Abstract
In this work, we investigated the in vivo activity of ravuconazole against the Y and Berenice-78 Trypanosoma cruzi strains using acutely infected dogs as hosts. Ravuconazole was well tolerated, as no significant side effects were observed during the treatment using 6.0 mg/kg twice a day (12 mg/kg/day) for up to 90 days. In all treated animals, parasitemia was permanently suppressed by the first day of treatment, independently of the parasite strain. Cultures of blood obtained posttreatment were negative for 90% of the animals, confirming that the drug induced a marked reduction in the parasite load. The results of PCR tests for T. cruzi in blood performed 1 month posttreatment were consistently negative for three of five and two of five animals infected with the Y and Berenice-78 strains, respectively. All ravuconazole-treated dogs consistently had negative serological test results during and until 30 days after treatment, regardless of the therapeutic scheme used. However, after the end of treatment, an increase in specific antibody levels was observed in all treated animals, although the antibody levels were always significantly lower than those of the nontreated control dogs. Despite being unable to induce a parasitological cure, ravuconazole treatment led to significant reductions in the levels of gamma interferon expression and lesions in cardiac tissues in animals infected with the Y strain, while the level of interleukin-10 mRNA expression increased. We conclude that ravuconazole has potent suppressive but not curative activity in the canine model of acute Chagas' disease, probably due to its unfavorable pharmacokinetic properties (half-life, 8.8 h). The longer half-life of ravuconazole in humans (4 to 8 days) makes it a promising drug for assessment for use as chemotherapy in human Chagas' disease.
Chagas' disease, a century after its discovery, remains an important health problem, being broadly dispersed in South and Central America (9). About 15 million people are currently estimated to be infected with Trypanosoma cruzi in Mexico and in Central American and South American countries, while 28 million remain at risk of infection (30). Due to international migration, a significant number of infected individuals now reside in countries where the disease is not endemic (9).
Recently, the success of programs to control the domiciliary vector has led to a decrease in the incidence and prevalence of Chagas' disease in some regions of the continent (20). However, despite the substantial advances in the control of the vectorial transmission of T. cruzi, little progress with the treatment of infected people has been made (27).
The specific chemotherapy for Chagas' disease, based on nitroheterocyclic compounds, is unsatisfactory. The 2-nitroimidazole benznidazole (BZ; Rochagan and Rodanil; Roche) and the 5-nitrofuran nifurtimox (Lampit; Bayer) were empirically developed more than 3 decades ago. Several clinical trials demonstrate that treatment with nifurtimox or benznidazole has low efficacy against the prevalent long-term chronic form of the disease, with cure rates varying from 0% to 19.1% (2, 6, 15, 28). Furthermore, both drugs cause frequent side effects, including anorexia, vomiting, peripheral polyneuropathy, and allergic dermopathy (6).
Given these limitations, the development of new drugs and evaluation of the efficacy of trypanocidal treatments in preventing morbidity are major challenges for Chagas' disease control (23). Ergosterol biosynthesis inhibitors (EBIs) are among the most promising anti-T. cruzi agents, as this parasite has an essential requirement for endogenous sterols for survival and proliferation and cannot use the abundant supply of cholesterol available in its mammalian hosts (23). Several steps in the sterol biosynthesis pathway in T. cruzi have been investigated as potential chemotherapeutic targets, both in vitro and in experimental infections (4, 23, 25, 26) The most advanced EBIs are azole compounds, comprising imidazole and triazole derivatives, which inhibit the C-14 demethylation of lanosterol. Novel triazole compounds, posaconazole (Schering Plough), ravuconazole (RAV; Eisai Co. Ltd., Tokyo, Japan), TAK 187 (Takeda Chemical Company), and albaconazole (Uriach), have in vitro MIC values against the clinically relevant intracellular amastigotes in the range of low nanomolar levels, being 30- to 100-fold lower than those of ketoconazole and itraconazole (24, 27). Studies performed in vivo in murine or dog models indicate that posaconazole, TAK-187, and albaconazole induced 50 to 100% parasitological cure rates in the acute phase and 50 to 60% in the chronic phase, even if the infecting T. cruzi strains were partially or fully resistant to benznidazole (11, 19, 23). Although ravuconazole has very potent anti-T. cruzi activity in vitro, its short terminal half-life in mice (4.5 h) limited its activity in this experimental model (24). On the other hand, the half-life of the compound in dogs is longer (8.8 h; Eisai Co. Ltd., data on file), and this suggested that it could have better activity against T. cruzi in this animal model.
In the present study, we made a head-to-head comparison of the anti-T. cruzi activities of ravuconazole and benznidazole in a dog model of acute Chagas' disease described previously (11, 14), using T. cruzi strains susceptible and resistant to EBIs (11). The effect of drug treatment on the immune response of the infected animals was also assessed.
MATERIALS AND METHODS
T. cruzi stocks.
In this study, two strains were used: the Y strain (Trypanosoma cruzi, lineage II [TcII]), isolated from an acute human case of Chagas' disease, and the Berenice-78 (Be-78) strain (TcII), isolated by xenodiagnosis in 1978 from what was considered the first clinical case of the disease in Brazil of a patient with an indeterminate form of the disease. We have previously shown that these strains present distinct levels of EBI resistance in the canine model, with the Y strain being EBI susceptible and the Berenice-78 strain being EBI resistant (11).
Experimental animals and infection.
Thirty-five mongrel dogs (4 months old) from the kennel at the Ouro Preto Federal University (UFOP), Minas Gerais State, Brazil, were used in this study. All procedures and experimental protocols were conducted in accordance with the guidelines issued by the Brazilian College of Animal Experimentation (COBEA) and were approved by the Ethics Committee in Animal Research at UFOP (number 2005/47). The animals were fed commercial dog food and were given water ad libitum. Prior to the study, the animals were dewormed and vaccinated against several infectious diseases. Thirty dogs were inoculated with 2.0 × 103 bloodstream trypomastigotes per kg of body weight of the Y (15 animals) or Berenice-78 (15 animals) T. cruzi strains. Five age-matched noninfected dogs were used as negative controls.
Drugs.
Ravuconazole ([R-(R*,R*)]-4-[2-[2-(2,4-difluorophenyl)-2-hydroxy-1-metlyl-3-(1H-1,2,4-triazol-1-yl)propyl]-4-thiazolyl]benzonitrile) was provided by Eisai Co. Ltd. Benznidazole (N-benzyl-2-nitro-1-imidazolacetamide) was synthesized at Produtos Roche Q.F.S.A., Rio de Janeiro, Brazil).
Treatment scheme and experimental groups.
The animals infected with each T. cruzi strain were divided into three experimental groups: (i) five dogs were treated with ravuconazole at 12 mg/kg twice a day (b.i.d.; i.e., every 12 h [q12h]) for 90 days; (ii) five dogs were treated with benznidazole, the reference drug, at 7.0 mg/kg b.i.d. (q12h) for 60 days; and (iii) five dogs were maintained as nontreated controls. Another five animals were maintained as a noninfected and nontreated control group. The treatment schemes were previously described by Guedes et al. (11, 14). In all therapeutic schemes, oral treatment was started 12 to 22 days postinfection, immediately after the appearance of parasitemia, detected by fresh blood examination.
Assessment of parasitological cure.
To verify the occurrence of parasitological cure, a battery of four independent tests was used: fresh blood examination, hemoculture, PCR assay, and a serological enzyme-linked immunosorbent assay (ELISA).
(i) Fresh blood examination.
The parasitemia of the animals was examined from the 10th day of infection until the parasites were no longer detectable by collecting fresh blood from the marginal ear vein. The mortality rate was expressed as the cumulative percentage of dead animals.
(ii) Blood culture.
Blood culture assays were performed at 1 and 6 months posttreatment for treated and controls animals, as described by Chiari et al. (7). Blood cultures were examined monthly for up to 120 days for the detection of parasites.
(iii) PCR assay.
Ten milliliters of blood from each animal was collected at 1 and 6 months posttreatment. The samples were immediately mixed with an equal volume of 6 M guanidine HCl-0.2 M EDTA solution, maintained at room temperature for 2 weeks, and boiled for 15 min to break the minicircles (3). Three DNA extractions were performed using 40 μg of glycogen (Boehringer Mannheim) to precipitate the DNA. The PCR conditions were the same as those described by Gomes et al. (10), but 20 pmol each of primer S35 [5′-AAATAATGTACGGG (T/G)GAGATGCATGA-3′] and primer S36 (5′-GGGTTCGATTGGGGTTGGTGT-3′) was used. Briefly, 2 μl of blood DNA template was added to 10 mM Tris-HCl (pH 9.0); 75 mM KCl; 3.5 mM MgCl2; 0.1% Triton X-100; 0.2 mM each dATP, dCTP, dGTP, and dTTP (Sigma Chemical Co.); 1.0 U of Taq DNA polymerase (Promega); and water in a 20-μl reaction volume. The reaction mixtures were overlaid with 30 μl of mineral oil and subjected to 35 amplification cycles in a research programmable thermal controller (MiniCycler). The temperature profile was 95°C for 5 min for denaturation, 2 cycles for annealing at 30°C for 2 min, followed by 33 cycles with the annealing temperature increased to 40°C, and a final extension at 72°C for 5 min. Five microliters of the PCR products was analyzed by electrophoresis on a 6% polyacrylamide gel and visualized by silver staining.
(iv) Serological profile.
Serum samples were collected from the blood of all dogs before and monthly after the parasite inoculation until 6 months posttreatment. The serum samples were stored at −80°C, and ELISA tests were performed as described by Voller et al. (29). Briefly, the ELISA plates were sensitized with T. cruzi antigen prepared by alkaline extraction of the Y strain, obtained at exponential growth in liver infusion-tryptose (LIT) medium. Sera were added, and the antibody binding was detected by using peroxidase-labeled anti-dog IgG. Total IgG or IgG1 and IgG2 isotypes conjugated to horseradish peroxidase (Bethyl Laboratories, Montgomery, AL) were used for the determination of antibody levels. The plates were read with a spectrophotometer with a 490-nm filter (model 3550; Bio-Rad Laboratories). The cutoff was determined using the mean absorbance of 10 uninfected animals plus 2 standard deviations.
Histopathology and morphometric analysis.
The animals were euthanized 6 months after the end of the treatment by injection with thionenbutal (Abbott, São Paulo, Brazil) at 0.5 ml/kg of body weight (0.03 g/ml in 0.89% saline solution). A fragment of approximately 1.0 cm by 1.0 cm by 0.2 cm from the middle of the right atrial wall of each dog was taken for histopathological analyses. The tissue fragments were fixed in 10% buffered formalin solution, dehydrated, cleared, and embedded in paraffin. The blocks were cut into 4-mm-thick sections and stained with hematoxylin-eosin (H&E) for inflammation assessment or Masson's trichrome for fibrosis quantitative evaluation. Twenty fields from each H&E- or Masson's trichrome-stained section were randomly chosen at a ×40 magnification, giving a total of 1.5 × 106 μm2 of myocardium area analyzed. Images were obtained through a Leica DM 5000 B microchamber (version 2.4.0 R1; Leica Application Suite, United Kingdom) and processed with Leica Qwin (version 3) image analyzer software. The inflammatory process was evaluated by use of a correlation index between the number of cell nuclei observed in the myocardium muscles from noninfected and infected animals (16). The fibrosis area was quantified using the image segmentation function. All pixels with blue hues in the Masson's trichrome-stained section were selected to build a binary image, and the total area occupied by connective tissue in noninfected and T. cruzi-infected dogs was subsequently calculated.
Semiquantitative analysis of IFN-γ and IL-10 expression in heart tissue.
RNA was isolated from the right atrium of each dog by acidic guanidinium thiocyanate-phenol-chloroform extraction (RNAgentsTotal RNA isolation system; Promega). One microgram of total RNA was treated with RQ1 RNase-free DNase (Promega), before reverse transcription by the addition of 100 U SuperScript II RNaseH (Invitrogen), 10 mM deoxynucleotide triphosphates (dNTPs; Invitrogen), 0.1 M dithiothreitol (DTT; Invitrogen), 1× RNase H-negative reverse transcriptase buffer (Invitrogen), 1 mg oligo(dT)12-18 primer (Invitrogen), and 100 U RNaseOUT RNase inhibitor (Invitrogen) in a total volume of 20 μl. The reaction proceeded for 1 h at 42°C. Two microliters of cDNA was used for amplification in a 12-ml PCR mixture containing 2.5 mM dNTPs (Invitrogen), a 20 mM concentration of the 30 and 50 external primers, 1.5 mM MgCl2, 1× PCR buffer, and 0.5 U Taq DNA polymerase (Invitrogen). The PCR conditions were as follows: 94°C for 5 min, 94°C for 1 min, 57°C for 1 min, 72°C for 2 min (3 steps; n cycles), 57°C for 1 min, and 72°C for 6 min (final extension). The primers used (sense and antisense) the sequence from 5′ to 3′ (the number of cycles and the expected product size of the PCR product are shown in brackets) and were specific for the cytokines gamma interferon (IFN-γ; primers CGGCCTAACTCTCTGAAACG and CCTCCCTCTTACTGGTGCTG [38 cycles, 380 bp]) and interleukin-10 (IL-10; AGCACCCTACTTGAGGACGA and GATGTCTGGGTCGTGGTTCT [40 cycles, 249 bp]; Dialab, Brazil) and the constitutive gene hypoxanthine phosphoribosyltransferase (HPRT; AAGCTTGCTGGTGAAAAGGA and CAATGGGACTCCAGATGCTT [28 cycles, 219 bp]; Dialab). The PCR products and molecular weight marker were run on 6% polyacrylamide gels and stained with silver nitrate. The PCR products on the silver-stained gels were quantified with a densitometer using the Quantity One program (The Discovery Series, 1998; Bio-Rad Laboratories). The densitometric values for each cytokine were divided by the average value for HPRT for the same sample.
Statistical analysis.
The results of the serological assays, mRNA cytokine expression in the right atrium, histological data, and weight gain were analyzed by the nonparametric Newman-Keuls multiple comparison test. Differences were considered significant if P was equal to or less than 0.05.
RESULTS
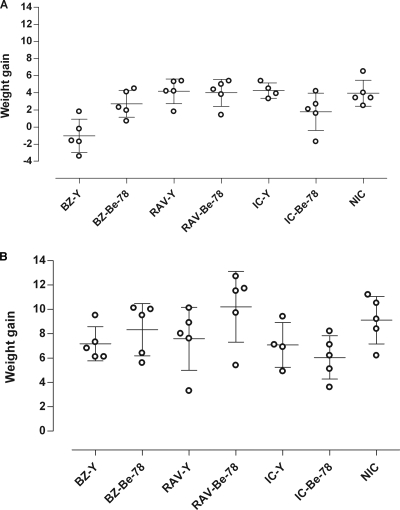
RAV and BZ oral treatment was started 12 to 22 days postinfection, immediately after the appearance of parasitemia, as detected by the examination of fresh blood. RAV was well tolerated by the dogs, and no side effects were observed during the treatment period. The data showed that 1 day after the end of the drug treatment, no statistically significant difference in weight gain (P < 0.05) was found among RAV-treated and nontreated animals (Fig. 1 A). In contrast, compared to the weight gain for the RAV-treated group, BZ led to a reduced weight gain in the Berenice-78-infected animals and a significant weight loss among animals infected with the Y strain (P < 0.01) (Fig. 1A). On the other hand, no weight differences were observed 6 months after the treatment (P > 0.05) between the two treatment groups (Fig. 1B). These data suggest that RAV has lower toxicity than BZ, the reference drug.
FIG. 1.
Weight gain (in kilograms) of animals infected with the Y and Berenice-78 Trypanosoma cruzi strains and treated with RAV or BZ compared with the weight gains of the infected untreated (infected control [IC]) and noninfected control (NIC) groups 1 day (A) and 180 days (B) after the end of treatment. The weight gain was calculated from the difference between the weights of the animals assessed on the first and the last days of treatment.
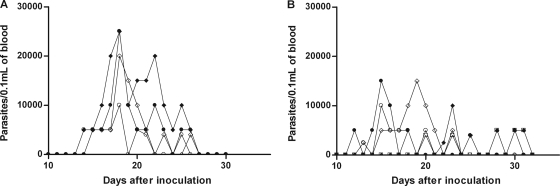
All infected animals showed prepatent periods of 12 to 22 days, having similar initial parasitemia levels (2,500 to 5,000 trypomastigotes/0.1 ml of blood) (Fig. 2). Both drugs were very effective in suppressing the proliferation of the Y and Be-78 T. cruzi strains, as the parasitemia was permanently suppressed after the first day posttreatment in all treated animals, with 100% survival in all cases. The control (untreated) animals inoculated with the Y strain showed the highest parasitemia levels, with peak values being 25,000 trypomastigotes/0.1 ml of blood and the patent period being 12 to 13 days (Fig. 2A), while Be-78 strain-infected animals had parasitemia level peaks of 15,000 trypomastigotes/0.1 ml of blood and a patent period of from 10 to 19 days (Fig. 2B).
FIG. 2.
Parasitemia curve for dogs inoculated with 2,000 blood trypomastigotes/kg of body weight of the Y (A) and Berenice-78 (B) T. cruzi strains and left untreated.
The potent suppression of parasitemia induced by RAV treatment was associated with negative results of blood culture performed 30 days after treatment in 80 and 100% of the animals infected with the Y and Berenice-78 strains, respectively (Table 1). The results of the PCR assay (Table 1) performed 1 month after treatment also showed a marked reduction of the parasite load: in animals inoculated with the Y or Berenice-78 strain and treated with RAV, three of five and two of five, respectively, had negative PCR results. An increase in the numbers of positive results by blood culture and PCR assays performed 6 months posttreatment was detected; this result was verified in 60% (three of five) and 80% (four of five) of the blood samples obtained from RAV-treated animals infected with Y and Be-78, respectively. For the BZ-treated animals, all blood culture and PCR tests were negative (Table 1), in agreement with the findings of previous studies (11, 13). In contrast, 100% of the infected and nontreated animals showed positive parasitological test results.
TABLE 1.
Rates of positive results of blood culture and PCR tests performed with the blood of dogs infected with the Y and Berenice-78 T. cruzi strains performed 30 days after RAV and BZ treatment
| T. cruzi strain | Drug | No. of dogs with positive result/total no. tested (%) |
|
|---|---|---|---|
| Blood culture | PCR | ||
| Y | RAV | 1/5 (20) | 2/5 (40) |
| BZ | 0/5 (0) | 0/5 (0) | |
| ICa | 4/4b (100) | 4/4b (100) | |
| Berenice-78 | RAV | 0/5 (0) | 3/5 (60) |
| BZ | 0/5 (0) | 0/5 (0) | |
| IC | 5/5 (100) | 5/5 (100) | |
IC, infected control.
The mortality rate was 20% (one of five dogs) during the acute phase of the infection for untreated animals.
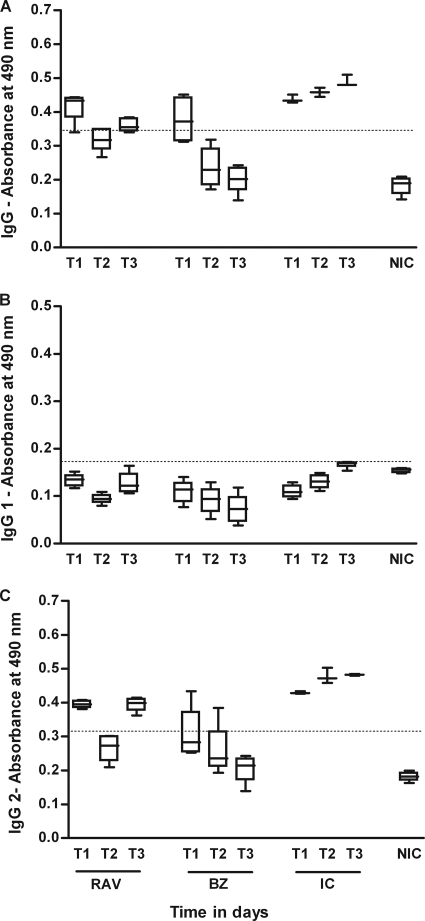
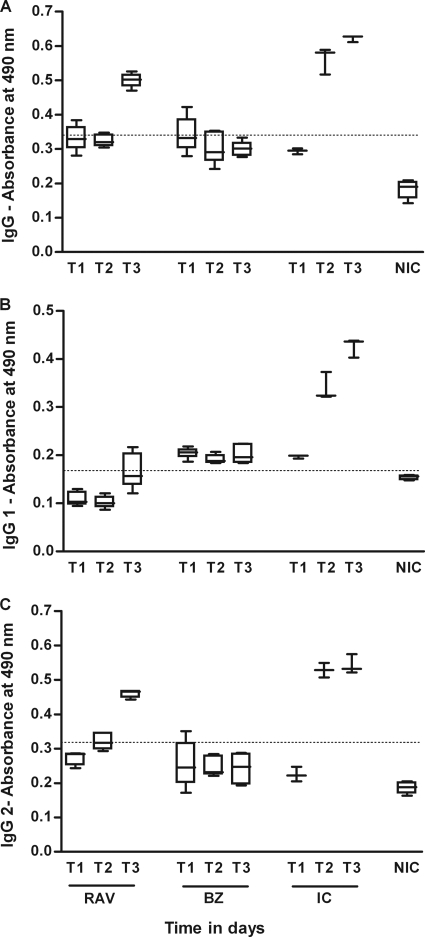
We evaluated the influence of the specific treatment with RAV and BZ on the humoral response kinetics during the acute and chronic phases of the disease. The results of the quantification of anti-T. cruzi IgG and isotypes IgG1 and IgG2 are shown in Fig. 3 and 4. The ELISA test revealed that all infected and nontreated dogs showed similar profiles for IgG and IgG2 antibodies (Fig. 3 and 4). The levels of this immunoglobulin increased until about the 90th day of infection and stabilized afterwards until the end of the experiment. Remarkably, the distinct production of IgG1 subclasses was observed, depending on the infecting strain: although IgG1 levels rose in dogs infected with the Berenice-78 strain, for those infected with the Y strain, the antibody levels were similar to those of the noninfected controls (Fig. 3 and 4). Specific treatment with RAV or BZ induced marked differences in the serological response compared with that of the nontreated control: all treated dogs showed negative serological test results during and until 30 days after treatment (data not shown), regardless of the therapeutic scheme used, confirming the potent suppressive effects of RAV on parasite proliferation. However, after the end of treatment an increase in the T. cruzi total IgG- and IgG2-specific antibody levels was observed in all RAV-treated animals, although the antibody levels were always significantly lower than those of the nontreated control dogs (P < 0.05) (Fig. 3 and 4). On the other hand, the antibody levels detected in serum samples from the BZ-treated animals were similar to the ones detected in the RAV-treated dogs (P > 0.05) during the treatment, but remained at those levels until the end of the observation period (6 months after treatment; Fig. 3 and 4).
FIG. 3.
IgG, IgG1, and IgG2 antibody levels in sera of dogs inoculated with 2,000 trypomastigotes per kg of body weight of the T. cruzi Y strain and treated with RAV at 12 mg/kg b.i.d. (q12h) for 90 days or BZ at 7 mg/kg b.i.d. (q12h) for 60 days compared with the levels in the infected and untreated (infected control [IC]) and noninfected control (NIC) groups. T1, 15 days after infection, before treatment; T2, 75 days of infection or 60 days of treatment; T3, 255 (BZ-treated animals) and 285 (RAV-treated animals) days after infection or 180 days posttreatment.
FIG. 4.
IgG, IgG1, and IgG2 antibody levels in the sera of dogs inoculated with 2,000 trypomastigotes per kg of body weight of the Berenice-78 T. cruzi strain and treated with RAV at 12 mg/kg b.i.d. (q12h) for 90 days or BZ at 7 mg/kg b.i.d. (q12h) for 60 days compared with the levels in the infected and untreated (infected control [IC]) and noninfected control (NIC) groups. T1, 15 days after infection or before treatment; T2, 75 days of infection or 60 days of treatment; T3, 255 (BZ-treated animals) and 285 (RAV-treated animals) days of infection or 180 days posttreatment. IgG cutoff, 0.338; IgG1 cutoff, 0.170; IgG2 cutoff, 0.315.
Taken together, the results of blood culture and PCR and the IgG antibody levels indicate that RAV treatment is efficient in reducing the parasitemia load in animals infected with T. cruzi but does not lead to a complete parasitological cure in any of the treated animals, while we confirmed our previous findings that BZ has curative activity in this dog model of acute Chagas' disease (11, 13).
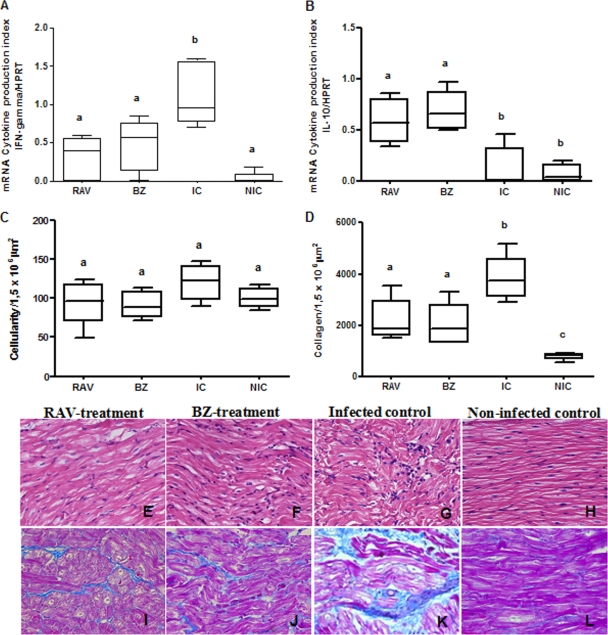
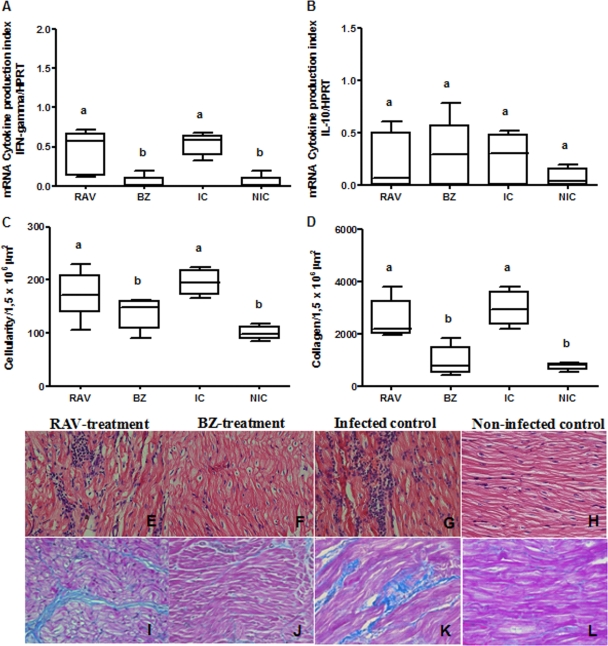
In order to evaluate the efficacy of early RAV treatment in preventing the development of chronic lesions in dogs infected with Y and Berenice-78 T. cruzi stocks, a quantitative analysis of the inflammation and fibrosis in the right atrium of the RAV- and BZ-treated, infected nontreated, and noninfected dogs was performed at the end of the observation period (6 months after the end of treatment). In animals infected with the Y strain, RAV treatment led to a reduction of about 20% of the inflammatory cells compared to the numbers in the nontreated animals (Fig. 5 C, E, and G). Additionally, the RAV treatment induced a significant (P < 0.05) reduction in fibrotic lesions (intrafascicular collagen deposition; Fig. 5D, I, and K) compared with that in nontreated animals. Similar results were observed for BZ (Fig. 5C, D, F, and J). On the other hand, in animals infected with EBI-resistant strain Berenice-78, RAV treatment was unable to reduce the intensity of the inflammatory and fibrotic cardiac lesions, while BZ was able to provide almost full protection (Fig. 6 C, D, E, F, I, and J). We also evaluated the IFN-γ and IL-10 cytokine mRNA expression in the cardiac tissues through semiquantitative reverse transcription-PCR (RT-PCR), in order to correlate cardiac alterations with cytokine profiles. In animals infected with the Y strain and treated with RAV or BZ, we detected a significant reduction in the level of IFN-γ mRNA expression and an increase in the level of IL-10 mRNA expression in relation to the levels in infected and nontreated dogs (P < 0.05) (Fig. 5A and B). Again, a different pattern was observed in RAV-treated dogs infected with the EBI-resistant Berenice-78 strain (Fig. 6A and B), as these animals had cardiac tissue IFN-γ and IL-10 cytokine mRNA expression levels indistinguishable from those of nontreated animals. In contrast, BZ-treated animals, which had significantly lower numbers of cardiac lesions, also presented significantly lower levels of IFN-γ mRNA expression in the cardiac tissue (P < 0.05). These data indicate that the early specific RAV treatment is effective in reducing the cardiac lesions as well as altering the pattern of the immune response in experimental Chagas' disease, but its success in preventing chronic cardiac alterations is T. cruzi strain dependent.
FIG. 5.
Effect of specific treatment on the intensity of lesions or cytokine expression in the heart tissue of dogs infected with Trypanosoma cruzi. Dogs were inoculated with 2,000 trypomastigotes per kg of body weight of the Y strain and treated with RAV at 12 mg/kg b.i.d. (q12h) for 90 days or BZ at 7 mg/kg b.i.d. (q12h) for 60 days; infected and untreated (infected control [IC]) and noninfected control (NIC) groups were also evaluated. IFN-γ (A) and IL-10 (B) mRNA expression in the right atrium was evaluated by semiquantitative reverse transcription-PCR 180 days posttreatment. Morphometric and histopatology analyses were performed with hematoxylin-eosin staining for inflammation quantification (C, E, F, G, and H) and Masson's trichrome staining for collagen quantification (D, I, J, K, and L). Magnifications, ×40. a, b, and c, different letters indicate a significant difference (P < 0.05).
FIG. 6.
Effect of specific treatment on the intensity of lesions or cytokine expression in the heart tissue of dogs infected with Trypanosoma cruzi. Dogs were inoculated with 2,000 trypomastigotes per kg of body weight of the Berenice-78 strain and treated with RAV at 12 mg/kg b.i.d. (q12h) for 90 days or BZ at 7 mg/kg b.i.d. (q12h) for 60 days; infected and untreated controls (infected control [IC]) and noninfected control (NIC) groups were also evaluated. IFN-γ (A) and IL-10 (B) mRNA expression in the right atrium was evaluated by semiquantitative reverse transcription-PCR 180 days posttreatment. Morphometric and histopatology analyses were performed with hematoxylin-eosin staining for inflammation quantification (C, E, F, G, and H) and Masson's trichrome staining for collagen quantification (D, I, J, K, and L). Magnifications, ×40. a and b, different letters indicate significant differences (P < 0.05).
DISCUSSION
Previous studies have demonstrated the potent in vitro anti-T. cruzi activity of RAV, a specific inhibitor of sterol C-14α-demethylase, although its in vivo action in murine models of acute and chronic Chagas' disease was limited (24). The MIC of this compound against intracellular amastigotes (1 nM) is 1,000 to 5,000 lower than the levels attainable in human plasma with multiple oral dosing, and its terminal half-life in humans is ≥120 h (1, 18).
In the present study, we used a canine model of infection with one EBI-sensitive strain (strain Y) and one EBI-resistant strain (strain Berenice-78) to establish the anti-T. cruzi efficacy of RAV in controlling the parasite load and preventing cardiac damage. We consider the dog to be an appropriate model for such an evaluation for two reasons: first, the results for experimentally infected dogs treated with BZ were similar to those observed for human patients in terms of therapeutic effectiveness and cure rates in both the acute and the chronic phases of the disease (14); second, RAV has a longer terminal half-life in this experimental animal (8.8 h) than in mice (4.5 h). Additionally, RAV displays a large volume of distribution (1, 18), which, together with its high level of intrinsic anti-T. cruzi activity (24), is of crucial importance for curative activity in vivo (27).
The benefit of the early RAV treatment was clearly demonstrated by the rapid and permanent suppression of the patent parasitemia, a fact confirmed by the results of the blood culture and PCR assays. The specific treatment also induced marked differences in the humoral immune response in relation to that for the nontreated controls, as all treated dogs had negative serological tests during and until 30 days after treatment, confirming the potent suppressive effects of RAV on parasite proliferation. However, after the end of treatment, the increase in the T. cruzi IgG- and IgG2-specific antibody levels observed in all RAV-treated animals clearly indicated a lack of parasitological cure. The surprising differences in the IgG1 responses to the two T. cruzi strains confirmed similar findings by Guedes et al. (13), who demonstrated that the levels of IgG1 antibodies in the sera of Y strain-infected animals untreated or treated with BZ were indistinguishable from those in the noninfected controls, suggesting that the IgG1 antibody titer might not be the appropriate marker of cure after specific treatment for T. cruzi infection (5).
Considering the hypothesis that the parasite triggers a chain of immune alterations, our results are consistent with a marked reduction in the parasite load during the treatment period. This idea is consistent with the marked reduction of the intensity of chronic cardiac lesions observed in animals infected with the EBI-sensitive Y strain and treated with either RAV or BZ (Fig. 5). Recent studies, using sensitive methodologies, have clearly associated the presence of parasites with the inflammatory processes that underlie the pathological processes associated with chronic Chagas' disease (17, 22), and this fact, coupled with the finding that T. cruzi-specific CD8+ and CD4+ T cells are consistently associated with inflammatory infiltrates rich in Th1 cytokines, has led to the notion that parasite persistence is a necessary and sufficient condition to generate and sustain a Th1-biased inflammatory response in infected tissues (8, 12). On the other hand, the specific RAV treatment was not able to significantly reduce the intensity of the heart inflammation and fibrotic area in animals inoculated with the EBI-resistant Berenice-78 strain. These results can be explained by the known resistance of the Berenice-78 strain to azole drugs, previously reported by Guedes et al. as a result of a study with albaconazole (11). In any case, these findings indicate that an effective antiparasitic treatment in the early stages of Chagas' disease can lead to a profound reduction in the frequency and severity of the parasite-induced chronic lesions, even if the parasite load is not completely eliminated.
The next step of the study was devoted to evaluation of the influence of the specific treatment on the pattern of cytokine expression in the heart tissue. We observed that the IFN-γ mRNA and IL-10 mRNA levels in the right atrium of the RAV- and BZ-treated dogs infected with the Y and Berenice-78 strain were related to cardiac damage. In the Y-strain-infected RAV- or BZ-treated animals, a reduction of heart lesions was associated with low IFN-γ mRNA and high IL-10 mRNA levels, while high IFN-γ mRNA and low IL-10 mRNA levels were detected in the heart tissue of the infected and nontreated animals. A similar correlation was observed in animals infected with the EBI-resistant Be-78 strain. These findings support a link between the effectiveness of the specific treatment in preventing the cardiac chronic lesions and the quality of the immune response, and it is possible that a fine balance of pro- and anti-inflammatory cytokines could be the major key in controlling morbidity from Chagas' disease following treatment. Additionally, it has been demonstrated that BZ has a selective impact on the host immune response, as demonstrated by its ability to deregulate cytokine and nitric oxide synthesis (21). Our results show that the efficacy of RAV treatment in preventing cardiac lesions is related to early modifications in the humoral and cellular immune responses.
In the present study, no RAV blood levels were measured, but data on file from Eisai Co. Ltd. indicate that the maximum concentration of drug in the plasma of beagle dogs dosed with 10 mg/kg is ca. 1 μg/ml, which is several orders of magnitude higher than the MIC against the intracellular amastigote forms (24). Thus, the lack of curative effects of RAV in the dog model, despite its potent intrinsic anti-T. cruzi activity, is most probably due to the relatively short (8.8-h) terminal half-life of the compound, as was arguably the reason for the similar results obtained with murine models (24). However, the significantly longer half-life of RAV in humans (4 to 8 days; Eisai Co. Ltd., data on file) offers a promising opportunity to assess the drug as chemotherapy for human Chagas' disease. Based on these considerations, the Drugs for Neglected Diseases Initiative (DNDi; Geneva, Switzerland) has recently announced an agreement with the Eisai Co. for the clinical development of E1224, a prodrug of RAV, for the specific treatment of chronic human Chagas' disease (http://www.dndi.org/press-releases/532-eisai-and-dndi-enter-into-a-collaboration.html and http://www.dndi.org/press-releases/673-new-agreement-to-tackle-chagas-disease.html).
Acknowledgments
This work received financial support from DNDi; Rede Mineira de Bioterismo (FAPEMIG), Universidade Federal de Ouro Preto; and research fellowships from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (to M. T. Bahia).
We are grateful to Robert Don, DNDi's senior project manager, for encouragement and excellent assistance. We thank Hideki Yoshitomi, Eisai Co. Ltd., for the generous provision of ravuconazole for this study.
Footnotes
Published ahead of print on 19 April 2010.
REFERENCES
- 1.Andes, D., K. Marchillo, T. Stamstad, and R. Conklin. 2003. In vivo pharmacodynamics of a new triazole, ravuconazole, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:1193-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braga, M. S., L. Lauria-Pires, E. R. Argañaraz, R. J. Nascimento, and A. R. L. Teixeira. 2000. Persistent infections in chronic Chagas' disease patients treated with anti-Trypanosoma cruzi nitroderivatives. Rev. Inst. Med. Trop. Sao Paulo 42:157-161. [DOI] [PubMed] [Google Scholar]
- 3.Britto, C., M. A. Cardoso, P. Wincker, and C. M. Morel. 1993. A simple protocol for the physical cleavage of Trypanosoma cruzi kinetoplast DNA present in blood samples and its use in polymerase chain reaction (PCR)-based diagnosis of chronic Chagas disease. Mem. Inst. Oswaldo Cruz 88:171-172. [DOI] [PubMed] [Google Scholar]
- 4.Buckner, F. S., J. H. Griffin, A. J. Wilson, and W. C. Van Voorhis. 2001. Potent anti-Trypanosoma cruzi activities of oxidosqualene cyclase inhibitors. Antimicrob. Agents Chemother. 45:1210-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caldas, I. S., A. Talvani, S. Caldas, C. M. Carneiro, M. Lana, P. M. Guedes, and M. T. Bahia. 2008. Benznidazole therapy during acute phase of Chagas disease reduces parasite load but does not prevent chronic cardiac lesions. Parasitol. Res. 103:413-421. [DOI] [PubMed] [Google Scholar]
- 6.Cançado, J. R. 2002. Long term evaluation of etiological treatment of Chagas disease with benznidazole. Rev. Inst. Med. Trop. Sao Paulo 44:29-37. [PubMed] [Google Scholar]
- 7.Chiari, E., J. C. Dias, M. Lana, and C. A. Chiari. 1989. Hemocultures for the parasitological diagnosis of human chronic Chagas' disease. Rev. Soc. Bras. Med. Trop. 22:19-23. [DOI] [PubMed] [Google Scholar]
- 8.Cunha-Neto, E., P. C. Teixeira, L. G. Nogueira, C. Mady, B. Lanni, N. Stolf, A. Fiorelli, R. Honorato, and J. Kalil. 2007. New concepts on the pathogenesis of chronic Chagas cardiomyopathy: myocardial gene and protein expression profiles. Rev. Soc. Bras. Med. Trop. 39:59-62. [PubMed] [Google Scholar]
- 9.Dias, J. C. 2007. Globalization, inequity and Chagas disease. Cad. Saude Publica 23:13-22. [DOI] [PubMed] [Google Scholar]
- 10.Gomes, M. L., A. M. Macedo, A. R. Vago, S. D. Pena, L. M. Galvão, and E. Chiari. 1998. Trypanosoma cruzi: optimization of polymerase chain reaction for detection in human blood. Exp. Parasitol. 88:28-33. [DOI] [PubMed] [Google Scholar]
- 11.Guedes, P. M., J. A. Urbina, M. Lana, L. C. C. Afonso, V. M. Veloso, W. L. Tafuri, G. L. Machado-Coelho, E. Chiari, and M. T. Bahia. 2004. Activity of the new triazole derivative albaconazole against Trypanosoma (Schizotrypanum) cruzi in dog hosts. Antimicrob. Agents Chemother. 48:4286-4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guedes, P. M., V. M. Veloso, L. C. C. Afonso, M. V. Caliari, C. M. Carneiro, L. F. Diniz, E. A. Marques-da-Silva, I. S. Caldas, M. A. Do Valle Matta, S. M. Souza, M. Lana, E. Chiari, L. M. Galvão, and M. T. Bahia. 2009. Development of chronic cardiomyopathy in canine Chagas disease correlates with high IFN-gamma, TNF-alpha, and low IL-10 production during the acute infection phase. Vet. Immunol. Immunopathol. 130:43-52. [DOI] [PubMed] [Google Scholar]
- 13.Guedes, P. M., V. M. Veloso, J. K. Gollob, L. C. C. Afonso, I. S. Caldas, P. Vianna, M. Lana, E. Chiari, M. Bahia, and L. M. C. Galvão. 2008. IgG isotype profile is correlated with cardiomegaly in beagle dogs infected with distinct Trypanosoma cruzi strains. Vet. Immunol. Immunopathol. 124:163-168. [DOI] [PubMed] [Google Scholar]
- 14.Guedes, P. M., V. M. Veloso, W. L. Tafuri, L. M. Galvão, C. M. Carneiro, M. Lana, E. Chiari, K. Ataide Soares, and M. T. Bahia. 2002. The dog as model for chemotherapy of the Chagas' disease. Acta Trop. 84:9-17. [DOI] [PubMed] [Google Scholar]
- 15.Lauria-Pires, L., M. S. Braga, A. C. Vexenat, N. Nitz, A. Simões-Barbosa, D. L. Tinoco, and A. R. L. Teixeira. 2000. Progressive chronic Chagas heart disease ten years after treatment with anti-Trypanosoma cruzi nitroderivatives. Am. J. Trop. Med. Hyg. 63:111-118. [DOI] [PubMed] [Google Scholar]
- 16.Maltos, K. L., G. B. Menezes, M. V. Caliari, O. A. Rocha, J. M. Santos, D. L. Alves, I. D. Duarte, and J. N. Francischi. 2004. Vascular and cellular responses to pro-inflammatory stimuli in rat dental pulp. Arch. Oral Biol. 49:443-450. [DOI] [PubMed] [Google Scholar]
- 17.Marin-Neto, J. A., E. Cunha-Neto, B. C. Maciel, and M. V. Simões. 2007. Pathogenesis of chronic Chagas heart disease. Circulation 115:1109-1123. [DOI] [PubMed] [Google Scholar]
- 18.Mikamo, H., X. H. Yin, Y. Hayasaki, Y. Shimamura, K. Uesugi, N. Fukayama, M. Satoh, and T. Tamaya. 2002. Penetration of ravuconazole, a new triazole antifungal, into rat tissues. Chemotherapy 48:7-9. [DOI] [PubMed] [Google Scholar]
- 19.Molina, J. T., Z. Brener, J. A. Urbina, and A. J. Romanha. 2000. Activity of TAK-187 triazole on mice infected with Trypanosoma cruzi strains differently susceptible to benznidazole. Mem. Inst. Oswaldo Cruz 95:304-308. [Google Scholar]
- 20.Moncayo, A. 2003. Chagas disease: current epidemiological trends after the interruption of vectorial and transfusional transmission in the Southern Cone countries. Mem. Inst. Oswaldo Cruz 98:577-591. [DOI] [PubMed] [Google Scholar]
- 21.Pascuti, M. F., M. Pitashny, A. L. Nocito, P. Guermonprez, S. Amigorena, J. Wietzerbin, E. Serra, O. Bottasso, and S. Revelli. 2004. Benznidazole, a drug used in Chagas' disease, ameliorates LPS-induced inflammatory response in mice. Life Sci. 76:685-697. [DOI] [PubMed] [Google Scholar]
- 22.Tarleton, R. L. 2001. Parasite persistence in the aetiology of Chagas disease. Int. J. Parasitol. 31:550-554. [DOI] [PubMed] [Google Scholar]
- 23.Urbina, J. A. 2009. Ergosterol biosynthesis and drug development for Chagas disease. Mem. Inst. Oswaldo Cruz 104:311-318. [DOI] [PubMed] [Google Scholar]
- 24.Urbina, J. A., G. Payares, C. Sanoja, J. Molina, R. Lira, Z. Brener, and A. J. Romanha. 2003. In vitro and in vivo activities of ravuconazole on Trypanosoma cruzi, the causative agent of Chagas disease. Int. J. Antimicrob. Agents 21:27-38. [DOI] [PubMed] [Google Scholar]
- 25.Urbina, J. A., J. L. Concepcion, A. Caldera, G. Payares, C. Sanoja, T. Otomo, and H. Hiyoshi. 2004. In vitro and in vivo activities of E5700 and ER-119884, two novel orally active squalene synthase inhibitors, against Trypanosoma cruzi. Antimicrob. Agents Chemother. 48:2379-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urbina, J. A., K. Lazardi, E. Marchan, G. Visbal, T. Aguirre, M. M. Piras, R. Piras, R. A. Maldonado, G. Payares, and W. de Souza. 1993. Mevinolin (lovastatin) potentiates the antiproliferative effects of ketoconazole and terbinafine against Trypanosoma (Schizotripanum) cruzi: in vitro and in vivo studies. Antimicrob. Agents Chemother. 37:580-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urbina, J. A., and R. Docampo. 2003. Specific chemotherapy of Chagas disease: controversies and advances. Trends Parasitol. 19:495-501. [DOI] [PubMed] [Google Scholar]
- 28.Viotti, R., C. Vigliano, H. Armenti, and E. Segura. 1994. Treatment of chronic Chagas disease with benznidazole: clinical and serological evolution of patients with long-term follow-up. Am. Heart J. 127:151-162. [DOI] [PubMed] [Google Scholar]
- 29.Voller, A., D. E. Bidwell, and A. Bartlett. 1976. Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull. World Health Organ. 53:55-65. [PMC free article] [PubMed] [Google Scholar]
- 30.WHO. 2007. Report of Scientific Group in Chagas Disease. Update July 2007. Special Programme for Research and Training in Tropical Diseases, WHO, Geneva, Switzerland.