Abstract
Plasmacytoid dendritic cells (pDCs) play a central role in antiviral immunity, detecting viruses via Toll-like receptors (TLR) and producing in response vast amounts of type I interferons (IFNs). Hepatitis B virus (HBV) causes chronic infection after vertical transmission. This study investigated whether an HBV-infected maternal environment might influence DC numbers and pDC function in uninfected infants. Blood was collected from inactive HBsAg carrier and control mothers and their infants at birth and 1 and 6 months of age. HBV DNA was measured in maternal and neonatal perinatal sera using real-time PCR. The circulating frequencies of myeloid DCs (mDCs) and pDCs were determined in the babies by flow cytometry. Peripheral blood mononuclear cells (PBMCs) and cord blood pDCs were stimulated with resiquimod, and alpha interferon (IFN-α) production and the pDC phenotype were assessed. The effect of the common-cold virus, rhinovirus (RV), on resiquimod stimulation was also determined. HBV DNA was detected in 62.3% of the mothers and 41% of their infants. DC numbers and pDC functions were similar between subjects and controls and were not correlated with maternal or neonatal viremia. RV infection did not induce pDC maturation until the age of 6 months, and it reduced TLR7-dependent resiquimod-induced IFN-α production similarly in both groups. Although the DC system is immature at birth, DCs of uninfected neonates of HBV-positive mothers are competent to initiate and maintain T-cell responses. RV is a weak inducer of IFN-α production until the age of 6 months and inhibits IFN-α responses triggered by the TLR7 pathway.
Hepatitis B virus (HBV) is a hepatotropic noncytopathic DNA virus of the family Hepadnaviridae that causes a high rate (90%) of chronic infection when acquired through mother-to-infant transmission (16). The increased incidence of chronicity is attributed to the immaturity of the neonatal immune system and, specifically, to the functional impairment of T cells (1, 16). Neonatal dendritic cells (DCs) exhibit functional alterations that could lead to secondary defects of adaptive T-cell responses (2, 9, 12). The importance of DCs has been demonstrated by experiments showing that neonatal T cells can reach adult-like responses when stimulated with isolated allogeneic adult DCs (2). The main dysfunctions of neonatal DCs include low circulating numbers, low levels of costimulatory-molecule expression, decreased induction of cytokine production, and decreased capacity to stimulate naïve T cells (3, 12, 28).
In humans, at least two distinct bone marrow-derived DC subsets have been characterized: those of myeloid (mDC) and of plasmacytoid (pDC) DC origin. In adults, DCs represent 0.8 to 1% of peripheral blood mononuclear cells (PBMCs) (5), whereas cord blood DCs (CB DCs) represent 0.3% of the CB mononuclear cells (CBMCs) (28). Upon exposure to pathogens, pDCs produce abundant amounts of type I/II interferons (IFNs), whereas mDCs produce high levels of interleukin 12 (IL-12). pDCs can produce 200 to 1,000 times more alpha interferon (IFN-α) than any other type of blood cell after they recognize viral genetic material through Toll-like receptors (TLRs) (11, 26). Thus, they represent a most important cell type in antiviral innate immunity. The favorable responses to IFN-α treatment in chronically infected HBV patients suggest that pDCs can play an important role in HBV infection. Indeed, several studies have found quantitative and qualitative impairment of pDCs in chronic carriers (5, 31).
Although the mechanisms of mother-to-infant HBV transmission remain unclear, several factors have been shown to be involved, including high perinatal maternal viremia and transplacental passage of virions and viral antigens, as well as viral infection of neonatal PBMCs in both infected and uninfected infants (17, 18, 23, 30). It has been shown that exposure of PBMCs to HBV DNA in uninfected neonates can lead to defective T-cell responses and to HBV vaccination failure (30). Therefore, it can be speculated that even in the absence of neonatal infection, the presence of HBV or its products in the maternal environment may alter the development of the DC systems of these newborns. Similarly, in utero exposure to HIV-1 has been shown to induce quantitative and qualitative changes in uninfected neonatal DCs (27).
Reports on the role of DCs in HBV infection have focused on adult life, after chronic infection has already been established (6). It is therefore important to study any alterations of the DC system during the neonatal period, when mother-to-infant HBV transmission may take place. The aim of the present study was to investigate whether the numbers and function of DCs may be altered in children of HBV-positive mothers compared with children born to healthy mothers. We measured the circulating frequencies of mDCs and pDCs and evaluated the capacity of pDCs to mature in response to resiquimod (R848), a well-known potent pDC activator. To understand if maternal viremia may influence the TLR7-dependent IFN-α-inducing pathway, we further assessed the effect of a common-cold virus, rhinovirus type 1b (RV1b), on TLR7 signaling post-R848 stimulation. RV is a single-stranded RNA (ssRNA) virus and hence a natural ligand of TLR7 (7, 14).
MATERIALS AND METHODS
Study population.
All subjects included in this study were born to HBsAg+/HBeAg−, HCV- and HIV-negative mothers who had no clinical or laboratory signs of active hepatitis B at the time of delivery and had not received any antiviral treatment during pregnancy. All newborns were born at term (between 37 and 41 gestation weeks), and birth weight was greater than 2,500 g. According to the national recommendations, all newborns received one dose of 200 IU of hepatitis B immune globulin (HBIG) and their first hepatitis B vaccine (Engerix; GlaxoSmithKline Biologicals, Rixensart, Belgium) within 24 h after birth. Hepatitis B vaccination was completed by another two doses of monovalent vaccine at the ages of 1 and 6 months. At the time of delivery, 40 ml of CB was collected in a subset of subjects by using sterile heparinized syringes. A peripheral blood sample was obtained from all subjects at birth (before the administration of passive-active immunoprophylaxis), at 1 month old (before the second vaccination dose), at 6 months old (10 to 14 days after the last vaccination dose), and at 7 months old. The control group consisted of infants born to healthy HBsAg− mothers followed in our outpatient clinic. According to national guidelines, they received 3 doses of hepatitis B vaccine using a combined vaccine (Infarix-Hexa; GlaxoSmithKline Biologicals, Rixensart, Belgium) at the ages of 2, 4, and 6 months. Blood samples were collected from control infants at the same time points as for the subject group. Each control infant was examined at two time points, and blood sampling was coupled with routine hematologic work up.
The research protocol was approved by the hospital's ethics committees, and all mothers gave written informed consent.
Serological HBV markers and HBV DNA.
Hepatitis B serological markers were determined in all mothers enrolled in the study and in their newborns on the day of delivery, and anti-HBs antibody levels were determined in all infants at 7 months of age. Perinatal HBV DNA was determined in all mothers and newborns. Plasma HBsAg, HBeAg, and anti-HBs were assayed with enzyme-linked immunosorbent assay (ELISA) kits (Axsym; Abbot Laboratories, Abbott Park, IL). HBV DNA was quantified using a sensitive in-house real-time PCR (RTD-PCR) assay. The HBV DNA was extracted from 0.5 ml of serum using the QIAamp UltraSens Virus Kit (Qiagen Inc., Valencia, CA), and the DNA was eluted in 60 μl of elution buffer. The RTD-PCR was carried out in a LightCycler II apparatus, and the conditions of the RTD-PCR were the same as described previously (21). The 95% HBV DNA detection endpoint of the assay was 22 IU/ml or 60 copies/ml (22).
Circulating-DC frequencies.
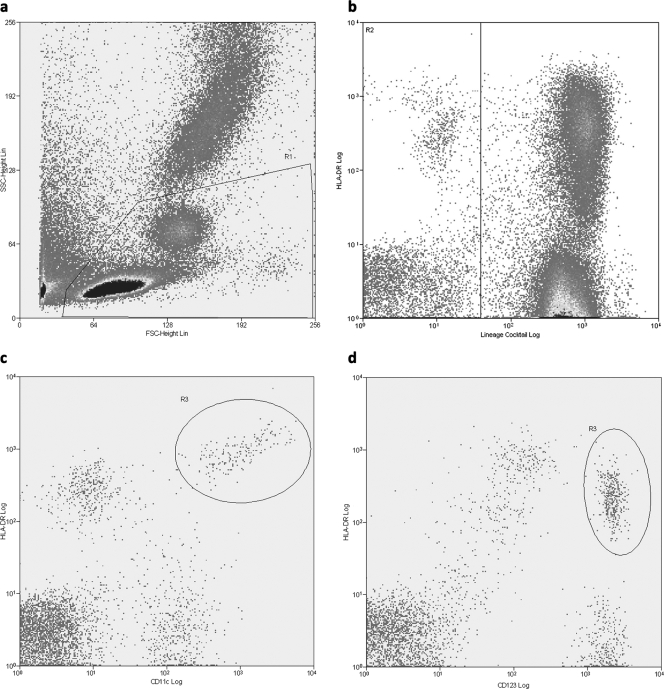
The frequencies of mDCs and pDCs were determined by using a three-color flow-cytometric analysis. Peripheral blood was collected in EDTA tubes and was processed within 4 h after collection. Whole blood was incubated with a mixed-lineage cocktail (Lin) of fluorescein isothiocyanate (FITC)-conjugated antibodies, which included antibodies to CD3, CD14, CD16, CD19, CD20, and CD56; peridinin chlorophyll protein (PerCP)-conjugated antibody to HLA-DR; phycoerythrein (PE)-conjugated antibody to either CD11c or CD123; and FcR-blocking reagent (Miltenyi Biotec). Isotype-matched antibodies were used as controls. All monoclonal antibodies were from BD Biosciences, San Diego, CA. After 30 min at 4°C in the dark, erythrocytes were lysed with 2 ml fluorescence-activated cell sorter (FACS) lysing solution (BD Biosciences, San Diego, CA) for 10 min at room temperature in the dark. After being washed with phosphate-buffered saline (PBS)-0.1% bovine serum albumin (BSA), the cells were fixed in 1% paraformaldehyde. In each case, the cells from 1 × 105 events were acquired on a FACSort Flow Cytometer (Becton Dickinson). The cells were gated by forward and side scatter characteristics, and data analysis was performed using Summit 4.2 software (DakoCytomation). Circulating mDCs were defined as Lin negative and HLA-DR and CD11c positive (Fig. 1 c), and pDCs were defined as Lin negative and HLA-DR positive and CD123-bright positive (Fig. 1d). The absolute numbers of mDCs and pDCs were then calculated using the percentage of cells with respect to the lymphocyte and monocyte absolute counts as determined by an automated hematoanalyzer.
FIG. 1.
Representative phenotypic analysis of mDC and pDC subsets by flow cytometry. (a) PBMCs were gated according to their forward (FSC) and side (SSC) scatter characteristics (R1). (b) Lin-negative but HLA-DR-positive cells were gated on region R2. (c and d) Gates R1 and R2 were selected, and mDCs were identified as Lin negative, HLA-DR and CD11c-positive (R3) (c), while pDCs were identified as Lin negative, HLA-DR and CD123 positive (R3) (d).
Isolation of PBMCs and CB pDCs.
Cord and peripheral blood was processed within 4 h from the time of blood collection and delivery. CBMCs and PBMCs were isolated by centrifugation on Ficoll-Histopaque (Sigma, St. Louis, MO). For CB pDC selection, CBMCs were resuspended in wash buffer containing PBS supplemented with 5% fetal calf serum (FCS) (HyClone, Logan, UT) and 2% EDTA (Sigma, St. Louis, MO). CB pDCs were isolated by a magnetic activated cell sorter, first by a negative-depletion step using a cocktail of monoclonal antibodies (anti-CD16, -CD19, -CD14, -CD3, -CD33, -CD15, and -glycophorin A) directly coupled to magnetic beads (Miltenyi Biotec), which were passed through an LD Column (Miltenyi Biotec). A positive-selection step from the depleted cell fraction followed, using anti-BDCA-4-conjugated magnetic microbeads (Miltenyi Biotec) passed over two MS columns (Miltenyi Biotec). The purity of CB pDCs was obtained by staining them with monoclonal antibodies to BDCA-2 conjugated to FITC (Miltenyi Biotec) and anti-CD123 antibody conjugated to PE (BD Biosciences, San Diego, CA) and was in all cases more than 95%.
PBMC and CB pDC infection and culture.
Freshly isolated PBMCs and CB pDCs were cultured in complete medium containing RPMI 1640 (Cambrex, Bio Sciences Walkersville, Inc.) supplemented with 10% FCS (HyClone, Logan, UT), 1% nonessential amino acids and 2 mM l-glutamine (Cambrex, Bio Sciences Walkersville, Inc.), 50 μM β-mercaptoethanol (Sigma, St. Louis, MO), and penicillin (100 U/ml) and streptomycin (100 μg/ml) (Cambrex, Bio Sciences Walkersville, Inc.).
RV1b was obtained from the ATCC and propagated in our institution as previously described (20). Cells were exposed to RV1b at a multiplicity of infection (MOI) of 1 for 1 h with gentle shaking. Infected and noninfected PBMCs and CB pDCs were cultured at 1 × 106 cells/ml and 2 × 105 cells/ml, respectively, in the presence or absence of 10 μg/ml R848 (Pharma Technologies, China). Recombinant IL-3 (R&D Systems Inc., Minneapolis, MN) was added under all conditions at 4-ng/ml concentration to optimize pDC viability. CB pDCs were harvested after 48 h and used for flow-cytometric analysis, while the supernatants were stored at −20°C until quantification of IFN-α was done.
Analysis of surface markers on pDCs.
For immunophenotypic analysis, monoclonal antibodies against CD86, HLA-DR, CCR7, and CD40 conjugated to FITC or PE (BD Biosciences, San Diego, CA) were added to pDC suspensions and incubated for 30 min at 4°C. After being washed with PBS-0.1% BSA, the cells were fixed in 1% paraformaldehyde. Data were obtained on a FACSort flow cytometer (Becton Dickinson) and analyzed using Summit 4.2 software (DakoCytomation). The results were expressed as means of mean fluorescence intensity (MFI) values (medians).
Cytokine quantification.
Levels of IFN-α were determined by sandwich ELISA using a commercial kit (Bender MedSystems, Vienna, Austria) according to the manufacturer's instructions. The detection limit of the assay was 4 pg/ml.
Statistical analysis.
Differences in numeric variables between groups were analyzed by the nonparametric Mann-Whitney test with two-tailed P values. The results were expressed as the mean ± standard error of the mean (SEM), and a P value of <0.05 was considered statistically significant. Statistical calculations were performed using GraphPad Prism Software (version 3).
RESULTS
Population description.
The study population included 30 neonate subjects born to HBsAg+/HBeAg− mothers, 17 of whom were reexamined at the age of 1 month while 10 were examined for the third time at the age of 6 months; 42 neonates of non-HBV-infected mothers served as controls (Table 1). No infant developed chronic HBV infection during follow-up, and all vaccinated subjects had detectable anti-HBs antibodies (>10 IU/liter) at the age of 7 months. Perinatal viremia was detected in 62.3% of the mothers (median, 3.7 ± 3.4 log10 viral copies [vc]/ml) and 41% of neonates (median 1.9 ± 1.13 log10 vc/ml).
TABLE 1.
Study population
| Groupa | No. of patients | Age | % of individuals with detectable viremia on day of delivery |
Viral loadb from individuals with detectable viremia on day of delivery |
||
|---|---|---|---|---|---|---|
| Mothers | Newborns | Mothers | Newborns | |||
| SB_CB | 4 | 0 | 100 | 50 | 4.3 ± 4.2 | 1.5 ± 1.1 |
| CT_CB | 5 | 0 | NAc | NA | NA | NA |
| SB_0 | 30 | 0-48 h | 60 | 33 | 3.7 ± 3.5 | 1.9 ± 1.4 |
| CT_0 | 20 | 0-48 h | NA | NA | NA | NA |
| SB_1 | 17 | 30-35 days | 59 | 47 | 3.1 ± 2.4 | 1.9 ± 1.3 |
| CT_1 | 7 | 30-35 days | NA | NA | NA | NA |
| SB_6 | 10 | 6 mo | 60 | 50 | 3.2 ± 2.7 | 1.8 ± 1.4 |
| CT_6 | 10 | 6 mo | NA | NA | NA | NA |
SB, subjects; CT, controls.
Viral loads are presented as the log10 of the medians of vc/ml.
NA: not applicable.
Frequencies of circulating DC subsets.
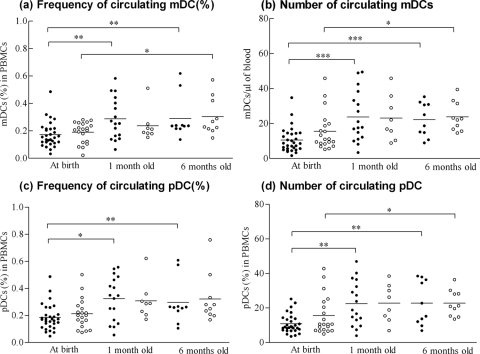
Circulating DC frequencies and numbers were calculated by flow cytometry (Table 1). The frequencies of DC subsets of total PBMCs, their absolute numbers, and mDC/pDC ratios in neonates of HBsAg+ mothers were similar to those observed in neonates of healthy mothers at all ages studied (Fig. 2 a to d). Moreover, there were no statistically significant differences between subjects with and without detectable HBV DNA levels and controls or between subjects born to viremic and nonviremic mothers and controls at all ages. However, mDC and pDC frequencies and numbers were significantly lower at birth than at 1 and 6 months, but not between 1- and 6-month old individuals, in both subjects and controls (Fig. 2a to d).
FIG. 2.
The frequencies and absolute numbers of myeloid DCs (a and b) and plasmacytoid DCs (c and d) from subject (•) and control (○) neonates in different age groups plotted from the data from the cytometric analysis. Each dot represents a single donor, and the horizontal bars represent the means. There were no significant differences between children from chronically HBV-infected mothers and controls in all age groups. mDC and pDC frequencies and numbers were significantly lower at birth than for 1- and 6-month-old neonates, independent of maternal HBV status. *, P < 0.05 between groups; **, P < 0.005 between groups; ***, P < 0.0005 between groups.
IFN-α production from PBMCs of neonates born to HBsAg+ mothers.
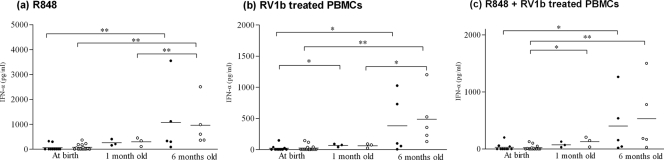
To assess DC function in infancy, the capacity of PBMCs to produce IFN-α was evaluated in 18 subject neonates, 50% of whom were positive for HBV DNA (median, 1.9 ± 1.6 log10 vc/ml) while 55.5% had viremic mothers (median, 4 ± 3.8 log10 vc/ml) on the day of delivery, and in 22 control neonates. The study included 10 subjects and 14 controls at birth, 3 subjects and 3 controls at the age of 1 month, and 5 subjects and 5 controls at the age of 6 months. There were no statistically significant differences observed between subjects and controls under different stimulations (Fig. 3), and IFN-α production was not correlated with maternal or neonatal viremia within the subject group. At birth, IFN-α was detectable at very low concentrations that increased significantly in 1- and 6-month-old subjects and controls under all stimulations (P < 0.05) (Fig. 3). Incubation with RV1b inhibited R848-induced IFN-α production to almost one-half; however, this was not statistically significant (P = 0.1 in CB pDCs; P = 0.4 at birth; P = 0.056 at 1 month; and P = 0.14 at 6 months of age).
FIG. 3.
IFN-α production from PBMCs in subject (•) and control (○) neonates after stimulation with R848 and/or RV1b. Each dot represents a single donor, and the horizontal bars represent the means. *, P < 0.05 between groups; **, P < 0.005 between groups.
The maturational capacity of CB pDCs from HBsAg+ mothers.
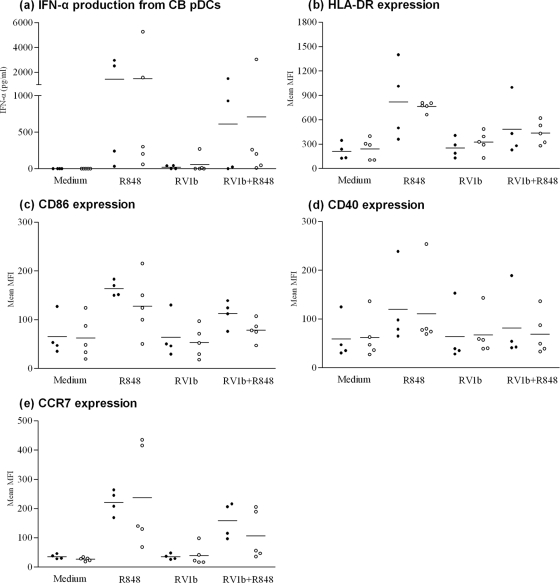
IFN-α production and the expression of CD86, HLA-DR, CD40, and CCR7 were determined on the surfaces of 4 CB pDCs from HBV-infected mothers, all of whom were viremic on the day of delivery while two of their subject newborns were positive for HBV DNA, as well as from 5 control mothers (Table 1). IFN-α production was increased, and all markers were upregulated post-R-848 stimulation at comparable levels in subject newborns positive or negative for HBV DNA and control newborns, indicating that pDCs were functional in all groups (Fig. 4). RV1b-stimulated pDCs had costimulatory expression and IFN-α production similar to those of resting pDCs, suggesting that RV1b avoided pDC recognition. However, marker expression and IFN-α production in pDCs that were stimulated in parallel with RV1b and R848 were reduced to almost one-half compared to pDCs stimulated with R848 alone in both subjects and controls, although this difference was not significant (Fig. 4).
FIG. 4.
IFN-α production from CB pDCs (a) and expression of costimulatory molecules (b to e) in subject (•) and control (○) neonates after stimulation with R848 and/or RV1b. Each dot represents a single donor, and the horizontal bars represent the means. No statistically significant differences were observed among the subjects and controls under similar conditions. Medium, nonstimulated CB pDCs.
DISCUSSION
Immaturity of innate immunity could explain neonatal vulnerability to infection. In the majority of studies, the function of neonatal DCs has been shown to be defective compared to adult DC function (3, 6). Increasing evidence suggests that neonates of HBV carrier mothers are exposed to virions and/or viral antigens in utero even if they do not acquire the infection (17, 18, 23, 30). In the present study, viral exposure before birth was demonstrated by the finding that 41% of the subjects showed detectable HBV DNA levels at birth. HBV exposure was also demonstrated by additional studies from our group in which PBMCs from 10 out of the 30 subject neonates were shown to respond to HBV core antigen (unpublished data). Although none of the subjects developed HBV infection, we hypothesized that this encounter could have further influenced the DC system in their early childhood. Our results showed that DC numbers and pDC function were not altered in uninfected neonates by possible HBV exposure.
The frequencies and ratio of mDCs and pDCs at birth and 1 and 6 months of age were similar in subjects and controls. More importantly, the DC numbers of neonates who had evidence of viral exposure as depicted by maternal and/or neonatal viremia were comparable to those of the control group. Therefore, the circulating DCs of infants born to chronically HBV-infected mothers seem to be numerically efficient at initiating innate and adaptive immune responses. However, it is worth noting that the infectivity of all HBV-infected mothers included in this study was low, since they were inactive carriers with low HBV DNA levels. Therefore, our results cannot be generalized for all infants born to HBV-infected mothers. Since neonatal viremia was quite low, it is possible that infants exposed to HBV were not infected because their innate systems were activated efficiently by pDCs to clear the virus during the initial period of infection. DCs were adequate to present HBV surface antigens from the vaccine and to initiate T-cell responses, as was also confirmed by their anti-HBs antibody levels (>10 IU/liter) at the age of 7 months. Both mDC and pDC numbers and frequencies were statistically lower in newborns than in 1- and 6-month-old individuals, independently of maternal HBsAg status. The fact that the observed differences within the group of babies born to HBsAg+ mothers were not significant might have been due to the small sample number. The mean frequencies of mDCs and pDCs did not exceed 0.4% of total PBMCs until the age of 6 months, and therefore, they are expected to reach adult levels (0.8 to 1%) later in life (6).
During viral infection, pDCs detect viral genetic material or its degradation products via interaction with TLRs. Activated pDCs can stimulate naïve T cells, but this activation is poor relative to that of mDCs (7). They exert their antiviral activities mainly by producing abundant amounts of type I IFNs and also a number of other cytokines (10). In this way, they modulate innate immune responses and shape the adaptive system by promoting Th1 responses and suppressing Th2 responses. The TLRs expressed in pDCs include TLR7 and TLR9, located in the endosomal compartments. TLR7 recognizes viral ssRNA, as well as imidazoquinolines, such as imiquimod and R848 (7, 8, 14). Several studies demonstrated IFN-α production in pDCs after incubation with a variety of ssRNA viruses, including influenza A virus (4, 15), Sendai virus (11), and vesicular stomatitis virus (15). Rhinoviruses are the major cause of the common cold, which is very common in young children but less so with increasing age and therefore is an important marker of a developing immune response (19, 29). Although there has been no prior direct evidence of RV-pDC interaction, it was considered a successful candidate to stimulate IFN-α production by pDCs because it is a naturally occurring ssRNA virus and has also been shown to efficiently activate IFN-α production in monocyte-derived DCs and PBMCs (13, 25).
Large blood volumes are necessary to isolate circulating pDCs, which is not feasible when studying newborns or young infants. Since pDCs are responsible for 90% of IFN-α secretion from PBMCs (26), we evaluated pDC function by assessing IFN-α production from PBMCs. The levels of IFN-α in R848- and RV-stimulated PBMCs were comparable between subjects positive and negative for HBV DNA and controls at all ages and under all conditions. At birth, RV1b infection and R848 stimulation did not induce high IFN-α production, but this production significantly increased in 1- and 6-month-old neonates, depicting the normal maturation process. RV escapes pDC recognition in early infancy, whereas at 6 months of age, it is sufficient to activate the innate-defense arm of pDCs. This phenomenon may reflect the physiologically functional DC maturational process during infancy, as shown by the results on DC frequencies at this age.
The maturational capacity of pDCs was further examined in cord blood of four carrier mothers by assessing IFN-α secretion and the expression of the costimulatory molecules CD86 and CD40, which signal T cells through CD28 receptor, the activator marker HLA-DR, and the chemokine receptor CCR7, which mediates homing to the lymph nodes. Similar to the PBMC results, although all subject mothers and two of their infants were viremic, CB pDC responses after R848 stimulation and RV infection were comparable between viremic and nonviremic subjects and controls.
In PBMCs and CB pDCs, R848-induced IFN-α production was reduced in RV-infected cells but was not blocked; it is therefore possible that RV has an inhibitory effect on the TLR7 signaling pathway, independent of HBV maternal infection. It can be assumed that RV does not avoid pDC recognition, at least at 6 months of age, but may inhibit an IFN-α response triggered by coinfecting pathogens that act through the TLR7 pathway. Up-to-date respiratory syncytial virus (RSV) and measles virus, both ssRNA viruses, have been shown to completely block the TLR7 signaling pathway, and thus, they have been suggested to infect pDCs (24).
Due to difficulties in following HBV-positive mothers and their neonates, our sample population was small, and this weakens our results. However, isolated pDCs were more than 95% pure, and resting pDCs and PBMCs did not induce any IFN-α secretion, whereas following R848 stimulation, cytokine production and marker expression increased dramatically in both subjects and controls. Therefore, the observed responses were not due to a nonspecific reaction or to generalized pDC dysfunction.
In conclusion, our results show that clinically uninfected neonates of HBsAg+/HBeAg− mothers develop normal circulating DC numbers and have normally functional pDCs early in life. The fact that neonates with transient viremia did not develop infection may be due to an efficient innate immune response to clear the low levels of the virus and/or to the absence of HBV e antigen. Our results reinforce the notion of an immature DC system in early infancy. Production of type I INFs by pDCs has a key role in antiviral immunity. Thus, impaired pDC function in response to RV1b and its interference in the TLR7 pathway in early infancy may result in defective T-cell responses against coinfection with other viruses or against treatment. Nevertheless, due to the small sample size and the low infectivity of HBV-infected mothers, these findings should be verified by larger studies.
Acknowledgments
We are particularly grateful to the study subjects and their mothers. We also thank Dominique De Wit and Bénédicte Danis for their assistance through consultation in developing the experimental techniques used in this study.
The study was cofunded by the European Social Fund and National Resources—(EPEAEK II) Pythagoras and was also supported by a training fellowship from the European Society for Pediatric Infectious Diseases (ESPID) and a fellowship from the European Society of Clinical Microbiology and Infectious Diseases (ESCMID).
Footnotes
Published ahead of print on 12 May 2010.
REFERENCES
- 1.Beasley, R. P., L. Y. Hwang, C. C. Lin, Y. C. Ko, and S. J. Twu. 1983. Incidence of hepatitis among students at a university in Taiwan. Am. J. Epidemiol. 117:213-222. [DOI] [PubMed] [Google Scholar]
- 2.Delespesse, G., L. P. Yang, Y. Ohshima, C. Demeure, U. Shu, D. G. Byun, and M. Sarfati. 1998. Maturation of human neonatal CD4+ and CD8+ T lymphocytes into Th1/Th2 effectors. Vaccine 16:1415-1419. [DOI] [PubMed] [Google Scholar]
- 3.De Wit, D., V. Olislagers, S. Goriely, F. Vermeulen, H. Wagner, M. Goldman, and F. Willems. 2004. Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human newborns. Blood 103:1030-1032. [DOI] [PubMed] [Google Scholar]
- 4.Diebold, S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 5.Duan, X., M. Wang, H. Li, H. Zhuang, D. Xu, and F. Wang. 2004. Decreased frequency and function of circulating plasmocytoid dendritic cells (pDC) in hepatitis B virus infected humans. J. Clin. Immunol. 24:637-646. [DOI] [PubMed] [Google Scholar]
- 6.Duan, X. Z., H. Zhuang, M. Wang, H. W. Li, J. C. Liu, and F. S. Wang. 2005. Decreased numbers and impaired function of circulating dendritic cell subsets in patients with chronic hepatitis B infection (R2). J. Gastroenterol. Hepatol. 20:234-242. [DOI] [PubMed] [Google Scholar]
- 7.Fuchsberger, M., H. Hochrein, and M. O'Keeffe. 2005. Activation of plasmacytoid dendritic cells. Immunol. Cell Biol. 83:571-577. [DOI] [PubMed] [Google Scholar]
- 8.Gibson, S. J., J. M. Lindh, T. R. Riter, R. M. Gleason, L. M. Rogers, A. E. Fuller, J. L. Oesterich, K. B. Gorden, X. Qiu, S. W. McKane, R. J. Noelle, R. L. Miller, R. M. Kedl, P. Fitzgerald-Bocarsly, M. A. Tomai, and J. P. Vasilakos. 2002. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 218:74-86. [DOI] [PubMed] [Google Scholar]
- 9.Harris, D. T., M. J. Schumacher, J. Locascio, F. J. Besencon, G. B. Olson, D. DeLuca, L. Shenker, J. Bard, and E. A. Boyse. 1992. Phenotypic and functional immaturity of human umbilical cord blood T lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 89:10006-10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hochrein, H., M. O'Keeffe, and H. Wagner. 2002. Human and mouse plasmacytoid dendritic cells. Hum. Immunol. 63:1103-1110. [DOI] [PubMed] [Google Scholar]
- 11.Hornung, V., J. Schlender, M. Guenthner-Biller, S. Rothenfusser, S. Endres, K. Conzelmann, and G. Hartmann. 2004. Replication-dependent potent IFN-alpha induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J. Immunol. 173:5935-5943. [DOI] [PubMed] [Google Scholar]
- 12.Hunt, D. W., H. I. Huppertz, H. J. Jiang, and R. E. Petty. 1994. Studies of human cord blood dendritic cells: evidence for functional immaturity. Blood 84:4333-4343. [PubMed] [Google Scholar]
- 13.Khaitov, M., V. Laza-Stanca, M. Edwards, R. Walton, G. Rohde, M. Contoli, A. Papi, L. Stanciu, S. Kotenko, and S. Johnston. 2009. Respiratory virus induction of alpha-, beta- and lambda-interferons in bronchial epithelial cells and peripheral blood mononuclear cells. Allergy 64:375-386. [DOI] [PubMed] [Google Scholar]
- 14.Krug, A., A. Towarowski, S. Britsch, S. Rothenfusser, V. Hornung, R. Bals, T. Giese, H. Engelmann, S. Endres, A. M. Krieg, and G. Hartmann. 2001. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur. J. Immunol. 31:3026-3037. [DOI] [PubMed] [Google Scholar]
- 15.Lund, J., L. Alexopoulou, A. Sato, M. Karow, N. Adams, N. Gale, A. Iwasaki, and R. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. U. S. A. 101:5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMahon, B. J., W. L. Alward, D. B. Hall, W. L. Heyward, T. R. Bender, D. P. Francis, and J. E. Maynard. 1985. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J. Infect. Dis. 151:599-603. [DOI] [PubMed] [Google Scholar]
- 17.Milich, D., and T. J. Liang. 2003. Exploring the biological basis of hepatitis B e antigen in hepatitis B virus infection. Hepatology 38:1075-1086. [DOI] [PubMed] [Google Scholar]
- 18.Milich, D. R., J. E. Jones, J. L. Hughes, J. Price, A. K. Raney, and A. McLachlan. 1990. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc. Natl. Acad. Sci. U. S. A. 87:6599-6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papadopoulos, N. G., and S. L. Johnston. 1998. Viruses and asthma exacerbations. Thorax 53:913-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papadopoulos, N. G., L. A. Stanciu, A. Papi, S. T. Holgate, and S. L. Johnston. 2002. Rhinovirus-induced alterations on peripheral blood mononuclear cell phenotype and costimulatory molecule expression in normal and atopic asthmatic subjects. Clin. Exp. Allergy 32:537-542. [DOI] [PubMed] [Google Scholar]
- 21.Paraskevis, D., C. Haida, N. Tassopoulos, M. Raptopoulou, D. Tsantoulas, H. Papachristou, V. Sypsa, and A. Hatzakis. 2002. Development and assessment of a novel real-time PCR assay for quantitation of HBV DNA. J. Virol. Methods 103:201-212. [DOI] [PubMed] [Google Scholar]
- 22.Paraskevis, D., C. Katsoulidou, Z. Moschidis, E. Hatzitheodorou, A. Varaklioti, and A. Hatzakis. 2007. Development of a flexible and sensitive in-house real-time PCR assay for the quantification of HBV DNA, p. 217. Abstr. XVIIth Regional Congr. Int. Soc. Blood Transfusion, Europe, Madrid, Spain, 23 to 27 June 2007.
- 23.Ranger-Rogez, S., S. Alain, and F. Denis. 2002. Hepatitis viruses: mother to child transmission. Pathol. Biol. (Paris) 50:568-575. [DOI] [PubMed] [Google Scholar]
- 24.Schlender, J., V. Hornung, S. Finke, M. Gunthner-Biller, S. Marozin, K. Brzozka, S. Moghim, S. Endres, G. Hartmann, and K. Conzelmann. 2005. Inhibition of toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J. Virol. 79:5507-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schrauf, C., S. Kirchberger, O. Majdic, M. Seyerl, G. Zlabinger, K. Stuhlmeier, M. Sachet, J. Seipelt, and J. Stöckl. 2009. The ssRNA genome of human rhinovirus induces a type I IFN response but fails to induce maturation in human monocyte-derived dendritic cells. J. Immunol. 183:4440-4448. [DOI] [PubMed] [Google Scholar]
- 26.Siegal, F. P., N. Kadowaki, M. Shodell, P. A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y. J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science 284:1835-1837. [DOI] [PubMed] [Google Scholar]
- 27.Velilla, P. A., C. J. Montoya, A. Hoyos, M. E. Moreno, C. Chougnet, and M. T. Rugeler. 2008. Effect of intrauterine HIV-1 exposure on the frequency and function of uninfected newborns' dendritic cells. Clin. Immunol. 126:243-250. [DOI] [PubMed] [Google Scholar]
- 28.Velilla, P. A., M. T. Rougeles, and C. A. Chougnet. 2006. Defective antigen-presenting cell function in human neonates. Clin. Immunol. 121:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xatzipsalti, M., S. Kyrana, M. Tsolia, S. Psarras, A. Bossios, V. Laza-Stanca, S. L. Johnston, and N. G. Papadopoulos. 2005. Rhinovirus viremia in children with respiratory infections. Am. J. Respir. Crit. Care Med. 172:1037-1040. [DOI] [PubMed] [Google Scholar]
- 30.Yue, Y., J. Meng, and S. Zhang. 2002. Mechanism of peripheral blood mononuclear cell invasion by HBV on artificial immunization in newborns. Chin. Med. J. (Engl.). 115:1380-1382. [PubMed] [Google Scholar]
- 31.Zhang, Z., H. Zhang, D. Chen, J. Yao, J. Fu, L. Jin, and F. Wang. 2007. Response to interferon-alpha treatment correlates with recovery of blood plasmacytoid dendritic cells in children with chronic hepatitis B. J. Hepatol. 47:751-759. [DOI] [PubMed] [Google Scholar]