Abstract
Ten penicillinase-producing Neisseria gonorrhoeae (PPNG) strains isolated from 2000 to 2008 were characterized by multilocus sequence typing, multiantigen sequence typing, and plasmid typing. Sequence analysis showed that 8 strains contained a TEM-1 β-lactamase gene. However, two other genetically distinct PPNG strains, isolated in 2004 and 2008, each contained a TEM-135 β-lactamase on different plasmids, a Toronto/Rio type R plasmid and an Asia type R plasmid, suggesting independent origins of these PPNG strains.
Antibiotic-resistant Neisseria gonorrhoeae is a major public health concern (15). An essential element in gonococcal-infection control is the availability of effective antimicrobial therapy. However, N. gonorrhoeae has developed resistance to multiple classes of antimicrobials. In Japan, the prevalence of fluoroquinolone-resistant N. gonorrhoeae strains is over 80% (12), and N. gonorrhoeae strains with reduced sensitivity and with resistance to cefixime (CFM) have emerged and spread nationwide (5, 7). In contrast to the high prevalence of N. gonorrhoeae strains with chromosomal β-lactam resistance genes, the prevalence of penicillinase-producing N. gonorrhoeae (PPNG) strains with a β-lactamase gene carried on a plasmid is relatively low in Japan. However, the prevalence of PPNG strains in other countries in Asia is high (16). To study the epidemiology of N. gonorrhoeae, nucleotide sequence-based typing methods, like multilocus sequence typing (MLST) and multiantigen sequence typing (MAST), are useful tools, since the analyses yield highly reproducible and easy-to-compare data from different laboratories.
Among the 719 N. gonorrhoeae strains isolated from January 2000 to December 2008 in the Nakano Sogo Hospital in Japan, 10 strains (1.4%) were found to be penicillinase-producing N. gonorrhoeae (PPNG) by the nitrocefin test (data not shown). The MICs of penicillin (PEN), cefixime (CFM), and ceftriaxone (CRO) were determined by the agar dilution method (6), suggesting that the strains were highly resistant to penicillin but not to cephalosporins (Table 1). This low prevalence was consistent with other reports (14, 16).
TABLE 1.
Penicillinase-producing Neisseria gonorrhoeae strains isolated in Tokyo from 2000 to 2008
| Strain | Time of isolation | Sex of patienta | Age (yr) of patient | Specimen typeb | MLST type | MAST type | Plasmid type | bla type | MIC (μg/ml) |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PEN | CFM | CRO | |||||||||
| NGON 00-002 | January 2000 | M | 26 | UD | ST-1590 | ST-270 | Africa | TEM-1 | 16 | 0.032 | 0.016 |
| NGON 00-027 | June 2000 | M | 42 | U | ST-1921 | ST-1817 | Asia | TEM-1 | >64 | 0.008 | 0.008 |
| NGON 04-025 | April 2004 | M | 27 | U | ST-1597 | ST-1549 | Toronto/Rio | TEM-135 | >64 | 0.004 | ≤0.004 |
| NGON 05-042 | August 2005 | F | 30 | VD | ST-1588 | ST-4012 | Africa | TEM-1 | 64 | 0.25 | 0.064 |
| NGON 06-041 | October 2006 | M | 56 | U | ST-1588 | ST-4012 | Africa | TEM-1 | 32 | 0.25 | 0.032 |
| NGON 08-003 | January 2008 | F | 31 | VD | ST-7823 | ST-4013 | Asia | TEM-135 | >64 | 0.032 | 0.032 |
| NGON 08-041 | September 2008 | M | 52 | U | ST-1584 | ST-1478 | Africa | TEM-1 | 64 | 0.008 | 0.004 |
| NGON 08-043 | September 2008 | M | 31 | U | ST-7823 | ST-1288 | Asia | TEM-1 | >64 | 0.064 | 0.064 |
| NGON 08-044 | September 2008 | F | 25 | VD | ST-7823 | ST-1288 | Asia | TEM-1 | >64 | 0.064 | 0.064 |
| NGON 08-046 | October 2008 | F | 59 | VD | ST-1584 | ST-1478 | Africa | TEM-1 | 64 | 0.25 | 0.032 |
M, male; F, female.
U, urine; VD, vaginal discharge; UD, urethral discharge.
MLST and MAST (3, 4) were used to characterize these PPNG strains. As shown in Table 1, both MLST and MAST divided the 10 PPNG strains into 7 types, with 4 (NGON 00-002, NGON 00-027, NGON 04-025, and NGON 08-003) of the PPNG strains having unique sequence types (ST) by both MLST and MAST. However, three pairs of strains (NGON 05-042 and NGON 06-041, NGON 08-041 and NGON08-046, and NGON 08-043 and NGON 08-044) had identical sequence types by MLST and by MAST (Table 1). Although we have no information linking the patients from whom each pair of strains was isolated, transmission of PPNG strains might be considered in these cases.
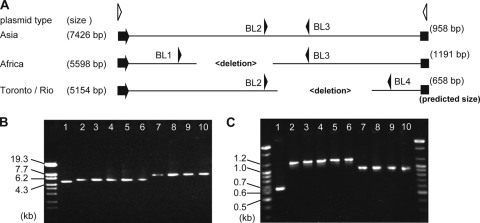
Plasmids of the PPNG strains carrying the β-lactamase gene (bla) have been typed based on plasmid size, since deletion mutants have been reported previously (9). To investigate plasmid diversity in the PPNG strains in this study, plasmid DNAs were purified using QIAprep Spin miniprep kits (Qiagen). To estimate β-lactamase plasmid size, we amplified the complete DNA of each plasmid by long PCR using LA Taq polymerase (TaKaRa) and primers bla-IR, 5′-TCGTGGTGTCACGCTCGTCG, and bla-IF, 5′-CTGCAGCAATGGCAACAACGTTG, which anneal to nucleotides 7426 to 7404 and 1 to 23, respectively, of the 7,426-bp pJD4 plasmid (Fig. 1A) (9). The PCR products were incubated for 2 min at 96°C followed by 30 cycles of 10 s at 96°C, 10 s at 63°C, and 8 min at 72°C. As shown in Fig. 1B, analysis of the amplified plasmid DNAs in a 1% agarose gel showed three plasmid sizes: 5.2, 5.6, and 7.4 kb. By use of a multiplex PCR method for plasmid typing (10), the 5.2-, 5.6-, and 7.4-kb plasmids were identified as Toronto/Rio, Africa, and Asia type R plasmids, respectively (Fig. 1A and C).
FIG. 1.
Typing of plasmids carrying β-lactamases from Neisseria gonorrhoeae strains. (A) Schematics of Asia, Africa, and Toronto/Rio type plasmids. Each β-lactamase gene is shown by an arrowhead. The annealing sites of the primers used in this study for plasmid size determination (white arrowheads) and for plasmid type determination (black arrowheads) are shown. (B) Products of whole-plasmid PCR amplification, separated on a 1% agarose gel. (C) Products of multiplex PCR, separated on a 2% agarose gel. The size marker lanes contain StyI-digested lambda DNA (Toyobo) (B) or a 100-bp DNA ladder (Bioneer) (C). Lane 1, NGON 04-025; lane 2, NGON 00-002; lane 3, NGON 05-042; lane 4, NGON 06-041; lane 5, NGON 08-041; lane 6, NGON 08-046; lane 7, NGON 00-027; lane 8, NGON 08-003; lane 9, NGON 08-043; lane 10, NGON 08-044.
Although the molecular sizes of N. gonorrhoeae R plasmids are diverse, plasmids carrying β-lactamases are genetically related and carry a TEM-1 type bla gene, blaTEM-1 (12). To confirm the conservation of blaTEM-1, the bla genes of the 10 PPNG isolates were analyzed by DNA sequencing (8). The primers used for amplification and sequencing were bla-F, 5′-CGCTCATGAGACAATAACCCTGG, and bla-R, 5′-GGGTCTGACGCTCAGTGGAACG. The PCR products were incubated for 2 min at 96°C followed by 30 cycles of 10 s at 96°C, 10 s at 60°C, and 1 min at 72°C. Nucleotide sequencing was carried out as described previously (8). As shown in Table 1, two distinct blaTEM alleles were found: 8 PPNG strains contained blaTEM-1, and the other 2 strains (NGON 04-025 and NGON 08-003) contained blaTEM-135, a TEM allele originally identified in Salmonella enterica serovar Typhimurium (11). These alleles, blaTEM-1 and blaTEM-135, had one base difference, which resulted in a single amino acid substitution, M182T (residue numbering follows that of Ambler et al. [1]).
Interestingly, the two PPNG strains with blaTEM-135 were genetically different: the sequence types of strain NGON 04-025 were MLST ST-1597 and MAST ST-1549, and those of strain NGON 08-003 were MLST ST-7823 and MAST ST-4013 (Table 1). The plasmids carried by strains NGON 04-025 and NGON 08-003 were also distinct: the plasmid for the former was a Toronto/Rio type, and that for the latter was an Asia type. Taken together, these findings suggest that blaTEM-135 may have been introduced independently into these two N. gonorrhoeae strains or may have emerged by a point mutation in each. Recently, Srifeungfung et al. (13) reported that a PPNG strain isolated in Thailand contained a blaTEM-135 allele. PPNG strains containing blaTEM-135 might be widespread in Asian countries, although further study is needed to determine the prevalence.
The TEM type β-lactamase genes, which are widely distributed in Gram-negative bacteria, are diverse in sequence and in substrate spectrum. Some types of TEM β-lactamases can hydrolyze extended-spectrum cephalosporins with an oxyimino side chain, including ceftriaxone, which is still an effective antibiotic for N. gonorrhoeae. The diverse substrate spectra of TEM β-lactamases are due to mutations in the blaTEM gene that alter the amino acid configuration around the β-lactamase active site. Since bacteria with blaTEM-135 have a restricted β-lactamase substrate spectrum, as reported in a previous study (10) and also in this study (Table 1), the selective pressure for emergence of N. gonorrhoeae blaTEM-135 is not known. It is noteworthy that there are other TEM β-lactamases with extended substrate spectra that may have arisen as a single point mutation in blaTEM-1 or blaTEM-135, e.g., blaTEM-29 and blaTEM-20 (2). Since point mutations in blaTEM-1 and blaTEM-135 could lead to emergence of N. gonorrhoeae β-lactamases with extended substrate spectra, the antibiotic resistance profiles of PPNG strains should be monitored, especially in areas of high PPNG prevalence.
Nucleotide sequence accession number.
The sequence data for the blaTEM-135 gene have been assigned DDBJ accession number AB551787.
Acknowledgments
We thank Hiroko Matsuoka for technical assistance.
This work was partly supported by grants-in-aid from the Ministry of Health, Labor and Welfare of Japan (H21-Shinkou-Ippan-001 and H21-Shinkou-Ippan-012).
Footnotes
Published ahead of print on 26 April 2010.
REFERENCES
- 1.Ambler, R. P., A. F. W. Coulson, J.-M. Frère, J.-M. Ghuysen, B. Joris, M. Forsman, R. C. Levesque, G. Tiraby, and S. G. Waley. 1991. A standard numbering scheme for the class A β-lactamases. Biochem. J. 276:269-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arlet, G., S. Goussard, P. Courvalin, and A. Philippon. 1999. Sequences of the genes for the TEM-20, TEM-21, TEM-22, and TEM-29 extended-spectrum β-lactamases. Antimicrob. Agents Chemother. 43:969-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jolley, K. A. 2001. Multi-locus sequence typing, p. 173-186. In A. J. Pollard and M. C. Maiden (ed.), Meningococcal disease: methods and protocols. Humana Press, Totowa, NJ.
- 4.Martin, I. M., C. A. Ison, D. M. Aanensen, K. A. Fenton, and B. G. Spratt. 2004. Rapid sequence-based identification of gonococcal transmission clusters in a large metropolitan area. J. Infect. Dis. 189:1497-1505. [DOI] [PubMed] [Google Scholar]
- 5.Muratani, T., S. Akasaka, T. Kobayashi, Y. Yamada, H. Inatomi, K. Takahashi, and T. Matsumoto. 2001. Outbreak of cefzopran (penicillin, oral cephems, and aztreonam)-resistant Neisseria gonorrhoeae in Japan. Antimicrob. Agents Chemother. 45:3603-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Committee for Clinical Laboratory Standards. 1999. Performance standards for antimicrobial susceptibility testing, 9th informational supplement M7-A4 (M100-S9). National Committee for Clinical Laboratory Standards, Wayne, PA.
- 7.Ochiai, S., H. Ishiko, M. Yasuda, and T. Deguchi. 2008. Rapid detection of the mosaic structure of the Neisseria gonorrhoeae penA gene, which is associated with decreased susceptibilities to oral cephalosporins. J. Clin. Microbiol. 46:1804-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohnishi, M., Y. Watanabe, E. Ono, C. Takahashi, H. Oya, T. Kuroki, K. Shimuta, N. Okazaki, S. Nakayama, and H. Watanabe. 2010. Spread of a chromosomal cefixime-resistant penA gene among different Neisseria gonorrhoeae lineages. Antimicrob. Agents Chemother. 54:1060-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pagotto, F., A. T. Aman, L. K. Ng, K. H. Yeung, M. Brett, and J. A. Dillon. 2000. Sequence analysis of the family of penicillinase-producing plasmids of Neisseria gonorrhoeae. Plasmid 43:24-34. [DOI] [PubMed] [Google Scholar]
- 10.Palmer, H. M., J. P. Leeming, and A. Turner. 2000. A multiplex polymerase chain reaction to differentiate β-lactamase plasmids of Neisseria gonorrhoeae. J. Antimicrob. Chemother. 45:777-782. [DOI] [PubMed] [Google Scholar]
- 11.Pasquali, F., C. Kehrenberg, G. Manfreda, and S. Schwarz. 2005. Physical linkage of Tn3 and part of Tn1721 in a tetracycline and ampicillin resistance plasmid from Salmonella Typhimurium. J. Antimicrob. Chemother. 55:562-565. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Pescador, R., M. S. Stempien, and M. S. Urdea. 1988. Rapid chemiluminescent nucleic acid assays for detection of TEM-1 β-lactamase-mediated penicillin resistance in Neisseria gonorrhoeae and other bacteria. J. Clin. Microbiol. 26:1934-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srifeungfung, S., A. Roongpisuthipong, S. Asavapiriyanont, R. Lolekha, C. Trubyddharat, S. Lokpichart, P. Sungthong, and P. Tongtep. 2009. Prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae in HIV-seropositive and gonococcal antimicrobial susceptibility: an update in Thailand. Jpn. J. Infect. Dis. 62:467-470. [PubMed] [Google Scholar]
- 14.Tanaka, M., H. Nakayama, T. Notomi, S. Irie, Y. Tsunoda, A. Okadome, T. Saika, and I. Kobayashi. 2004. Antimicrobial resistance of Neisseria gonorrhoeae in Japan, 1993-2002: continuous increasing of ciprofloxacin-resistant isolates. Int. J. Antimicrob. Agents 24(Suppl. 1):S15-S22. [DOI] [PubMed] [Google Scholar]
- 15.Tapsall, J. W., F. Ndowa, D. A. Lewis, and M. Unemo. 2009. Meeting the public health challenge of multidrug- and extensively drug-resistant Neisseria gonorrhoeae. Expert Rev. Anti Infect. Ther. 7:821-834. [DOI] [PubMed] [Google Scholar]
- 16.WHO Western Pacific Gonococcal Antimicrobial Surveillance Programme. 2008. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the WHO Western Pacific Region, 2006. Commun. Dis. Intell. 32:48-51. [DOI] [PubMed] [Google Scholar]