, for most patients, these levels are still likely to be protective (5), so these people would likely not experience tetanus even if exposed. The defect may also be manifested more strongly with MCC vaccine than with other vaccines or with exposure to infectious disease itself. Infection, along with other vaccines, comes with additional stimulation via TLRs or other pattern recognition receptors provided by vaccine adjuvants or by pathogen-associated molecules. Such additional stimulation may well complement this subtle defect. MCC vaccine is adsorbed to alum without any additional, more potent, adjuvant. Such additional stimulation could also explain the efficient response to MC polysaccharide in these patients after disease.
Our finding that the anti-TT response is lower in the MCC vaccine failure cohort may at first glance appear to partially contradict the findings of Auckland et al. (1), who found 13/20 (65%) MCC vaccine failures generated an anti-TT response of >0.1 IU/ml following vaccination, compared with 24/28 controls (86%). This difference was not significant (P = 0.09); however, this study compared vaccine failures with controls who had also suffered meningococcal disease. In addition, these controls were not closely matched with the vaccine failure patients for age (medians, 3.4 and 10.2 years for vaccine failure and control groups) or for gender (54% versus 38% male) and ethnicity.
Vaccine failures after vaccination with the Hib conjugate vaccine have also been described. Investigation of such cohorts has suggested that there is inadequate priming despite administration of 3 or 4 doses of vaccine (6), leading to a qualitative deficiency of Hib-specific memory B cells and poor avidity and antibody function (14). Most adolescents in the current study were too old to have been included in the United Kingdom Hib campaign, but there was a nonsignificant trend for anti-Hib responses to be lower in the MCC vaccine failure patients. Of course responses to Hib PRP in this cohort would not have been a result of vaccination but of an unknown number of contacts with the organism, which would tend to increase the variability within each group. In addition, contact with H. influenzae would be in the context of TLR agonists, such as lipopolysaccharide (LPS), and the true T-cell dependence/T-cell independence of responses to polysaccharide delivered on live organisms remains a matter of debate (8, 11).
The statistical power of this study was limited by the number of available patients, who all experienced their disease in the period 1999 to 2004, following MCC vaccine introduction in 1999. By 2004, serogroup C disease had become vanishingly rare (20), a state that has likely been maintained by herd immunity (15), though this effect would not have been present prior to 2004.
In conclusion, we devised and used a novel immunological assay to reveal possible immune defects hitherto undetectable and which may explain the phenomenon of MCC vaccine failure in adolescents. The assay detected a defect in T-cell responsiveness, which could lead to impaired induction of a bactericidal antibody response in some individuals following MCC vaccination (and which may have relevance to other conjugate vaccines). Through better understanding of the cellular and molecular causes of vaccine failure, we may be able to produce vaccines in the future that provide better protection to individuals such as these.
Acknowledgments
We thank Sue Newton and Kay Hopkinson of the School of Medicine Flow Cytometry Core Facility for help and advice.
R.A.F. was supported by the Binney Fund. J.C. and A.W.H. were supported by the Wellcome Trust (061268) and Yorkshire Cancer Research (S286). R.C.R., A.W.H., and R.B. were supported by the Meningitis Research Foundation (0604.0).
R.A.F. and J.C. performed the cellular assays and analyzed data, A.L. provided α-δ-dex, and A.W.H., M.W.M., and R.C.R. conceived the study, analyzed data, and wrote the manuscript with R.F. M.R. and E.M. provided information on vaccine failures, and R.B. and E.K. performed serological assays. All authors contributed to the final manuscript.
We have no conflicts of interest.
Footnotes
Published ahead of print on 12 May 2010.
REFERENCES
- 1.Auckland, C., S. Gray, R. Borrow, N. Andrews, D. Goldblatt, M. Ramsay, and E. Miller. 2006. Clinical and immunologic risk factors for meningococcal C conjugate vaccine failure in the United Kingdom. J. Infect. Dis. 194:1745-1752. [DOI] [PubMed] [Google Scholar]
- 2.Balmer, P., R. Borrow, and E. Miller. 2002. Impact of meningococcal C conjugate vaccine in the UK. J. Med. Microbiol. 51:717-722. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard Rohner, G., M. D. Snape, D. F. Kelly, T. John, A. Morant, L. M. Yu, A. Borkowski, F. Ceddia, R. Borrow, C. A. Siegrist, and A. J. Pollard. 2008. The magnitude of the antibody and memory B cell responses during priming with a protein-polysaccharide conjugate vaccine in human infants is associated with the persistence of antibody and the intensity of booster response. J. Immunol. 180:2165-2173. [DOI] [PubMed] [Google Scholar]
- 4.Borrow, R., P. Balmer, and E. Miller. 2005. Meningococcal surrogates of protection—serum bactericidal antibody activity. Vaccine 23:2222-2227. [DOI] [PubMed] [Google Scholar]
- 5.Borrow, R., P. Balmer, and M. H. Roper. 2007. The immunological basis for immunization series, module 3: tetanus (update 2006). World Health Organization, Geneva, Switzerland.
- 6.Breukels, M. A., E. Jol-van der Zijde, M. J. van Tol, and G. T. Rijkers. 2002. Concentration and avidity of anti-Haemophilus influenzae type b (Hib) antibodies in serum samples obtained from patients for whom Hib vaccination failed. Clin. Infect. Dis. 34:191-197. [DOI] [PubMed] [Google Scholar]
- 7.Cartwright, K. A., J. M. Stuart, D. M. Jones, and N. D. Noah. 1987. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol. Infect. 99:591-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, Q., J. L. Cannons, J. C. Paton, H. Akiba, P. L. Schwartzberg, and C. M. Snapper. 2008. A novel ICOS-independent, but CD28- and SAP-dependent, pathway of T-cell-dependent, polysaccharide-specific humoral immunity in response to intact Streptococcus pneumoniae versus pneumococcal conjugate vaccine. J. Immunol. 181:8258-8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster, R. A., J. Carlring, M. W. McKendrick, A. Lees, R. Borrow, R. C. Read, and A. W. Heath. 2009. Evidence of a functional B-cell immunodeficiency in adults who experience serogroup C meningococcal disease. Clin. Vaccine Immunol. 16:692-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldblatt, D. 2000. Conjugate vaccines. Clin. Exp. Immunol. 119:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan, A. Q., A. Lees, and C. M. Snapper. 2004. Differential regulation of IgG anti-capsular polysaccharide and antiprotein responses to intact Streptococcus pneumoniae in the presence of cognate CD4+ T cell help. J. Immunol. 172:532-539. [DOI] [PubMed] [Google Scholar]
- 12.Lal, G., P. Balmer, H. Joseph, M. Dawson, and R. Borrow. 2004. Development and evaluation of a tetraplex flow cytometric assay for quantitation of serum antibodies to Neisseria meningitidis serogroups A, C, Y, and W-135. Clin. Diagn. Lab. Immunol. 11:272-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lal, G., P. Balmer, E. Stanford, S. Martin, R. Warrington, and R. Borrow. 2005. Development and validation of a nonaplex assay for the simultaneous quantitation of antibodies to nine Streptococcus pneumoniae serotypes. J. Immunol. Methods 296:135-147. [DOI] [PubMed] [Google Scholar]
- 14.Lee, Y. C., D. F. Kelly, L. M. Yu, M. P. Slack, R. Booy, P. T. Heath, C. A. Siegrist, R. E. Moxon, and A. J. Pollard. 2008. Haemophilus influenzae type b vaccine failure in children is associated with inadequate production of high-quality antibody. Clin. Infect. Dis. 46:186-192. [DOI] [PubMed] [Google Scholar]
- 15.Maiden, M. C., A. B. Ibarz-Pavon, R. Urwin, S. J. Gray, N. J. Andrews, S. C. Clarke, A. M. Walker, M. R. Evans, J. S. Kroll, K. R. Neal, D. A. Ala'aldeen, D. W. Crook, K. Cann, S. Harrison, R. Cunningham, D. Baxter, E. Kaczmarski, J. Maclennan, J. C. Cameron, and J. M. Stuart. 2008. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J. Infect. Dis. 197:737-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maslanka, S. E., L. L. Gheesling, D. E. Libutti, K. B. Donaldson, H. S. Harakeh, J. K. Dykes, F. F. Arhin, S. J. Devi, C. E. Frasch, J. C. Huang, P. Kriz-Kuzemenska, R. D. Lemmon, M. Lorange, C. C. Peeters, S. Quataert, J. Y. Tai, and G. M. Carlone. 1997. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The Multilaboratory Study Group. Clin. Diagn. Lab. Immunol. 4:156-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pecanha, L. M., C. M. Snapper, F. D. Finkelman, and J. J. Mond. 1991. Dextran-conjugated anti-Ig antibodies as a model for T cell-independent type 2 antigen-mediated stimulation of Ig secretion in vitro. I. Lymphokine dependence. J. Immunol. 146:833-839. [PubMed] [Google Scholar]
- 18.Pickering, J. W., T. B. Martins, M. C. Schroder, and H. R. Hill. 2002. Comparison of a multiplex flow cytometric assay with enzyme-linked immunosorbent assay for quantitation of antibodies to tetanus, diphtheria, and Haemophilus influenzae type b. Clin. Diagn. Lab. Immunol. 9:872-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson, K., K. R. Neal, C. Howard, J. Stockton, K. Atkinson, E. Scarth, J. Moran, A. Robins, I. Todd, E. Kaczmarski, S. Gray, I. Muscat, R. Slack, and D. A. Ala'Aldeen. 2002. Characterization of humoral and cellular immune responses elicited by meningococcal carriage. Infect. Immun. 70:1301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trotter, C. L., N. J. Andrews, E. B. Kaczmarski, E. Miller, and M. E. Ramsay. 2004. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet 364:365-367. [DOI] [PubMed] [Google Scholar]
- 21.Trotter, C. L., and M. E. Ramsay. 2007. Vaccination against meningococcal disease in Europe: review and recommendations for the use of conjugate vaccines. FEMS Microbiol. Rev. 31:101-107. [DOI] [PubMed] [Google Scholar]