Abstract
We evaluated the pharmacokinetics of lopinavir-ritonavir with and without nonnucleoside reverse transcriptase inhibitors (NNRTIs) in Ugandan adults. The study design was a three-period crossover study (3 tablets [600 mg of lopinavir/150 mg of ritonavir {600/150 mg}], 4 capsules [533/133 mg], and 2 tablets [400/100 mg] twice a day [BD]; n = 40) of lopinavir-ritonavir with NNRTIs and a parallel one-period study (2 tablets BD; n = 20) without NNRTIs. Six-point pharmacokinetic sampling (0, 2, 4, 6, 8, and 12 h) was undertaken after observed intake with a standardized breakfast. Ugandan DART trial participants receiving efavirenz (n = 20), nevirapine (n = 18), and no NNRTI (n = 20) had median ages of 41, 35, and 37 years, respectively, and median weights of 60, 64, and 63 kg, respectively. For the no-NNRTI group, the geometric mean (percent coefficient of variation [%CV]) lopinavir area under the concentration-time curve from 0 to 12 h (AUC0-12) was 110.1 (34%) μg·h/liter. For efavirenz, the geometric mean lopinavir AUC0-12 (%CV) values were 91.8 μg·h/liter (58%), 65.7 μg·h/liter (39%), and 54.0 μg·h/liter (65%) with 3 tablets, 4 capsules, and 2 tablets BD, respectively, with corresponding (within-individual) geometric mean ratios (GMR) for 3 and 2 tablets versus 4 capsules of 1.40 (90% confidence interval [CI], 1.18 to 1.65; P = 0.002) and 0.82 (90% CI, 0.68 to 0.99; P = 0.09), respectively, and the apparent oral clearance (CL/F) values were reduced by 58% and 1%, respectively. For nevirapine, the geometric mean lopinavir AUC0-12 (%CV) values were 112.9 μg·h/liter (30%), 68.1 μg·h/liter (53%), and 61.5 μg·h/liter (52%), respectively, with corresponding GMR values of 1.66 (90% CI, 1.46 to 1.88; P < 0.001) and 0.90 (90% CI, 0.77 to 1.06; P = 0.27), respectively, and the CL/F was reduced by 57% and 7%, respectively. Higher values for the lopinavir concentration at 12 h (C12) were observed with 3 tablets and efavirenz-nevirapine (P = 0.04 and P = 0.0005, respectively), and marginally lower C12 values were observed with 2 tablets and efavirenz-nevirapine (P = 0.08 and P = 0.26, respectively). These data suggest that 2 tablets of lopinavir-ritonavir BD may be inadequate when dosed with NNRTIs in Ugandan adults, and the dosage should be increased by the addition of an additional adult tablet or a half-dose tablet (100/25 mg), where available.
When efavirenz (a CYP450 inducer) and lopinavir-ritonavir (metabolized by CYP450) are coadministered, a decrease in lopinavir plasma concentrations has been observed (8). In theory, this could lead to subtherapeutic lopinavir concentrations, the development of virological failure, and, potentially, the emergence of resistance mutations, particularly in the absence of viral load monitoring. Therefore, an increase from the standard dose of 3 capsules (400 mg of lopinavir/100 mg of ritonavir [400/100 mg]) to 4 capsules (533/133 mg) twice daily was recommended during the coadministration of lopinavir-ritonavir capsules (Kaletra) with nonnucleoside reverse transcriptase inhibitors (NNRTIs) in HIV-infected patients.
A solid formulation of lopinavir-ritonavir tablets (Aluvia tablets; based on Meltrex technology) is now approved for the treatment of HIV infection. Each tablet contains 200 mg/50 mg lopinavir-ritonavir, so the standard dose is 2 tablets (rather than 3 capsules) twice daily (BD). The tablets are preferred to the capsules because of heat stability, a lack of a food effect, and lower pill burden.
However, there are few data on the pharmacokinetic (PK) interactions between NNRTIs and lopinavir-ritonavir tablets, particularly in African populations, and the previously recommended 533/133-mg twice-daily capsule dose cannot be achieved with 200/50-mg tablets. In the United States, original recommendations for lopinavir-ritonavir tablets with NNRTIs were 400/100 mg twice daily, but a dose of 600/150 mg twice daily was to be considered if decreased lopinavir susceptibility was suspected. In Europe, a dose of 600/150 mg twice daily with close monitoring was recommended. These recommendations were based on two healthy-volunteer studies that found that the administration of lopinavir-ritonavir tablets at 400/100 mg twice daily with efavirenz led to decreases in the lopinavir area under the concentration-time curve (AUC) by 20% and a decrease in the trough concentration (Ctrough) by 27% but suggested that the increased lopinavir bioavailability with the tablet may compensate for this effect (9). In contrast, administration of lopinavir-ritonavir tablets at 600/150 mg twice daily with efavirenz led to 36% increases in lopinavir AUC and Ctrough values (11) compared to a dose of 400/100 mg twice daily without efavirenz. A half-dose (100/25-mg) lopinavir-ritonavir tablet is now licensed, and late in 2008, both U.S. and European recommendations changed to a dose of 500/125 mg (two 200/50-mg tablets plus one 100/25-mg half-dose tablet) twice daily based on a third healthy volunteer study, which found exposures with this dosing scheme and efavirenz similar to those for 400/100 mg twice daily without efavirenz (15).
Lopinavir-ritonavir is the main protease inhibitor used for second-line therapy in resource-limited settings, where the vast majority of patients receive antiretroviral therapy (ART) under the public health approach to ART (7) without routine viral load monitoring and where half-dose lopinavir-ritonavir tablets may not always be available. Concerns about low plasma lopinavir concentrations with 2 tablets twice daily and NNRTIs are therefore particularly important in these settings. However, increasing the dose to 3 tablets twice a day may also lead to long-term toxicity, and there is contradictory evidence on whether low trough levels are associated with virological failure (1, 2). Therefore, early in 2008 (before the change in dosing recommendations), we performed a three-period crossover study of Ugandan patients receiving lopinavir-ritonavir with NNRTIs and a parallel one-period pharmacokinetic study of those receiving lopinavir-ritonavir without NNRTIs within the Development of Anti-Retroviral Therapy in Africa (DART) trial.
MATERIALS AND METHODS
The DART trial was a randomized controlled trial evaluating ART management strategies for 3,316 symptomatic, previously untreated HIV-infected adults with CD4 cell counts of <200 cells/mm3 in Uganda and Zimbabwe (3). The main comparison was clinically driven monitoring (CDM) versus laboratory and clinical monitoring (LCM). All participants initiated triple-drug ART with coformulated zidovudine-lamivudine (Combivir) plus tenofovir, nevirapine, or abacavir. All participants switching to second-line therapy following clinical or immunological failure (in the LCM group only) received lopinavir-ritonavir (Kaletra capsules or Aluvia tablets) supported by an NNRTI, if this was not in the failing regimen, and further nucleoside reverse transcriptase inhibitors (NRTI). The DART trial received ethics approval in Uganda, Zimbabwe, and the United Kingdom, and all participants gave informed consent.
After the introduction of lopinavir-ritonavir tablets in Uganda and Zimbabwe, additional ethics approval was obtained for a pharmacokinetic (PK) study with two parts in Ugandan DART centers, including the Joint Clinical Research Centre, Kampala, Uganda; its satellite at the Infectious Diseases Institute, Mulago Hospital; and the MRC/UVRI Uganda Research Unit on AIDS (second part only). The first part was a three-period crossover study recruiting 20 participants receiving efavirenz and 20 participants receiving nevirapine with lopinavir-ritonavir as a second-line therapy (following three first-line NRTIs). As all participants on NNRTIs and lopinavir-ritonavir at these clinics were already receiving 3 tablets of lopinavir-ritonavir twice a day when the PK study started, the order was not randomized. A full six-point PK curve was generated at enrollment, when participants were receiving 3 lopinavir-ritonavir tablets BD plus NNRTI (with or without an additional NRTI[s]), and then after 2 weeks of 4 lopinavir-ritonavir capsules BD and 2 weeks of 2 lopinavir-ritonavir tablets BD. Venous blood (5 ml) was sampled 0, 2, 4, 6, 8, and 12 h after observed intake with a standardized meal of two fried samosas (moderate-fat meal) and tea after the blood drawing at time zero. The sampling schedule was based on a pragmatic design previously utilized for studying lopinavir-NNRTI interactions in patients from the United Kingdom (5). The second part was a single PK study recruiting 20 participants from the same Ugandan DART centers who received 2 lopinavir-ritonavir tablets BD without NNRTIs (also as a second-line therapy following first-line treatment with 2 NRTIs and an NNRTI) and who underwent a single full six-point PK curve as described above. Participants for this second part of the study were also recruited from the DART center in Entebbe, Uganda. To more closely reflect real-life conditions, participants were prescribed lopinavir-ritonavir tablets and lopinavir-ritonavir capsules, which they stored at home and brought to the clinic on each day of PK testing. However, each participant was visited at home to confirm that capsules were stored at the appropriate temperature, using either a refrigerator or clay pots. All participants were checked for the absence of gastrointestinal disease before each day of PK testing, and none were taking rifampin concurrently. Plasma was stored at −20°C before shipment to Liverpool, United Kingdom, for analysis. Details of age, gender, and body weight were obtained from the DART database. HIV-1 RNA was not routinely assessed within the DART trial. All participants provided additional informed consent for participation in the PK substudy.
Lopinavir (and ritonavir) concentrations were determined by a validated high-performance liquid chromatography (HPLC)-tandem mass spectrometry method as described previously (4). Briefly, an internal standard (20 μl [500 ng/ml]) (catalog no. Ro31-9564; Roche Discovery, Welwyn, United Kingdom) and acetonitrile (400 μl; VWR Laboratory Supplies, Poole, United Kingdom) were added to aliquots (100 μl) of calibrators, quality controls (QCs), and patient plasma. After mixing, centrifugation, and the addition of ammonium formate buffer (100 μl [20 mM]; Fisher Scientific, Loughborough, United Kingdom), samples were analyzed by HPLC-tandem mass spectrometry (Waters Quattro Premier XE; Waters Corporation, MA) via an Acuity UPLC bridged ethyl hybrid C18 column (Waters Corporation, MA). The interassay variation was between 6.9% and 8.4% for low, medium, and high QCs. Intra-assay variabilities were 5.7%, 4.7%, and 2.3%, with accuracies of 100%, 99.9%, and 98.5% for low, medium, and high QCs, respectively. The limits of quantitation of the plasma lopinavir and ritonavir assays are 103 and 26 ng/ml, respectively. The Liverpool laboratory participates in an external quality assurance program (KKGT, Netherlands).
Noncompartmental analysis.
Standard analyses were done by using Stata 10 software. For each day of PK monitoring and each drug (lopinavir and ritonavir), the area under the concentration-time curve over 0 to 12 h (AUC0-12) using the trapezoidal rule with scheduled time points, maximum concentration of drug in plasma (Cmax), time to maximum concentration of drug in plasma (Tmax), and lopinavir concentration at 12 h (C12) (trough) were calculated. Values under the lower limit of quantitation (LLQ) (103 and 26 ng/ml for lopinavir and ritonavir, respectively) were imputed as LLQ-1 in the AUC0-12 calculation. Separate analyses were conducted for patients taking nevirapine, efavirenz, and no NNRTIs. Geometric mean ratios (GMRs) and their 90% confidence intervals for lopinavir and ritonavir AUC0-12, Cmax, and C12 comparing data for 2 lopinavir-ritonavir tablets BD and 3 lopinavir-ritonavir tablets BD with 4 lopinavir-ritonavir capsules BD were generated by using a paired t test on log transformations and then back-transforming values to the normal scale. Unpaired t tests were used to compare NNRTI and no-NNRTI groups. The impact of age, gender, hemoglobin, and body weight was assessed in linear-mixed regression models for AUC0-12, Cmax, and C12, including patient-level random effects.
Population PK modeling of lopinavir and ritonavir.
Nonlinear mixed-effects modeling was performed by using NONMEM (version VI 2.0, level 1.1, double precision; ICON Development Solutions, Ellicott City, MD) with first-order conditional estimation with interaction (FOCE-I). Model fit was assessed by statistical and graphical methods. As the “no-NNRTI” control group contained different patients from each of the NNRTI groups, we developed separate models for lopinavir and ritonavir in the presence of nevirapine and efavirenz and made a primary comparison between 2 and 3 tablets versus 4 capsules BD, which was the standard of care prior to the change of the formulation. The main pharmacokinetic parameter of interest was the apparent oral clearance (CL/F).
Samples lower than the LLQ (<LLQ) of the drug assays were included in the analysis as LLQ/2 (51.5 ng/ml and 13 ng/ml for lopinavir and ritonavir, respectively). A one-compartment model with first-order absorption best described both lopinavir and ritonavir data, parameterized by apparent oral clearance (CL/F), volume of distribution (V/F), and absorption rate constant (ka). Interoccasion variability on CL/F significantly improved the fit; interindividual variability on CL/F was removed, as it was negligible (∼10−6). For lopinavir models, the ka was fixed to 1.22 h−1 (10). No parameters were fixed for the model for ritonavir plus efavirenz, whereas V/F was fixed to 42.1 liters (the value obtained for the ritonavir-efavirenz model) due to estimation issues for the ritonavir-plus-nevirapine model. Interoccasion variability was described by an exponential model, and residual error was described by a proportional model for lopinavir and a combined proportional-additive model for ritonavir. The ritonavir AUC0-12 was significantly associated with the lopinavir CL/F, and the lopinavir AUC0-12 was significantly associated with the ritonavir CL/F; these associations were described by power relationships. The inclusion of a factor for 3 or 2 tablets versus 4 capsules BD significantly improved the fit.
For each model, the association between CL/F and other model parameters was described by the following equations: CL/Fij = θ1·(RTVij/cons)θ2·θ3I(3 tablets BD)·θ4I(2 tablets BD)·exp(κij) (cons = 3.16 for efavirenz and 2.84 for nevirapine [median ritonavir AUC0-12 in each group]) for lopinavir and CL/Fij = θ1·(LPVij/cons)θ2·θ3I(3 tablets BD)·θ4I(2 tablets BD)·exp(κij) (cons = 71.92 for efavirenz and 79.78 for nevirapine [median lopinavir AUC0-12 in each group]) for ritonavir, where CL/Fij is the CL/F of the ith individual on the jth occasion, θ1 is the population parameter estimate, θ2 is the factor associated with the ritonavir (RTV) AUC0-12 on the lopinavir (LPV) CL/F and the lopinavir AUC0-12 on the ritonavir CL/F, RTVij and LPVij are the AUC0-12 of ritonavir and lopinavir, respectively, for the ith individual on the jth occasion (constants normalize these AUC0-12 values [as described above]), θ3 is the relative change in CL/F for 3 tablets versus 4 capsules BD, θ4 is the relative change in CL/F for 2 tablets versus 4 capsules BD, and κij is the interoccasion variability (mean of zero; variance, π2). Residual error was described as follows: Y = F × (1 + ɛ1) for lopinavir and Y = F × (1 + ɛ1) + ɛ2 for ritonavir, where Y is the observed concentration, F is the predicted concentration, and ɛ1 and ɛ2 are the proportional and additive random effects, which are assumed to have a mean of zero and variances of σ12 and σ22, respectively.
Ninety-five percent prediction intervals (P2.5 to P97.5) were constructed from 1,000 simulated patients using the fixed and random effects of the final models. A total of 93% and 94% of the observed concentrations were within the prediction intervals for the efavirenz and nevirapine models, respectively.
RESULTS
In total, 60 participants taking lopinavir-ritonavir with efavirenz (n = 21), nevirapine (n = 19), or no NNRTIs (n = 20) were recruited. However, two participants (one taking efavirenz and one taking nevirapine) had very low lopinavir (4/6 PK samples <LLQ) and ritonavir (6/6 PK samples <LLQ) measurements on the day of PK evaluation with 4 lopinavir-ritonavir capsules BD and were therefore excluded from further analyses because the group receiving 4 lopinavir-ritonavir capsules BD was the reference group for comparing bioequivalence and also due to suspected noncompliance despite reported observed intake. However, both excluded participants had not reported missed pills according to nurse pill counts and the self-reported adherence questionnaire and had pharmacokinetic parameters with 2 and 3 tablets BD that were within (but toward the lower end of) the ranges observed for other participants. Only 3 (0.4%) of the remaining 682 lopinavir plasma measurements were <LLQ (103 ng/ml), with two being 12-h values for tablets for one patient and the other being an initial value (t = 0) for 2 tablets BD. A total of 8/682 (1.2%) plasma ritonavir measurements were <LLQ (26 ng/ml) (6 at 12 h and 2 at 0 h). Enrollment characteristics are shown in Table 1. A total of 6/20 participants (30%) taking efavirenz, versus 13/19 participants (72%) taking nevirapine and 14/20 participants (70%) with no NNRTI, were women, reflecting issues with the use of efavirenz in women who may become pregnant.
TABLE 1.
Patient characteristics at enrollment
| Parameter | Value for group |
||
|---|---|---|---|
| Efavirenz | Nevirapine | No NNRTIs | |
| No. of patients in enrolled in PK substudy | 21 | 19 | 20 |
| No. of patients included in analysis (%)a | 20 (100) | 18 (100) | 20 (100) |
| No. of patients at center (%) | |||
| Joint Clinical Research Centre, Uganda | 17 (85) | 10 (56) | 15 (75) |
| Academic Alliance, Uganda | 3 (15) | 8 (44) | |
| Entebbe, Uganda | 5 (25) | ||
| No. of male patients (%) | 14 (70) | 5 (28) | 6 (30) |
| No. of female patients (%) | 6 (30) | 13 (72) | 14 (70) |
| Median age (yr) (range) | 41 (32-52) | 35 (27-63) | 37 (28-68) |
| Median wt (range) | 59.8 (49-75) | 63.6 (52-85) | 63.3 (48-111) |
| Median BMI (kg/m2) (range) | 23.2 (17.1-26.7) | 24.8 (20.0-33.2) | 24.3 (19.2-37.1) |
| Median hemoglobin level (g/dl) (range) | 13.9 (11.2-16.5) | 13.6 (8.7-16.8) | 13.5 (7.9-15.7) |
| No. of patients taking other NRTIs (%) | |||
| None | 1 (5) | ||
| Lamivudine | 1 (5) | ||
| Abacavir | 4 (20) | 2 (11) | |
| Didanosine | 14 (70) | 14 (78) | |
| Tenofovir | 1 (6) | ||
| Lamivudine + tenofovir | 1 (6) | ||
| Lamivudine + zidovudine | 2 (10) | ||
| Lamivudine + abacavir | 8 (40) | ||
| Zidovudine + abacavir | 1 (5) | ||
| Didanosine + abacavir | 9 (45) | ||
Excluding two patients with low levels of lopinavir and ritonavir receiving 4 capsules of lopinavir-ritonavir BD.
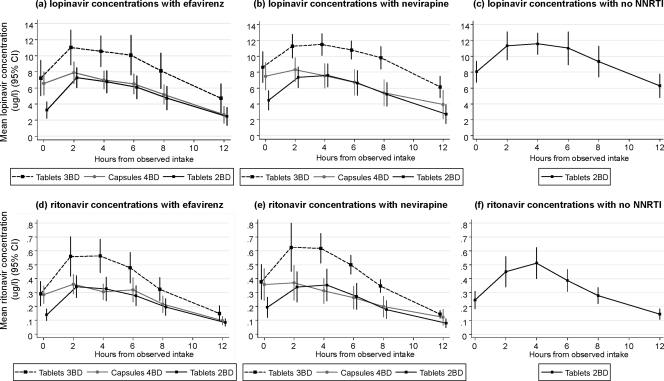
Mean plasma concentrations of lopinavir and ritonavir are shown over time for those receiving concurrent efavirenz, nevirapine, or no NNRTIs in Fig. 1. There was little difference between the two NNRTIs efavirenz and nevirapine in summary PK parameters for lopinavir (Table 2) and ritonavir (Table 3), and we found no independent effect of sex, age, weight, hemoglobin level, body mass index (BMI), or body surface area (BSA) on pharmacokinetic parameters. For AUC0-12, Cmax, and C12, standard deviations were similar across tablet and capsule formulations, being somewhat higher only for participants taking efavirenz with 3 lopinavir-ritonavir tablets BD. In particular, there was no suggestion of higher standard deviations with 4 capsules of lopinavir-ritonavir BD compared to the tablet formulations (12).
FIG. 1.
Mean plasma concentrations (95% CI) of lopinavir and ritonavir over time.
TABLE 2.
Plasma lopinavir pharmacokinetic parametersb
| Parameter | Value for group |
||||||
|---|---|---|---|---|---|---|---|
| Efavirenz (n = 20) + lopinavir-ritonavir dose of: |
Nevirapine (n = 18) + lopinavir-ritonavir dose of: |
No NNRTIs (n = 20) + 2 tablets of lopinavir-ritonavir (400/100 mg) BD | |||||
| 3 tablets (600/150 mg) BD | 4 capsules (533/133 mg) BD | 2 tablets (400/100 mg) BD | 3 tablets (600/150 mg) BD | 4 capsules (533/133 mg) BD | 2 tablets (400/100 mg) BD | ||
| Geometric mean AUC0-12 (μg·h/ml) (%CV) [range] | 91.8 (58) [23.1-263.6] | 65.7 (39) [27.1-150.6] | 54.0 (65) [11.9-166.5] | 112.9 (30) [66.9-165.8] | 68.1 (53) [30.6-166.5] | 61.5 (52) [26.9-157.9] | 110.1 (34) [65.3-226.1] |
| No. of patients (%) with AUC0-12 (μg·h/ml) ofa: | |||||||
| <67.5 | 4 (20) | 11 (55) | 13 (65) | 1 (6) | 10 (56) | 12 (67) | 1 (5) |
| 67.5-90 | 5 (25) | 6 (30) | 2 (10) | 4 (22) | 2 (11) | 2 (11) | 5 (25) |
| 90-121.5 | 5 (25) | 2 (10) | 4 (20) | 4 (22) | 4 (22) | 2 (11) | 7 (35) |
| >121.5 | 6 (30) | 1 (5) | 1 (5) | 9 (50) | 2 (11) | 2 (11) | 7 (35) |
| Geometric mean Cmax (μg/ml) (%CV) [range] | 11.1 (46) [4.5-27.0] | 8.3 (37) [4.1-16.6] | 7.5 (43) [2.2-13.6] | 12.2 (23) [8.1-17.5] | 8.4 (43) [4.5-15.5] | 7.7 (39) [3.5-14.3] | 12.2 (30) [7.8-25.7] |
| No. of patients (%) with Tmax (h) of: | |||||||
| 0 | 4 (20) | 2 (11) | 6 (33) | 1 (6) | |||
| 2 | 10 (50) | 9 (45) | 11 (55) | 5 (28) | 6 (33) | 7 (39) | 7 (35) |
| 4 | 5 (25) | 2 (10) | 5 (25) | 8 (44) | 3 (17) | 7 (39) | 10 (50) |
| 6 | 5 (25) | 4 (20) | 4 (20) | 2 (11) | 3 (17) | 3 (17) | 3 (15) |
| 8 | 1 (5) | 1 (6) | |||||
| Geometric mean C12 (μg/ml) (%CV) [range] | 2.9 (189) [0.1-13.8] | 1.9 (116) [0.1-11.5] | 1.2 (256) [0.1-9.6] | 5.4 (64) [1.4-10.2] | 2.3 (159) [0.3-13.0] | 1.8 (119) [0.3-10.8] | 5.6 (52) [2.0-17.6] |
| No. of patients (%) with C12 (μg/ml) of: | |||||||
| <1 | 3 (15) | 3 (15) | 8 (40) | 4 (22) | 5 (28) | ||
| 1-<5 | 9 (45) | 16 (80) | 9 (45) | 8 (44) | 9 (50) | 10 (56) | 8 (40) |
| 5-<10 | 5 (25) | 3 (15) | 8 (44) | 3 (17) | 2 (11) | 11 (55) | |
| 10+ | 3 (15) | 1 (5) | 2 (11) | 2 (11) | 1 (6) | 1 (5) | |
| Geometric mean t1/2 (h) (%CV) [range] | 4.6 (81) [1.5-24.6] | 3.8 (55) [1.3-9.4] | 3.5 (78) [1.4-12.0] | 7.1 (63) [2.5-18.2] | 6.4 (133) [2.0-65.2] | 4.0 (54) [1.7-14.5] | 7.7 (60) [3.1-29.1] |
Categories prespecified as 75%, 100%, and 135% of the target AUC0-12, 90 μg·h/ml.
Note that the limit of assay detection was 0.102 μg/ml. Also note that the coefficient of variation (%CV) was calculated using the formula 100 × [sqrt(exp{[SD of ln(var)2} − 1)].
TABLE 3.
Plasma ritonavir pharmacokinetic measurementsa
| Parameter | Value for group |
||||||
|---|---|---|---|---|---|---|---|
| Efavirenz (n = 20) + lopinavir-ritonavir dose of: |
Nevirapine (n = 18) + lopinavir-ritonavir dose of: |
No NNRTIs (n = 20) + lopinavir-ritonavir dose of 2 tablets (400/100 mg) BD | |||||
| 3 tablets (600/150 mg) BD | 4 capsules (533/133 mg) BD | 2 tablets (400/100 mg) BD | 3 tablets (600/150 mg) BD | 4 capsules (533/133 mg) BD | 2 tablets (400/100 mg) BD | ||
| Geometric mean AUC0-12 (μg·h/ml) (%CV) [range] | 4.14 (61) [1.15-9.58] | 2.86 (41) [1.35-6.96] | 2.43 (59) [0.72-6.84] | 4.89 (38) [2.45-8.63] | 2.60 (63) [1.12-8.26] | 2.43 (55) [1.13-10.13] | 3.69 (49) [1.31-9.13] |
| Geometric mean Cmax (μg/ml) (%CV) [range] | 0.58 (51) [0.22-1.23] | 0.41 (44) [0.20-0.79] | 0.36 (48) [0.12-0.86] | 0.71 (40) [0.38-1.67] | 0.39 (63) [0.17-1.06] | 0.35 (50) [0.14-1.24] | 0.50 (51) [0.19-1.11] |
| No. of patients (%) with Tmax (h) of: | |||||||
| 0 | 6 (30) | 2 (11) | 6 (33) | 1 (6) | |||
| 2 | 9 (45) | 7 (35) | 11 (55) | 7 (39) | 7 (39) | 7 (39) | 6 (30) |
| 4 | 5 (25) | 2 (10) | 6 (30) | 6 (33) | 2 (11) | 9 (50) | 12 (60) |
| 6 | 6 (30) | 5 (25) | 3 (15) | 3 (17) | 3 (17) | 1 (6) | 2 (10) |
| Geometric mean C12 (μg/ml) (%CV) [range] | 0.11 (103) [0.03-0.45] | 0.08 (66) [0.03-0.32] | 0.06 (87) [0.03-0.23] | 0.13 (54) [0.05-0.24] | 0.08 (109) [0.03-0.43] | 0.06 (70) [0.03-0.33] | 0.13 (59) [0.04-0.39] |
| Geometric mean t1/2 (h) (%CV) [range] | 3.3 (32) [2.2-7.3] | 3.7 (50) [2.1-10.2] | 3.3 (26) [2.0-6.2] | 3.3 (25) [1.9-5.0] | 4.6 (46) [2.7-12.8] | 3.3 (17) [2.5-4.1] | 4.1 (35) [2.5-11.7] |
Note that the limit of assay detection was 0.025 μg/ml.
The mean lopinavir AUC0-12 was substantially lower than the expected 83 μg·h/ml (Kaletra capsules summary of product characteristics, August 2008 [also see reference 16]) for both the group taking 4 lopinavir-ritonavir capsules BD (geometric means of 65.7 μg·h/ml with efavirenz and 68.1 μg·h/ml with nevirapine) and the group taking 2 tablets of lopinavir-ritonavir BD (geometric means, 54.0 μg·h/ml with efavirenz and 61.5 μg·h/ml with nevirapine). In contrast, the geometric mean lopinavir AUC0-12 values were 110.1 μg·h/ml for those receiving 2 tablets of lopinavir-ritonavir BD without NNRTIs and 91.8 μg·h/ml and 112.9 μg·h/ml for those receiving 3 tablets BD with efavirenz or nevirapine, respectively, which is more similar to the 113.2 μg·h/ml expected for lopinavir-ritonavir tablets (Aluvia summary of product characteristics, December 2009). Similarly, the mean ritonavir AUC0-12 was substantially lower for both the group taking 4 capsules of lopinavir-ritonavir BD (geometric means, 2.86 μg·h/ml with efavirenz and 2.60 μg·h/ml with nevirapine) and the group taking 2 lopinavir-ritonavir tablets BD (geometric means, 2.43 μg·h/ml with efavirenz and 2.43 μg·h/ml with nevirapine), whereas the geometric mean ritonavir AUC0-12 values were 3.69 μg·h/ml for those receiving 2 tablets of lopinavir-ritonavir BD without NNRTIs and 4.14 μg·h/ml and 4.89 μg·h/ml for those receiving 3 tablets BD with efavirenz or nevirapine, respectively. Estimated half-lives (t1/2) of lopinavir differed across groups similarly to the lopinavir AUC0-12; however, ritonavir half-lives were more similar across groups.
We found that 15%, 15%, and 40% of those receiving efavirenz with 3 lopinavir-ritonavir tablets, 4 lopinavir-ritonavir capsules, and 2 lopinavir-ritonavir tablets BD, respectively, had C12 values of <1 μg/ml, a minimum trough concentration suggested previously by some groups to be an important threshold (1, 13) but not supported by data from the manufacturer (2). Equivalent percentages for patients receiving nevirapine were 0%, 22%, and 28%, respectively, and none of those receiving 2 tablets of lopinavir-ritonavir BD without NNRTIs had a C12 of <1 μg/ml. However, 40%, 5%, and 15% of those receiving efavirenz and 56%, 28%, and 17% of those receiving nevirapine had C12 values >5 μg/ml, compared to 60% of those receiving 2 tablets of lopinavir-ritonavir BD without NNRTIs.
Comparison of data for the lopinavir-ritonavir 2- and 3-tablet BD groups to data for the 4-capsule BD group as a reference (as this was the recommended dose for lopinavir-ritonavir capsules with NNRTIs) showed that the AUC0-12, Cmax, and C12 values were at least 30 to 40% higher for the group receiving 3 tablets of lopinavir-ritonavir BD and were generally 10 to 20% lower for the group receiving 2 tablets of lopinavir-ritonavir BD (Table 4). Only the Cmax for the group receiving 2 lopinavir-ritonavir tablets BD with efavirenz was formally bioequivalent to 4 lopinavir-ritonavir capsules BD. Comparison of data for the group receiving 3 lopinavir-ritonavir tablets BD with NNRTI to data for the group receiving 2 lopinavir-ritonavir tablets BD without NNRTIs showed that the levels were generally similar those for the group receiving 3 tablets BD, although only the lopinavir AUC0-12 with nevirapine met formal criteria for bioequivalence (90% confidence interval [CI] within the range of 0.8 to 1.25). In contrast, both the AUC0-12 and C12 were statistically significantly reduced by over 50% for the group receiving 2 tablets BD and NNRTIs compared to the group receiving 2 tablets BD without NNRTIs.
TABLE 4.
GMR of plasma lopinavir and ritonavir pharmacokinetic measurements compared with data for 4 lopinavir-ritonavir capsules BD and 2 lopinavir-ritonavir tablets BD without NNRTIsa
| Dose and parameter | Dose for comparison | Value for group |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Lopinavir plus: |
Ritonavir plus: |
||||||||
| Efavirenz (n = 20) |
Nevirapine (n = 18) |
Efavirenz (n = 20) |
Nevirapine (n = 18) |
||||||
| GMR (90% CI) | P | GMR (90% CI) | P | GMR (90% CI) | P | GMR (90% CI) | P | ||
| 4 capsules BD | |||||||||
| AUC0-12 (μg/ml) | 3 tablets BD | 1.40 (1.18, 1.65) | 0.002 | 1.66 (1.46, 1.88) | <0.001 | 1.44 (1.19, 1.75) | 0.003 | 1.88 (1.56, 2.26) | <0.001 |
| 2 tablets BD | 0.82 (0.68, 0.99) | 0.09 | 0.90 (0.77, 1.06) | 0.27 | 0.85 (0.69, 1.04) | 0.18 | 0.93 (0.77, 1.13) | 0.54 | |
| Cmax (μg/ml) | 3 tablets BD | 1.34 (1.16, 1.54) | 0.002 | 1.45 (1.28, 1.66) | 0.0001 | 1.41 (1.14, 1.73) | 0.01 | 1.81 (1.50, 2.19) | <0.001 |
| 2 tablets BD | 0.90 (0.78, 1.05) | 0.26 | 0.92 (0.80, 1.06) | 0.31 | 0.88 (0.72, 1.08) | 0.30 | 0.91 (0.71, 1.15) | 0.49 | |
| C12 (μg/ml) | 3 tablets BD | 1.48 (1.09, 2.02) | 0.04 | 2.31 (1.64, 3.24) | 0.0005 | 1.33 (1.06, 1.67) | 0.04 | 1.59 (1.18, 2.15) | 0.01 |
| 2 tablets BD | 0.62 (0.39, 0.98) | 0.08 | 0.80 (0.57, 1.12) | 0.26 | 0.80 (0.60, 1.06) | 0.18 | 0.78 (0.61, 1.00) | 0.10 | |
| t1/2 (h) | 3 tablets BD | 1.14 (0.92, 1.41) | 0.31 | 1.05 (0.74, 1.49) | 0.81 | 0.88 (0.73, 1.06) | 0.24 | 0.71 (0.60, 0.83) | 0.002 |
| 2 tablets BD | 0.87 (0.67, 1.13) | 0.36 | 0.63 (0.47, 0.84) | 0.01 | 0.89 (0.74, 1.08) | 0.32 | 0.71 (0.60, 0.85) | 0.004 | |
| 2 tablets BD with no NNRTI | |||||||||
| AUC0-12 (μg/ml) | 3 tablets BD | 0.83 (0.66, 1.05) | 0.21 | 1.03 (0.87, 1.21) | 0.81 | 1.12 (0.86, 1.47) | 0.48 | 1.33 (1.06, 1.66) | 0.05 |
| 2 tablets BD | 0.49 (0.38, 0.63) | <0.001 | 0.56 (0.45, 0.70) | <0.001 | 0.66 (0.51, 0.86) | 0.01 | 0.66 (0.51, 0.85) | 0.01 | |
| Cmax (μg/ml) | 3 tablets BD | 0.91 (0.75, 1.11) | 0.45 | 1.00 (0.87, 1.15) | 0.98 | 1.15 (0.89, 1.47) | 0.37 | 1.40 (1.11, 1.77) | 0.02 |
| 2 tablets BD | 0.62 (0.51, 0.74) | <0.001 | 0.63 (0.53, 0.76) | <0.001 | 0.72 (0.57, 0.92) | 0.03 | 0.70 (0.54, 0.90) | 0.03 | |
| C12 (μg/ml) | 3 tablets BD | 0.51 (0.31, 0.83) | 0.03 | 0.96 (0.72, 1.27) | 0.80 | 0.87 (0.60, 1.25) | 0.53 | 1.03 (0.78, 1.37) | 0.85 |
| 2 tablets BD | 0.21 (0.12, 0.37) | <0.001 | 0.33 (0.22, 0.49) | <0.001 | 0.52 (0.37, 0.73) | 0.003 | 0.51 (0.37, 0.70) | 0.001 | |
| t1/2 (h) | 3 tablets BD | 0.60 (0.43, 0.83) | 0.01 | 0.93 (0.69, 1.26) | 0.70 | 0.80 (0.68, 0.95) | 0.04 | 0.80 (0.68, 0.93) | 0.02 |
| 2 tablets BD | 0.45 (0.33, 0.62) | <0.001 | 0.52 (0.39, 0.69) | <0.001 | 0.82 (0.70, 0.95) | 0.04 | 0.81 (0.70, 0.93) | 0.02 | |
Note that boldface type indicates parameters meeting the formal definition of bioequivalence (90% CI within the range of 0.8 to 1.25). Comparison to data for 4 capsules BD is a within-person comparison and is powered for bioequivalence. Comparison to data for 2 tablets BD without NNRTIs is a between-person comparison and is not powered for equivalence.
Population PK modeling of interactions between lopinavir-ritonavir and nevirapine or efavirenz.
Compared to 4 capsules (533/133 mg) BD, the lopinavir CL/F for patients also receiving efavirenz was reduced by 58% and 1% for 3 tablets (600/150 mg) and 2 tablets (400/100 mg) BD, respectively, and was reduced by 57% and 7%, respectively, for those also receiving nevirapine (Table 5). Compared to data for the group receiving 4 capsules BD, the ritonavir CL/F for patients also receiving efavirenz was reduced by 58% and 14% for the patients receiving 3 and 2 tablets BD, respectively, and by 55% and 13% for those also receiving nevirapine, respectively (Table 5). No other factors (age, gender, hemoglobin level, and body weight) were associated with the lopinavir or ritonavir CL/F.
TABLE 5.
Population pharmacokinetic model parameter estimates and standard errors for lopinavir and ritonavir in the presence of nevirapine or efavirenza
| Drug and parameter | Value |
|||||||
|---|---|---|---|---|---|---|---|---|
| Efavirenz |
Nevirapine |
|||||||
| Estimate | RSE (%) | IOV (%) | RSE (%) | Estimate | RSE (%) | IOV (%) | RSE (%) | |
| Lopinavir | ||||||||
| CL/F (liters/h) | 6.81 | 6.0 | 17.4 | 47.4 | 6.54 | 4.1 | 11.0 | 43.9 |
| V/F (liters) | 104 | 13.0 | 138 | 11.5 | ||||
| ka (h−1) | 1.22 | 1.22 | ||||||
| Factor associated with RTV AUC0-12 on CL/F | −0.633 | 13.2 | −0.612 | 11.0 | ||||
| Relative change in LPV CL/F for 600/150 mg | 0.415 | 5.1 | 0.434 | 7.2 | ||||
| Relative change in LPV CL/F for 400/100 mg | 0.986 | 6.8 | 0.929 | 5.3 | ||||
| Residual error, proportional (%) | 39.5 | 14.3 | 33.3 | 15.9 | ||||
| Ritonavir | ||||||||
| CL/F (liters/h) | 37.7 | 7.2 | 14.2 | 51.0 | 36.6 | 5.5 | 15.1 | 43.5 |
| V/F (liters) | 42.1 | 30.9 | 42.1 | |||||
| ka (h−1) | 0.111 | 13.7 | 0.0819 | 17.0 | ||||
| Factor associated with LPV AUC0-12 on CL/F | −0.882 | 9.9 | −1.12 | 7.4 | ||||
| Relative change in RTV CL/F for 600/150 mg | 0.421 | 7.6 | 0.452 | 5.9 | ||||
| Relative change in RTV CL/F for 400/100 mg | 0.856 | 7.6 | 0.872 | 8.0 | ||||
| Residual error | ||||||||
| Proportional (%) | 29.5 | 40.0 | 41.5 | 20.2 | ||||
| Additive (mg/liter) | 0.058 | 46.6 | 0.039 | 40.1 | ||||
CL/F, apparent oral clearance of lopinavir; V/F, apparent volume of distribution; ka, absorption rate constant; LPV, lopinavir; RTV, ritonavir; AUC0-12, area under the concentration-time curve over the dosing interval; RSE, relative standard error; IOV, interoccasion variability [RSE = (SEestimate/estimate) × 100].
DISCUSSION
When coadministered with efavirenz or nevirapine in HIV-infected Ugandan patients, the lopinavir AUC0-12 and C12 are significantly higher and the CL/F is significantly lower with 3 tablets BD than for the previously recommended dose of 4 lopinavir-ritonavir capsules BD. The lopinavir AUC0-12 and C12 are marginally lower with 2 tablets BD than with the previously recommended dose of 4 lopinavir-ritonavir capsules BD, although the CL/F values were similar. However, the lopinavir AUC0-12 and C12 for patients taking 3 tablets BD plus NNRTIs are similar to those for patients taking 2 tablets BD without NNRTIs in whom lopinavir and ritonavir exposures were similar to that observed for studies in resource-rich settings (4). Low plasma C12 values for patients taking 2 tablets BD plus NNRTIs may increase the risk of virological failure, although these levels are similar to the Ctrough values seen for patients taking 800/200 mg (4 tablets) four times a day (QD), which has shown good efficacy in patients without lopinavir resistance (6). Nevertheless, the fact that levels were lower than both those for patients taking 4 capsules BD with NNRTIs and those for patients taking 2 tablets BD without NNRTIs supports the most recent licensing information that a dose increment above 2 lopinavir-ritonavir tablets BD with concurrent NNRTIs should be considered.
Our study was powered on within-patient comparisons of the different doses and/or formulations with each NNRTI, efavirenz and nevirapine, and not the between-patient comparisons with the “no-NNRTI” group, which would have required substantially more patients to allow for between-patient variability. Rather, the “no-NNRTI” control group was intended to provide an independent estimate of the expected lopinavir exposure in Ugandan patients receiving tablets compared with historical Caucasian controls. We found similar AUC0-12 values for these patients receiving 2 tablets BD without NNRTIs (geometric mean of 110.1 versus 113.2 μg·h/ml in the most recent summary of product characteristics), more similar to values for those receiving 3 tablets of lopinavir-ritonavir BD with NNRTIs.
While current labeling now recommends 500/125 mg (two 200/50-mg tablets plus one 100/25-mg half-dose tablet) BD with NNRTIs, this may be difficult for resource-limited settings, particularly where half-dose lopinavir-ritonavir tablets are not available. In this situation, our study has shown that plasma PK levels for patients receiving 3 tablets BD with NNRTIs are similar to those for patients taking 2 tablets BD without NNRTIs, suggesting that this dosing scheme could be used with levels of toxicity (diarrhea, lipid abnormalities, and cardiovascular risk) similar to that seen for the population receiving lopinavir-ritonavir tablets without NNRTIs. Clearly, however, such a regimen will increase costs by 50%. An alternative option not evaluated in this study would be split dosing with 3 tablets in the morning and 2 tablets in the evening. As there is diurnal variation, with evening troughs lower than morning troughs upon even dosing, this would be the most logical split-dosing regimen but may be difficult for patients to follow and may be liable to dosing errors.
Our study has several limitations. Due to financial constraints, patients were not admitted the previous night, and therefore, only the dose at time zero, and not the preceding dose, was observed. However, concentrations at time zero were generally slightly higher than the C12, which does not support nonadherence as a driver of low trough levels. In healthy volunteers, the C0 (morning) was also slightly higher than the C12 (evening). Plasma concentrations were not measured an hour after intake, and therefore, our estimates of Tmax are only approximate, particularly for those patients taking NNRTIs, where this appeared to be somewhat closer to intake than in the group taking lopinavir-ritonavir tablets without NNRTIs. While the AUC0-12 may also be slightly underestimated, this should not affect differences between groups because the sampling schedule was the same for each day of PK evaluation. We also found surprisingly low lopinavir and ritonavir exposures with 4 capsules BD, particularly given the somewhat higher levels than expected for those receiving 2 tablets BD without NNRTIs. This finding may suggest problems with either the stability of the capsule formulation or its bioavailability in tropical settings. We also found lower levels of lopinavir with 3 tablets BD than those found by a previous study of efavirenz in a resource-rich setting (11). In particular, it is unclear why ritonavir levels were reduced so much by NNRTIs, with both capsule and tablet lopinavir-ritonavir, since these have generally been considered less-strong enzyme inducers than other concomitant medications such as rifampin. However, potentially, this could be a combined effect of the NNRTI and lopinavir, as lopinavir does induce ritonavir concentrations. Compliance was measured during this substudy (and throughout the DART trial) by using nurse pill counts and patient self-reporting; a missing dose(s) in the last 4 days was an exclusion criterion, and none of the included patients reported missing lopinavir-ritonavir doses in the last month during the substudy period. Finally, lipids were not routinely measured, limiting the assessment of short-term toxicity at the different doses used in this PK crossover study.
In general, the data from the population PK analysis are consistent with data from the noncompartmental analysis. The pharmacokinetic parameters have similarities with those reported in the literature (10, 14); however, the CL/F was higher. This is not unusual since here the population estimates of lopinavir and ritonavir CL/F values are those in the presence of NNRTIs at a dose of 533/133 mg twice daily. The use of different formulations, doses, and enzyme inducers, i.e., efavirenz versus nevirapine, together with a different control group from those receiving NNRTIs precluded the use of a single combined model for all data. Moreover, it was shown previously that a model incorporating the inhibition of lopinavir CL/F by ritonavir concentrations at each sampling time better describes lopinavir pharmacokinetics (14); however, the available data did not allow this type of analysis. Thus, the population PK models suggest that the differences in AUC0-12 values are mostly likely due to differences in CL/F and unlikely to be due to differences in absorption, although this could not be investigated directly due to a lack of data for the absorption phase.
Overall, therefore, this study suggests that the revised recommendation of a regimen of 500/125 mg (two 200/50-mg tablets plus one 100/25-mg half-dose tablet) lopinavir-ritonavir BD with NNRTIs is appropriate. Where half-dose lopinavir-ritonavir tablets are not available, the dose should be increased by the adding an additional adult tablet in the morning; alternatively, a dose of 3 tablets BD with NNRTIs is likely to provide exposure similar to that with 2 tablets BD without NNRTIs but is considerably more expensive. This study highlights the importance of conducting pharmacokinetic evaluations of target populations in resource-limited settings, where comparable plasma pharmacokinetic characteristics, and, consequently, the impact of an adverse drug-drug interaction, cannot be assumed.
Acknowledgments
We thank all the patients and staff from all the centers participating in the DART trial. We also acknowledge helpful comments from Marta Boffito and David Burger on the interpretation of these data and Ceppie Merry in help in training and setting up the study.
Members of the DART Trial Team are P. Mugyenyi, C. Kityo, F. Ssali, D. Tumukunde, T. Otim, J. Kabanda, H. Musana, J. Akao, H. Kyomugisha, A. Byamukama, J. Sabiiti, J. Komugyena, P. Wavamunno, S. Mukiibi, A. Drasiku, R. Byaruhanga, O. Labeja, P. Katundu, S. Tugume, P. Awio, A. Namazzi, G. T. Bakeinyaga, H. Katabira, D. Abaine, J. Tukamushaba, W. Anywar, W. Ojiambo, E. Angweng, S. Murungi, W. Haguma, S. Atwiine, and J. Kigozi, from the Joint Clinical Research Centre, Kampala, Uganda; E. Katabira, A. Ronald, A. Kambungu, F. Lutwama, I. Mambule, A. Nanfuka, J. Walusimbi, E. Nabankema, R. Nalumenya, T. Namuli, R. Kulume, I. Namata, L. Nyachwo, A. Florence, A. Kusiima, E. Lubwama, R. Nairuba, F. Oketta, E. Buluma, R. Waita, H. Ojiambo, F. Sadik, J. Wanyama, and P. Nabongo, from the Infectious Diseases Institute (formerly the Academic Alliance), Makerere University, Mulago, Uganda; H. Grosskurth, P. Munderi, G. Kabuye, D. Nsibambi, R. Kasirye, E. Zalwango, M. Nakazibwe, B. Kikaire, G. Nassuna, R. Massa, K. Fadhiru, M. Namyalo, A. Zalwango, L. Generous, P. Khauka, N. Rutikarayo, W. Nakahima, A. Mugisha, J. Todd, J. Levin, S. Muyingo, A. Ruberantwari, P. Kaleebu, D. Yirrell, N. Ndembi, F. Lyagoba, P. Hughes, M. Aber, A. Medina Lara, S. Foster, J. Amurwon, and B. Nyanzi Wakholi, from the MRC/UVRI Uganda Research Unit on AIDS, Entebbe, Uganda; A. Latif, J. Hakim, V. Robertson, A. Reid, E. Chidziva, R. Bulaya-Tembo, G. Musoro, F. Taziwa, C. Chimbetete, L. Chakonza, A. Mawora, C. Muvirimi, G. Tinago, P. Svovanapasis, M. Simango, O. Chirema, J. Machingura, S. Mutsai, M. Phiri, T. Bafana, M. Chirara, L. Muchabaiwa, and M. Muzambi, from the University of Zimbabwe, Harare, Zimbabwe; R. Ochai and D. Muhweezi, from The AIDS Support Organization (TASO), Uganda; C. Gilks, K. Boocock, C. Puddephatt, D. Winogron, and J. Bohannon, from the Imperial College, London, United Kingdom; and J. Darbyshire, D. M. Gibb, A. Burke, D. Bray, A. Babiker, A. S. Walker, H. Wilkes, M. Rauchenberger, S. Sheehan, L. Peto, K. Taylor, M. Spyer, A. Ferrier, B. Naidoo, D. Dunn, R. Goodall, from the MRC Clinical Trials Unit, London, United Kingdom. Independent DART Trial monitors were R. Nanfuka and C. Mufuka-Kapuya. Members of the DART Virology Group are P. Kaleebu (cochair), D. Pillay (cochair), V. Robertson, D. Yirrell, S. Tugume, M. Chirara, P. Katundu, N. Ndembi, F. Lyagoba, D. Dunn, R. Goodall, and A. McCormick. Members of the DART Health Economics Group are A. Medina Lara (chair), S. Foster, J. Amurwon, B. Nyanzi Wakholi, J. Kigozi, L. Muchabaiwa, and M. Muzambi. Members of the Trial Steering Committee are I. Weller (chair), A. Babiker (trial statistician), S. Bahendeka, M. Bassett, A. Chogo Wapakhabulo, J. Darbyshire, B. Gazzard, C. Gilks, H. Grosskurth, J. Hakim, A. Latif, C. Mapuchere, O. Mugurungi, and P. Mugyenyi; observers were C. Burke, M. Distel, S. Jones, E. Loeliger, C. Newland, G. Pearce, S. Rahim, J. Rooney, M. Smith, W. Snowden, and J.-M. Steens. Members of the Data and Safety Monitoring Committee are A. Breckenridge (chair), A. McLaren (chair) (deceased), C. Hill, J. Matenga, A. Pozniak, and D. Serwadda. Members of the Endpoint Review Committee are T. Peto (chair), A. Palfreeman, M. Borok, and E. Katabira.
DART is funded by the United Kingdom Medical Research Council, the United Kingdom Department for International Development (DFID), and the Rockefeller Foundation. GlaxoSmithKline, Gilead, and Boehringer-Ingelheim donated first-line drugs for DART, and Abbott provided lopinavir-ritonavir (Kaletra/Aluvia) as part of the second-line regimen for DART.
Footnotes
Published ahead of print on 26 April 2010.
REFERENCES
- 1.Ananworanich, J., P. Kosalaraksa, A. Hill, U. Siangphoe, A. Bergshoeff, C. Pancharoen, C. Engchanil, K. Ruxrungtham, and D. Burger. 2005. Pharmacokinetics and 24-week efficacy/safety of dual boosted saquinavir/lopinavir/ritonavir in nucleoside-pretreated children. Pediatr. Infect. Dis. J. 24:874-879. [DOI] [PubMed] [Google Scholar]
- 2.Chiu, Y., M. King, J. Li, C. Klein, and G. Hanna. 2007. Trough lopinavir concentrations do not predict virologic response to lopinavir/ritonavir-based three-drug regimens in antiretroviral-naïve patients, poster 38. Abstr. 8th Int. Wkshp. Clin. Pharmacol. HIV Ther., 16 to 18 April 2007, Budapest, Hungary.
- 3.DART Trial Team. 2009. Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial. Lancet 375:123-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickinson, L., L. Robinson, J. Tjia, S. Khoo, and D. Back. 2005. Simultaneous determination of HIV protease inhibitors amprenavir, atazanavir, indinavir, lopinavir, nelfinavir, ritonavir and saquinavir in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 829:82-90. [DOI] [PubMed] [Google Scholar]
- 5.Else, L., T. Mahungu, Z. Cuthbertson, C. Smith, D. Podlekareva, P. Hay, U. B. Dragsted, S. Khoo, M. Johnson, D. Back, and M. Youle. 2008. An open-label multi-centre pilot study evaluating the pharmacokinetics of co-administered lopinavir and nevirapine in HIV-infected adults. The NRTI-sparing study, abstr. P250. Abstr. 9th Int. Congr. Drug Ther. HIV Infect., Glasgow, United Kingdom, November 2008.
- 6.Gathe, J., B. A. da Silva, D. E. Cohen, M. R. Loutfy, D. Podzamczer, R. Rubio, S. Gibbs, T. Marsh, C. Naylor, L. Fredrick, and B. Bernstein. 2009. A once-daily lopinavir/ritonavir-based regimen is noninferior to twice-daily dosing and results in similar safety and tolerability in antiretroviral-naive subjects through 48 weeks. J. Acquir. Immune Defic. Syndr. 50:474-481. [DOI] [PubMed] [Google Scholar]
- 7.Gilks, C. F., S. Crowley, R. Ekpini, S. Gove, J. Perriens, Y. Souteyrand, D. Sutherland, M. Vitoria, T. Guerma, and K. De Cock. 2006. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet 368:505-510. [DOI] [PubMed] [Google Scholar]
- 8.Hsu, A., J. Isaacson, S. Brun, B. Bernstein, W. Lam, R. Bertz, C. Foit, K. Rynkiewicz, B. Richards, M. King, R. Rode, D. J. Kempf, G. R. Granneman, and E. Sun. 2003. Pharmacokinetic-pharmacodynamic analysis of lopinavir-ritonavir in combination with efavirenz and two nucleoside reverse transcriptase inhibitors in extensively pretreated human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 47:350-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein, C., Y. Cai, Y. Chiu, S. Causemaker, T. Doan, T. Podsadecki, B. Bernstein, and G. Hanna. 2006. Pharmacokinetics of lopinavir and ritonavir after multiple dose administration of lopinavir/ritonavir tablet co-administered with efavirenz, abstr. P286. Abstr. 8th Int. Congr. Drug Ther. HIV Infect., Glasgow, United Kingdom, 12 to 16 November 2006.
- 10.Klein, C., J. Ng, Y. L. Chiu, P. M. Diderichsen, B. da Silva, B. Bernstein, and W. Awni. 2008. Population pharmacokinetic/pharmacodynamic analyses of lopinavir and ritonavir in subjects receiving the tablet formulation, abstr. P245. Abstr. 9th Int. Congr. Drug Ther. HIV Infect., Glasgow, United Kingdom, November 2008.
- 11.Klein, C., T. Zhu, Z. Chiu, T. Doan, G. Hanna, W. Awni, and S. Brun. 2005. Effect of efavirenz on lopinavir/ritonavir pharmacokinetics from a new tablet formulation, poster PE4.3/2. European AIDS Clinical Society, Paris, France.
- 12.Klein, C. E., Y. L. Chiu, W. Awni, T. Zhu, R. S. Heuser, T. Doan, J. Breitenbach, J. B. Morris, S. C. Brun, and G. J. Hanna. 2007. The tablet formulation of lopinavir/ritonavir provides similar bioavailability to the soft-gelatin capsule formulation with less pharmacokinetic variability and diminished food effect. J. Acquir. Immune Defic. Syndr. 44:401-410. [DOI] [PubMed] [Google Scholar]
- 13.la Porte, C., D. Back, T. Blaschke, C. Boucher, C. Fletcher, C. Flexner, J. Gerber, A. Kashuba, J. Schapiro, and D. Burger. 2006. Updated guideline to perform therapeutic drug monitoring for antiretroviral agents. Rev. Antivir. Ther. 3:4-14. [Google Scholar]
- 14.Molto, J., M. J. Barbanoj, C. Miranda, A. Blanco, J. R. Santos, E. Negredo, J. Costa, P. Domingo, B. Clotet, and M. Valle. 2008. Simultaneous population pharmacokinetic model for lopinavir and ritonavir in HIV-infected adults. Clin. Pharmacokinet. 47:681-692. [DOI] [PubMed] [Google Scholar]
- 15.Ng, J., C. Klein, J. Xiong, Y.-L. Chiu, T. Doan, C. Rolle, C. Holas, R. Stryker, and B. Bernstein. 2008. Lopinavir/ritonavir (LPV/r) 500/125 mg BID plus efavirenz approximate the pharmacokinetic exposure of LPV/r 400/100 mg BID administered alone in healthy adult subjects, abstr. 765. Abstr. 15th Conf. Retroviruses Opportun. Infect., Boston, MA.
- 16.Stek, A. M., M. Mirochnick, E. Capparelli, B. M. Best, C. Hu, S. K. Burchett, C. Elgie, D. T. Holland, E. Smith, R. Tuomala, A. Cotter, and J. S. Read. 2006. Reduced lopinavir exposure during pregnancy. AIDS 20:1931-1939. [DOI] [PubMed] [Google Scholar]