Abstract
Serum samples from 151 healthy individuals aged from 15 to 89 years were investigated by enzyme-linked immunosorbent assay (ELISA) for IgG levels against 11 different purified antigens from Staphylococcus aureus. Surface antigens, such as teichoic acid, clumping factors A and B, and bone sialoprotein binding protein, and extracellular proteins, such as alpha-toxin, lipase, enterotoxin A, toxic shock syndrome toxin, scalded-skin syndrome toxin, fibrinogen binding protein, and extracellular adherence protein, were used. The IgG values were analyzed in relation to the state of nasal carriage at the time of sampling. There was great individual variation in antibody levels in both young and elderly healthy subjects. Occurrence of S. aureus in the nares at the time of sampling was correlated with higher antibody levels, while elderly individuals over 65 years of age showed slightly lower levels than younger adults. More individuals than was expected from random probability calculations showed high antibody levels against several antigens, and more individuals than would be expected showed low levels against several antigens. Certain extracellular proteins had more often induced IgG levels of the same magnitude in the same individuals, indicating that among these individuals, there was a tendency to respond to certain antigens in the same way. Most individuals had circulating IgG antibodies to the 11 tested antigens, and some individuals had the tendency to be “good responders” to several antigens, while others were “poor responders.” These findings constitute basic knowledge for the development of improved serological diagnostics, immune prophylaxis, individual prognosis tools, and therapy against invasive Staphylococcus aureus infections.
Staphylococcus aureus, a versatile pathogen, is one of the common causes of both nosocomial and community-acquired infections. Treatment of S. aureus is increasingly more difficult due to development of multidrug resistance (10), so an alternative treatment based on passive and/or active immunoprophylaxis is highly desirable.
The presence of circulating antibodies in patients with S. aureus infections has been intensively studied (4, 8, 9, 11, 12, 18, 22, 25). The protective roles of these antibodies, as well as their capacities to neutralize extracellular toxins, are still poorly understood. Twenty percent of the population are persistently colonized with S. aureus, and another 40% are transiently colonized (26, 30).
Individuals carrying S. aureus in the nose are at higher risk than noncarrying individuals for developing bacteremia, since 80% of the colonized patients who develop deep-seated infections are infected with endogenous strains, but on the other hand, they are at lower risk of S. aureus bacteremia-related death (28, 31). Holtfreter et al. reported that S. aureus carriers neutralize superantigens via antibodies specific for their colonizing strains, and this may be the explanation for the improved prognosis in severe sepsis for carriers (14). It has also been demonstrated that S. aureus carriers show higher levels of antibodies against toxic shock syndrome toxin (TSST), staphylococcal enterotoxin A (SEA), clumping factor A (ClfA), and ClfB (27, 31), and other studies showed that patients with deep-seated S. aureus infections initially had lower levels of antibodies against some antigens in acute-phase sera than the healthy population (8, 11, 27, 31). Furthermore, it has been reported that antibody levels in healthy individuals are stable for years and are functional, i.e., have neutralizing or opsonizing functions (11).
The serological diagnosis may contribute to the choice of treatment of the patient, e.g., by determination of the bacteriological diagnosis through discrimination between soft tissue and bone infections and by monitoring the progression of the infection (20) or in diagnosis of endocarditis (29). Today, S. aureus serological diagnosis encounters many problems, such as identification of the most relevant antigens and the choice of different methods to be used (neutralization, radioimmunoassay [RIA], enzyme-linked immunosorbent assay [ELISA], and Luminex technology). Different calculation models have been used to express the antibody levels, and there are uncertainties about the “normal” antibody levels for comparison. All these factors make the use of serology difficult in inexperienced hands (9, 22, 27).
The aim of this study was to investigate the antibody levels in a healthy population and to compare the antibody repertoire between carriers and noncarriers. Possible relevant antigens were selected, and a reproducible ELISA with calculation methods for quantitative analysis was chosen. The methods and the results may be used for the improvement of serological diagnosis in clinical practice and/or development of new immunoprophylactic and immunotherapeutic tools.
MATERIALS AND METHODS
Materials.
Antibody levels against 11 different antigens were investigated in 151 healthy individuals. The main part of this material (115 samples) was collected as reference material (matched ages) in a prospective study regarding invasive S. aureus infections (16). These individuals attended a vaccine center and were screened for nasal carriage of S. aureus according to standard laboratory procedures. In order to compensate for the skewed age distribution of the individuals, another 36 samples from younger blood donors were included. The gender distribution was 90 men and 60 women, with average ages of 56 and 50 years. The age distribution of the total material was as follows: 29% ages 15 to 35 years, 21% ages 35 to 65, and 49% ages 65 to 90 years.
Antibody determination: ELISA.
Serum IgG levels were determined by ELISA as described previously (8). The working volume was 100 μl, and after each step, the microtiter plates were washed three times with phosphate-buffered saline (PBS) (pH 7.4) plus 0.05% Tween 20 (PBS-T). Briefly, microplates were coated with the appropriate antigen diluted in PBS and incubated overnight at 20°C. The next day, the microplates coated with the antigens ClfB, extracellular adherence protein (Eap), and Bsp were blocked with 2% bovine serum albumin (BSA) for 1 h at 20°C. Serum samples diluted in PBS-T were applied and incubated for 1 h at 37°C; each patient sample was titrated in 4 steps in 2-fold dilutions (Table 1). Alkaline phosphatase conjugated to monoclonal mouse anti-human IgG γ-chain-specific antibodies (Sigma Chemical Co., St. Louis, MO) diluted 1/30,000 in PBS-T was then added, and incubation was continued for 2 h at 37°C. After the final wash, the reaction was developed by the addition of p-nitrophenylphosphate substrate (Sigma Chemical Co.). The enzymatic reaction was measured at 405 nm in a Titertek Multiscan microplate reader (Flow Laboratories, Irvine, Scotland) after approximately 20 min of incubation. The absorbance values were transmitted online to a computer, and calculations were performed with Unitcalc software (PhPlate Stockholm AB, Stockholm, Sweden).
TABLE 1.
Investigated antigens
| Antigen | Abbreviation | Compound | Properties | Coating concn (μg/ml) | Starting serum dilution in ELISA | Supplier | Reference |
|---|---|---|---|---|---|---|---|
| Surface antigens | |||||||
| Teichoic acid | TA | Ribitol polymer, native | Cell wall component | 1 | 1/2,500 | PhPlate, Stockholm | 9 |
| Clumping factor | ClfA | Recombinant protein | Surface-located fibrinogen binding protein | 1 | 1/250 | J. I. Flock, Karolinska Institutet | 8 |
| Clf-B | Recombinant protein | Surface-located fibrinogen binding protein | 1 | 1/125 | J. I. Flock | 27 | |
| Bone sialoprotein binding protein | Bbp | Recombinant protein | Surface-located protein that binds bone sialoprotein | 4 | 1/125 | C. Rydén, Uppsala University | 24 |
| Extracellular proteins and toxins | |||||||
| Alpha-toxin | At | Native protein | Hemolytic toxin, cytotoxin | 3.5 | 1/250 | PhPlate, Stockholm | 9 |
| Lipase | Lip | Native protein | Hydrolysis of low-density lipoproteins | 2 | 1/2,500 | S. Tyski, University of Warsaw | 25 |
| Staphylococcal enterotoxin A | SEA | Native protein | Emetic toxin, superantigen | 1 | 1/250 | Toxin Technology, FLl | 18 |
| Toxic shock syndrome toxin | TSST | Native protein | Pyrogenic toxin, superantigen | 1 | 1/250 | Toxin Technology, Florida | 18 |
| Scalded-skin syndrome toxin | SSS | Native protein | Exfoliative toxin, epidermolytic toxin | 1 | 1/250 | Toxin Technology, Florida | 19 |
| Fibrinogen binding protein | Efb | Native protein | Fibrinogen binding protein, binds to complement factor C3b and C3d | 0.6 | 1/250 | J. I. Flock | 8 |
| Extracellular adherence protein | Eap | Recombinant protein | Binds to extracellular matrix molecules; blocks neutrophil and T-cell recruitment | 1 | 1/125 | J. I. Flock | 15 |
For every two plates, eight 2-fold dilutions of a reference serum (Golden Standard) consisting of pooled sera from six patients with confirmed S. aureus sepsis were included. The first dilution varied with the respective antigens (Table 1). Three control sera (two positive and one negative) were also included at single dilutions in duplicate wells to monitor the reaction.
Antigens.
Eleven highly purified antigens from S. aureus were used in separately developed assays (Table 1).
Interpretation of ELISA results in units.
The antibody levels were expressed as arbitrary units by using the reference line unit calculation method (21). The dilution curve of each sample (four dilutions) was adapted into a straight line parallel to that of the reference serum, after which the distance, expressed in dilution steps, between the two lines was determined. The reference serum was given the value of 1,000 U, and the antibody levels of the tested serum were related to these units, e.g., a level of 2,000 U in a sample means that the sample could be diluted twice to generate the same dilution curve as the reference serum (8).
Statistical methods.
Antibody levels were not normally distributed, and therefore, the Mann-Whitney nonparametric method was used. Comparisons with obtained and expected numbers of individuals showing high or low antibody levels according to a binomial distribution were performed with standard χ2 tests. For comparisons of levels between individuals, the unit values were normalized into quotients of their respective mean values, upon which pairwise correlation coefficients were determined. Figures and calculations were performed with GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA) and Microsoft Excel.
RESULTS
Antibody levels against 11 antigens in healthy individuals.
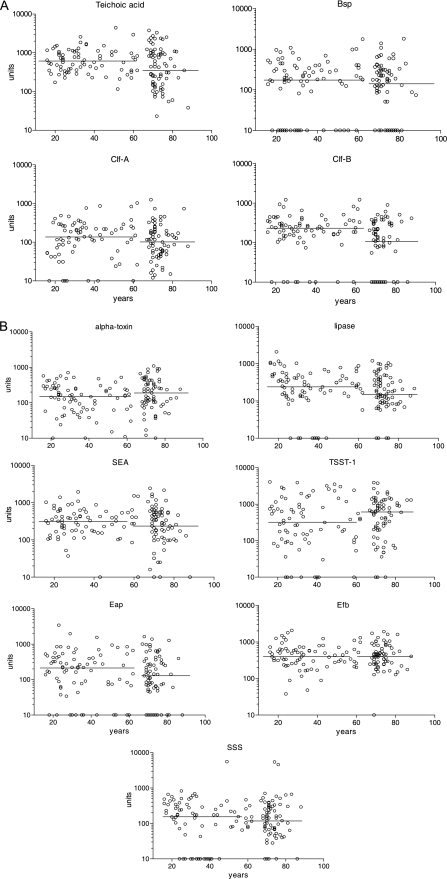
Staphylococcus aureus produces different virulence factors during various stages of infection. The present study considered antibodies against two main types of factors: the 11 different antigens were sorted into surface antigens and extracellular proteins and toxins. Furthermore, the selected healthy population matched the ages of individuals with deep-seated infections. The antibody levels against the 11 antigens as a function of age are shown in Fig. 1 A and B. It should be noted that the units are the same on all y axes but that different basic serum dilutions were applied for testing against different antigens, as shown in Table 1.
FIG. 1.
Serum IgG levels against different antigens. (A) Surface antigens. (B) Extracellular proteins. Each graphic shows the levels expressed in units, as described in Materials and Methods. The circles on the x axis indicate individuals lacking measurable levels of antibodies to the respective antigen; the horizontal lines crossing the dots show the median values for individuals younger and older than 65 years of age. Great variability against different antigens at all ages can be observed.
Antibody levels in relation to age.
There were no distinct differences in antibody levels related to age. However, the median values decreased with increasing age for most of the antigens, but this difference was significant only for clumping factor B. An exception to this was found for antibodies against TSST, where the median levels were twice as high in individuals over 65 years of age as in those below 65 years of age. In a number of antibody determinations, it was not possible to detect any antibodies at all; these are marked as circles on the x axes. The majority of these samples originated from subjects below 65 years of age for ClfA, lipase, scalded-skin syndrome toxin (SSS), and TSST, while most of the negative serum samples were obtained from individuals over 65 years of age for ClfB.
Antibody levels in relation to colonization of the nares.
In general, individuals who were colonized with S. aureus at the time of blood sampling showed higher median antibody levels against all antigens tested, except for extracellular fibrinogen binding protein (Efb). For five of the analyzed antigens, the difference was statistically significant (see Table 3), e.g., the antibody levels against extracellular adherence protein were three times higher in healthy individuals colonized with Staphylococcus aureus than in individuals not colonized.
TABLE 3.
Antibody levels in colonized versus noncolonized individuals
| Antigen | Level [median (SEMa)] |
P value | |
|---|---|---|---|
| Colonized (n = 26) | Not colonized (n = 89) | ||
| Surface antigens | |||
| Teichoic acid | 912 (123) | 422 (91) | 0.013 |
| ClfA | 170 (56) | 134 (21) | 0.239 |
| ClfB | 191 (45) | 163 (25) | 0.092 |
| Bsp | 233 (91) | 161 (31) | 0.067 |
| Extracellular proteins | |||
| Alpha-toxin | 325 (62) | 165 (17) | 0.056 |
| Lipase | 364 (45) | 176 (27) | 0.007 |
| SEA | 483 (94) | 271 (45) | 0.006 |
| TSST | 1043 (196) | 378 (91) | 0.022 |
| SSS | 161 (178) | 115 (86) | 0.275 |
| Efb | 394 (83) | 438 (37) | 0.402 |
| Eap | 268 (90) | 85 (52) | 0.009 |
SEM, standard error of the mean.
Antibody levels in relation to gender.
No differences were seen in antibody levels by gender (data not shown).
High or low levels of antibodies against more than one antigen.
From Fig. 1A and B, it is evident that there were great variations between individuals in the antibody levels against all antigens, in some cases, there was no detectable reactivity at all, even at the lowest dilution, 1/125. Some individuals showed low antibody levels against several antigens.
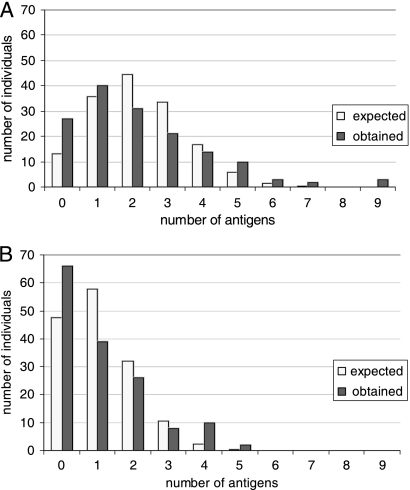
Figure 2A and B show the distributions of individuals who had low and very high antibody levels against one or more of the antigens. The achieved distribution is compared to the expected distribution, if these events were completely randomly distributed.
FIG. 2.
Distribution of observed versus expected numbers of individuals showing low or high levels of antibodies against one or more antigens. The x axis shows the number of antigens, and the y axis indicates the number of individuals who showed low or high levels of antibodies against this number of antigens. The observed and expected numbers of individuals were calculated based upon the random probability according to the binomial distribution. (A) Low levels, defined as levels below the 20th percentile for each antigen. (B) High levels, defined as levels above the 90th percentile for each antigen. The expected numbers were calculated using 20% and 10% random possibility, respectively, considering 11 different antigens.
Figure 2A shows the distribution of individuals with low antibody levels against one or more antigens. A “low level” was arbitrarily defined as a level below the 20th percentile for the respective antigen. This definition means that for each antigen there is a “random” possibility of 20% that the level will be considered low. In comparison with the random distribution of such individuals, based upon 20% probability of being “low,” it can be seen that there were more individuals with low or no reactions against several antigens than would occur by random chance (P = 0.001), e.g., 3 individuals out of 151 showed low or no antibody levels against 9 out of the 11 antigens tested. This finding might indicate that certain individuals have a special tendency not to react to staphylococcal antigens. None of the individuals (n = 32) showing low antibody levels to more than four antigens were colonized (P = 0.04). Certain antigens did not give rise to any antibody response at all in certain individuals, a situation that occurred most often for Bsp, ClfB, and Eap (Fig. 1).
Similarly, there was a skewed distribution of the number of individuals that showed the highest levels of antibodies against several of the studied antigens (Fig. 2B). A “high level” was arbitrarily defined as a level above the 90th percentile for the respective antigen, giving a 10% random probability of obtaining a high level. Again, 12 individuals had high levels of antibodies against 4 or 5 antigens, a situation that is highly unlikely to occur by random chance (P = 0.001). Thus, this finding might indicate that certain individuals have a special tendency to react strongly to some staphylococcal antigens. Also, 6 out of 11 of these individuals, who were sampled for carriage, were colonized with Staphylococcus aureus (P = 0.02). It should also be noted that for both low and high levels there were more individuals with levels to 1 or 0 antigens than expected (Fig. 2A and B).
Correlations between antibody levels against different antigens.
For each possible pairwise combination of the 11 antigens (n = 55), the correlation coefficient for the antibody levels in the 151 individuals was calculated. In the correlation matrix (see Table 4), it can be seen that levels of antibodies against teichoic acid and alpha-toxin most often covaried with lipase, but also, levels against the extracellular toxins SEA and SSS were included in this cluster. Another “cluster” of antigens giving similar levels of antibodies contained the surface-bound proteins ClfB and BSP, together with the extracellular adherence protein Eap. On the other hand TSST and Efb appeared to stimulate the formation of antibodies more independently of the other antigens. The mean correlation coefficient was 0.21.
TABLE 4.
Correlations (r) between the antibody levels against 11 antigens in 151 individualsa
| Antigen | LIP | AT | SEA | SSS | TA | Eap | Clf-B | BSP | TSST | Efb |
|---|---|---|---|---|---|---|---|---|---|---|
| LIP | ||||||||||
| AT | 0.47* | |||||||||
| SEA | 0.40* | 0.37* | ||||||||
| SSS | 0.38* | 0.34† | 0.27† | |||||||
| TA | 0.47* | 0.20 | 0.28† | 0.23 | ||||||
| Eap | 0.25 | 0.23 | 0.36† | 0.18 | 0.15 | |||||
| ClfB | 0.28† | 0.19 | 0.24 | 0.18 | 0.18 | 0.32† | ||||
| BSP | 0.11 | 0.03 | 0.25 | 0.01 | 0.02 | 0.41* | 0.28† | |||
| TSST | 0.17 | 0.14 | 0.19 | 0.19 | 0.29† | 0.21 | 0.10 | 0.08 | ||
| Efb | 0.14 | 0.12 | 0.08 | 0.17 | 0.12 | 0.25 | 0.21 | 0.16 | 0.08 | |
| ClfA | 0.02 | 0.11 | 0.34† | 0.12 | 0.04 | 0.20 | 0.18 | 0.13 | 0.03 | −0.08 |
For each possible pair of combinations for the 11 antigens (n = 55), the correlation coefficient for the antibody levels in the 151 individuals was calculated. The mean correlation coefficient was 0.21. Asterisks indicate high correlation (0.37 ≤ r < 0.47), and daggers indicate median correlation (0.27 < r < 0.37). It should be noted that a significant correlation is considered to be obtained at an r value of >0.18.
DISCUSSION
Staphylococcus aureus produces a wide variety of antigens and virulence factors. This great variation in the antibody levels is not only the result of the different individual responses to the different antigens they might be exposed to during life, but also, in the case of colonized individuals, the diversity of the colonizing strains that could influence the great variation we found (1, 6, 27).
An important portion of the serum samples were collected as reference material (matched ages) in a previous study with the purpose of analyzing the antibody responses in patients with deep-seated S. aureus infections (16). Eight of the 11 antigens presented in this study were used. Since we observed great variations in the antibody levels in healthy individuals, we decided to include three more antigens and to analyze the relationships between antibody levels, age, colonization, and antigens.
In this study, we used 11 highly purified antigens in a conventional ELISA to determine the levels of serum IgG to each antigen in a healthy population. The antigens were selected to represent surface antigens that might be relevant for colonization; the adherent protein and the potent anti-inflammatory Eap are virulence factors involved in wound healing, while such antigens as fibrinogen binding proteins and specific toxins may be involved in invasive disease. The specific toxins SEA, TSST, and SSS are not always expressed by all the strains, a fact that might influence (diminish) the occurrence of antibodies in the healthy population, but the results (Fig. 1) do not corroborate this assumption.
In general the antibody levels were of the same magnitude against surface antigens as against the extracellular proteins. In fact, as can be seen in Fig. 1, there were individuals who did not show any antibody response against individual antigens, notably Bsp, Eap, ClfB, and SSS, but in general, most individuals showed antibodies against all antigens tested and all individuals showed antibodies against at least some staphylococcal antigens, although at different levels (3, 4, 11, 13, 17, 20, 23). This indicates that most healthy adult individuals have been exposed to S. aureus and that the antigens studied were expressed during this exposure.
The ELISA and the reference line unit method used in this study give excellent reproducible and quantitative data on the antibody levels measured and make it possible to perform proper statistical calculations on the units obtained to compare antibody levels between healthy and diseased individuals in investigations of staphylococcal infections of various kinds.
However, the relative amounts of antibodies produced against the various antigens in the same individual are difficult to quantify with the present method, since they are all related to one gold standard serum containing various amounts of antibodies to the respective antigen and they are analyzed at three different starting concentrations in order to obtain optimal dilution curves to relate to the standard. For example, Dryla et al. (11) stated that most of the antibodies were produced against surface antigens. In our study, the protein surface antigens used did not appear to produce particularly high levels of antibodies; ClfA antibodies had to be tested for at an initial dilution of 1/250 and ClfB and Bsp antibodies at the lowest initial dilution of 1/125. In contrast, teichoic acid was tested for at the high initial dilution of 1/2,500, which still resulted in the same absorbance values as the other surface antigens.
Various results are available on the influence of age on antibody levels. It is generally agreed that the levels increase during childhood and adolescence to reach a steady level in the adult. However, several studies have shown that levels in the healthy elderly decrease with age (11, 12, 17). From Fig. 1, it can be seen that there was a tendency toward a decrease in antibody levels above 65 years of age. However, this decrease was significant only for the antigen ClfB, and the levels actually increased 100% against TSST, although this was not significant due to the large variation (Table 2). It could be surmised that elderly individuals have experienced longer exposure to the bacterium, possibly including earlier invasive infections, but on the other hand, the immune system is expected to become less reactive with age.
TABLE 2.
Antibody levels in individuals before and after 65 years of age
| Antigen | Level [median (SEMa)] at age: |
P value | |
|---|---|---|---|
| <65 yr (n = 78) | >65 yr (n = 73) | ||
| Surface antigens | |||
| Teichoic acid | 606 (76) | 436 (98) | 0.058 |
| ClfA | 140 (23) | 117 (23) | 0.299 |
| ClfB | 229 (28) | 116 (22) | 0.0006 |
| Bsp | 195 (35) | 165 (37) | 0.215 |
| Extracellular proteins | |||
| Alpha toxin | 160 (18) | 204 (28) | 0.089 |
| Lipase | 268 (39) | 179 (33) | 0.056 |
| SEA | 324 (41) | 282 (54) | 0.049 |
| TSST | 336 (115) | 623 (91) | 0.18 |
| SSS | 174 (72) | 139 (96) | 0.444 |
| Efb | 435 (44) | 430 (46) | 0.878 |
| Eap | 218 (56) | 134 (42) | 0.210 |
SEM, standard error of the mean.
van Belkum and coworkers stated that only individuals with persistent colonization by S. aureus showed increased antibody levels (26). Our study is based upon one-time sampling, and therefore, the issue of persistent colonization was not possible to evaluate. However, there evidently was a correlation between carrying the bacteria in the nares at the time of sampling and having higher antibody levels against all antigens investigated, significantly against 5 of 11 (Table 3). It could be anticipated that the protein surface antigens possibly taking part in the colonization should have activated the immune system more than extracellular proteins and toxins, but this was not the case. Teichoic acid showed more than twice the levels of antibodies in individuals carrying S. aureus, though on the other hand, the high levels of antibody against Eap in colonized individuals are in concordance with the anti-inflammatory properties of this protein that contribute to bacterial colonization (2).
Cole et al. demonstrated that colonized persons had defects in their local innate immunity to S. aureus (7). However, Clarke et al. (5) found higher levels of reactive IgG to the iron-responsive surface determinants IsdA and IsdH from noncarriers. Carriers have better prognosis than noncarriers in bacteremia, and Holtfreter et al. have explained the improved prognosis through the increased levels of preformed antibodies, and for toxins like TSST and SEA, the antibody levels are clearly protective (14). The view that humoral immune responses do not protect from colonization could be challenged by the work of Clarke et al., who showed beneficial effects of vaccination with IsdA or IsdH against nasal carriage in an animal model (5).
It is well known that certain individuals are at risk to develop toxic shock syndrome from S. aureus due to their lack of capacity to produce neutralizing antibodies against TSST. In this study, we showed that certain individuals had a stronger or weaker tendency to produce antibodies against some of the 11 antigens tested. The individuals who were high producers of antibodies to more than three antigens were significantly more often colonized in the nares, but none of the individuals who were low producers against more than four antigens were colonized. These data could be interpreted as showing that certain individuals have a tendency to produce and some not to produce antibodies against S. aureus antigens, and they support the findings by Cole that serum IgG antibodies are not protective against nasal colonization (7). Individuals who lack antibodies against several antigens might have been in less contact with Staphylococcus aureus, but this is difficult to anticipate for healthy individuals. The relevance of such low levels of antibodies against S. aureus to lowered protection against invasive S. aureus infections has recently been discussed (11, 14).
Related to the discussion above is the question of whether certain groups of antigens are more likely to induce antibody production than others, which is why the relative quantitative responses against all antigens were compared among all individuals (Table 4). The results clearly indicated that antibody levels against the extracellular proteins alpha-toxin, lipase, enterotoxin A, and extracellular adhesive protein more often were similar in the same individuals, most often together with antibodies against the surface-bound teichoic acid. If the same correlations seen in Table 4 are also relevant for patients with invasive staphylococcus infections, they should be considered in the development of improved serological diagnosis tools for these infections.
In conclusion, this study has pointed out the great variations in antibody levels in healthy young and elderly individuals. The occurrence of S. aureus in the nares at the time of sampling was correlated with higher antibody levels, but individuals aged over 65 years showed only slightly lower levels. Certain individuals were more prone to produce or not to produce antibodies than others, and certain antigens more often induced high levels in the same individuals.
These findings are important for the development of improved serological diagnostics and constitute important knowledge for future immune prophylaxis and therapy of invasive Staphylococcus aureus infections.
Footnotes
Published ahead of print on 5 May 2010.
REFERENCES
- 1.Cespedes, C., B. Said-Salim, M. Miller, S. H. Lo, B. N. Kreiswirth, R. J. Gordon, P. Vavagiakis, R. S. Klein, and F. D. Lowy. 2005. The clonality of Staphylococcus aureus nasal carriage. J. Infect. Dis. 191:444-452. [DOI] [PubMed] [Google Scholar]
- 2.Chavakis, T., M. Hussain, S. M. Kanse, G. Peters, R. G. Bretzel, J. I. Flock, M. Herrmann, and K. T. Preissner. 2002. Staphylococcus aureus extracellular adherence protein serves as anti-inflammatory factor by inhibiting the recruitment of host leukocytes. Nat. Med. 8:687-693. [DOI] [PubMed] [Google Scholar]
- 3.Christensson, B., A. Boutonnier, U. Ryding, and J. M. Fournier. 1991. Diagnosing Staphylococcus aureus endocarditis by detecting antibodies against S. aureus capsular polysaccharide types 5 and 8. J. Infect. Dis. 163:530-533. [DOI] [PubMed] [Google Scholar]
- 4.Christensson, B., F. J. Fehrenbach, and S. A. Hedstrom. 1985. A new serological assay for Staphylococcus aureus infections: detection of IgG antibodies to S. aureus lipase with an enzyme-linked immunosorbent assay. J. Infect. Dis. 152:286-292. [DOI] [PubMed] [Google Scholar]
- 5.Clarke, S. R., K. J. Brummell, M. J. Horsburgh, P. W. McDowell, S. A. Mohamad, M. R. Stapleton, J. Acevedo, R. C. Read, N. P. Day, S. J. Peacock, J. J. Mond, J. F. Kokai-Kun, and S. J. Foster. 2006. Identification of in vivo-expressed antigens of Staphylococcus aureus and their use in vaccinations for protection against nasal carriage. J. Infect. Dis. 193:1098-1108. [DOI] [PubMed] [Google Scholar]
- 6.Clarke, S. R., and S. J. Foster. 2006. Surface adhesins of Staphylococcus aureus. Adv. Microb. Physiol. 51:187-224. [DOI] [PubMed] [Google Scholar]
- 7.Cole, A. M., S. Tahk, A. Oren, D. Yoshioka, Y. H. Kim, A. Park, and T. Ganz. 2001. Determinants of Staphylococcus aureus nasal carriage. Clin. Diagn. Lab. Immunol. 8:1064-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colque-Navarro, P., M. Palma, B. Soderquist, J. I. Flock, and R. Mollby. 2000. Antibody responses in patients with staphylococcal septicemia against two Staphylococcus aureus fibrinogen binding proteins: clumping factor and an extracellular fibrinogen binding protein. Clin. Diagn. Lab. Immunol. 7:14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colque-Navarro, P., B. Soderquist, H. Holmberg, L. Blomqvist, P. Olcen, and R. Mollby. 1998. Antibody response in Staphylococcus aureus septicaemia—a prospective study. J. Med. Microbiol. 47:217-225. [DOI] [PubMed] [Google Scholar]
- 10.de Lencastre, H., D. Oliveira, and A. Tomasz. 2007. Antibiotic resistant Staphylococcus aureus: a paradigm of adaptive power. Curr. Opin. Microbiol. 10:428-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dryla, A., S. Prustomersky, D. Gelbmann, M. Hanner, E. Bettinger, B. Kocsis, T. Kustos, T. Henics, A. Meinke, and E. Nagy. 2005. Comparison of antibody repertoires against Staphylococcus aureus in healthy individuals and in acutely infected patients. Clin. Diagn. Lab. Immunol. 12:387-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granström, M., I. G. Julander, S. A. Hedstrom, and R. Mollby. 1983. Enzyme-linked immunosorbent assay for antibodies against teichoic acid in patients with staphylococcal infections. J. Clin. Microbiol. 17:640-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollsing, A. E., M. Granstrom, and B. Strandvik. 1987. Prospective study of serum staphylococcal antibodies in cystic fibrosis. Arch. Dis. Child. 62:905-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holtfreter, S., K. Roschack, P. Eichler, K. Eske, B. Holtfreter, C. Kohler, S. Engelmann, M. Hecker, A. Greinacher, and B. M. Broker. 2006. Staphylococcus aureus carriers neutralize superantigens by antibodies specific for their colonizing strain: a potential explanation for their improved prognosis in severe sepsis. J. Infect. Dis. 193:1275-1278. [DOI] [PubMed] [Google Scholar]
- 15.Hussain, M., A. Haggar, G. Peters, G. S. Chhatwal, M. Herrmann, J. I. Flock, and B. Sinha. 2008. More than one tandem repeat domain of the extracellular adherence protein of Staphylococcus aureus is required for aggregation, adherence, and host cell invasion but not for leukocyte activation. Infect. Immun. 76:5615-5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobsson, G., P. Colque-Navarro, E. Gustafsson, R. Andersson, and R. Mollby. 2010. Antibody responses in patients with invasive Staphylococcus aureus infections. Eur. J. Clin. Microbiol. Infect. Dis. 29:715-725. [DOI] [PubMed] [Google Scholar]
- 17.Julander, I. G., M. Granstrom, S. A. Hedstrom, and R. Mollby. 1983. The role of antibodies against alpha-toxin and teichoic acid in the diagnosis of staphylococcal infections. Infection 11:77-83. [DOI] [PubMed] [Google Scholar]
- 18.Kanclerski, K., B. Soderquist, M. Kjellgren, H. Holmberg, and R. Mollby. 1996. Serum antibody response to Staphylococcus aureus enterotoxins and TSST-1 in patients with septicaemia. J. Med. Microbiol. 44:171-177. [DOI] [PubMed] [Google Scholar]
- 19.Ladhani, S. 2001. Recent developments in staphylococcal scalded skin syndrome. Clin. Microbiol. Infect. 7:301-307. [DOI] [PubMed] [Google Scholar]
- 20.Persson, L., C. Johansson, and C. Ryden. 2009. Antibodies to Staphylococcus aureus bone sialoprotein-binding protein indicate infectious osteomyelitis. Clin. Vaccine Immunol. 16:949-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reizenstein, E., H. O. Hallander, W. C. Blackwelder, I. Kuhn, M. Ljungman, and R. Mollby. 1995. Comparison of five calculation modes for antibody ELISA procedures using pertussis serology as a model. J. Immunol. Methods 183:279-290. [DOI] [PubMed] [Google Scholar]
- 22.Ryding, U., F. Espersen, B. Soderquist, and B. Christensson. 2002. Evaluation of seven different enzyme-linked immunosorbent assays for serodiagnosis of Staphylococcus aureus bacteremia. Diagn. Microbiol. Infect. Dis. 42:9-15. [DOI] [PubMed] [Google Scholar]
- 23.Ryding, U., J. Renneberg, J. Rollof, and B. Christensson. 1992. Antibody response to Staphylococcus aureus whole cell, lipase and staphylolysin in patients with S. aureus infections. FEMS Microbiol. Immunol. 4:105-110. [DOI] [PubMed] [Google Scholar]
- 24.Tung, H., B. Guss, U. Hellman, L. Persson, K. Rubin, and C. Ryden. 2000. A bone sialoprotein-binding protein from Staphylococcus aureus: a member of the staphylococcal Sdr family. Biochem. J. 345:611-619. [PMC free article] [PubMed] [Google Scholar]
- 25.Tyski, S., P. Colque-Navarro, W. Hryniewicz, M. Granstrom, and R. Mollby. 1991. Lipase versus teichoic acid and alpha-toxin as antigen in an enzyme immunoassay for serological diagnosis of Staphylococcus aureus infections. Eur. J. Clin. Microbiol. Infect. Dis. 10:447-449. [DOI] [PubMed] [Google Scholar]
- 26.van Belkum, A., N. J. Verkaik, C. P. de Vogel, H. A. Boelens, J. Verveer, J. L. Nouwen, H. A. Verbrugh, and H. F. Wertheim. 2009. Reclassification of Staphylococcus aureus nasal carriage types. J. Infect. Dis. 199:1820-1826. [DOI] [PubMed] [Google Scholar]
- 27.Verkaik, N. J., C. P. de Vogel, H. A. Boelens, D. Grumann, T. Hoogenboezem, C. Vink, H. Hooijkaas, T. J. Foster, H. A. Verbrugh, A. van Belkum, and W. J. van Wamel. 2009. Anti-staphylococcal humoral immune response in persistent nasal carriers and noncarriers of Staphylococcus aureus. J. Infect. Dis. 199:625-632. [DOI] [PubMed] [Google Scholar]
- 28.von Eiff, C., K. Becker, K. Machka, H. Stammer, and G. Peters. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N. Engl. J. Med. 344:11-16. [DOI] [PubMed] [Google Scholar]
- 29.Watkin, R. W., S. Lang, P. A. Lambert, W. A. Littler, and T. S. Elliott. 2006. The serological diagnosis of staphylococcal infective endocarditis. J. Infect. 53:301-307. [DOI] [PubMed] [Google Scholar]
- 30.Wertheim, H. F., D. C. Melles, M. C. Vos, W. van Leeuwen, A. van Belkum, H. A. Verbrugh, and J. L. Nouwen. 2005. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 5:751-762. [DOI] [PubMed] [Google Scholar]
- 31.Wertheim, H. F., E. Walsh, R. Choudhurry, D. C. Melles, H. A. Boelens, H. Miajlovic, H. A. Verbrugh, T. Foster, and A. van Belkum. 2008. Key role for clumping factor B in Staphylococcus aureus nasal colonization of humans. PLoS Med. 5:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]