Abstract
Concentrative nucleoside transporter 2 (CNT2) (encoded by the SLC28A2 gene) transports various antiviral or antitumor purine nucleoside analogs to be involved in their pharmacokinetics and pharmacological actions. The results of our study showed that mouse hepatocytes hardly expressed CNT2 mRNA and no CNT2-dependent nucleoside uptake was observed, while rat hepatocytes exhibited high CNT2-dependent nucleoside uptake activity levels with abundant CNT2 mRNA expression. We concluded that CNT2 contributes considerably to nucleoside uptake in rat hepatocytes but not in mouse hepatocytes.
Concentrative nucleoside transporter 2 (CNT2) (encoded by the SLC28A2 gene) is an active plasma membrane nucleoside transporter for which the driving force is the Na+ gradient (10). The activity of CNT2 is resistant to nitrobenzylmercaptopurine riboside (NBMPR), an inhibitor of equilibrative nucleoside transporters (ENTs), as are activities of other CNT members, such as CNT1 and CNT3 (7). CNT2 preferentially transports endogenous purine nucleosides and many antiviral and anticancer purine nucleoside analogs (7, 10).
CNT2 expressed in the liver is thought to be involved in extraction of nucleoside analogs from circulation to determine their pharmacokinetics and to be involved in the pharmacological action of anti-hepatitis virus nucleoside analogs since hepatic uptake is a prerequisite step for the analogs to exert their antiviral activity. Therefore, understanding the functions of hepatic CNT2 would be important for nucleoside analog-based chemotherapy and for the development of novel nucleoside analogs.
In this report, we provide experimental evidence showing a striking species difference of hepatic CNT2 expression and function between the mouse and rat.
[3H]ribavirin and [2,8-3H]adenosine were purchased from Moravek Biochemicals (Brea, CA). All other reagents were commercially available. C57BL/6J male mice (8 weeks old) and Sprague-Dawley male rats (6 weeks old) were purchased from Japan SLC Inc. (Shizuoka, Japan). This study was approved by the Animal Research Committee of Chiba University.
The procedures for total RNA preparation and first-strand cDNA synthesis were described previously (5). Reverse transcription-PCR (RT-PCR) was performed with a series of primers (see Table S1 in the supplemental material). The DNA sequences of the PCR products were determined as described previously (5).
Rodent hepatocytes were isolated using a two-step collagenase digestion method described by Seglen (9). The hepatocytes were resuspended in a Na+-plus Krebs-Henseleit buffer (Na+-plus KHB) or Na+-free KHB (see Table S2 in the supplemental material) to give a cell density of 1.2 × 106 cells/ml.
Adenosine or ribavirin uptake by hepatocytes was measured by an oil filtration assay as described previously (4). Uptake studies were initiated by adding to the cell suspension an equal volume of prewarmed Na+-plus or Na+-free KHB containing either 1 μCi/ml [2,8-3H]adenosine and nonradiolabeled adenosine or 1 μCi/ml [3H]ribavirin and nonradiolabeled ribavirin at the indicated concentrations. Incubation of rodent hepatocytes with adenosine and incubation with ribavirin were performed for 30 s and 1 min, respectively, in all experiments, since the rate of adenosine or ribavirin uptake into rodent hepatocytes was linear for at least 1 min (data not shown). NBMPR, inosine, thymidine, and/or uridine (Sigma, St. Louis, MO) was also included in inhibition analysis. After incubation at 37°C, the assay mixture was layered on top of the oil layer (a mixture of silicone oil and mineral oil [Sigma] with a density of 1.0143) and centrifuged briefly (10,000 × g for 30 s). The hepatocytes passed through the filtration oil into the lower layer of 2 N NaOH, which served to solubilize the cell membrane and release the radiolabeled substrate. After overnight incubation, the compartment containing dissolved cells of the tube was transferred to a scintillation vial. Dissolved cells were mixed with Clear sol II (Nacalai Tesque, Kyoto, Japan), and radioactivity was measured by a liquid scintillation counter (LSC 6100; Aloka Co., Tokyo, Japan). All assays were also performed at 4°C, and the results were calculated by subtracting the activity at 4°C from the activity at 37°C.
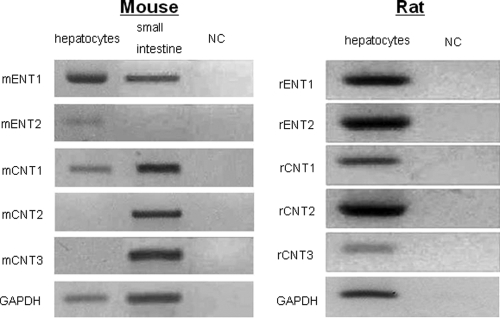
Expression of nucleoside transporter mRNAs in mouse and rat hepatocytes was examined by RT-PCR (Fig. 1). Mouse CNT1 (mCNT1) mRNA was only weakly detected and mCNT2 and mCNT3 mRNAs were not detected in mouse hepatocytes, while rat CNT2 (rCNT2) mRNA was substantially expressed and rCNT1 and rCNT3 mRNAs were moderately and weakly expressed in rat hepatocytes. mCNT2 and mCNT3 mRNA expression in mouse hepatocytes was not detected by real-time PCR analysis (data not shown). According to mRNA expression profiles, the results of Western blotting showed that rCNT2 protein was expressed in rat hepatocytes, while mCNT2 protein was not expressed in mouse hepatocytes (see Fig. S1 in the supplemental material).
FIG. 1.
Expression of nucleoside transporter mRNAs in mouse and rat hepatocytes. Nucleoside transporter mRNAs and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression were detected by RT-PCR. Mouse small intestine cDNA was used as a PCR control. NC, nontemplate control.
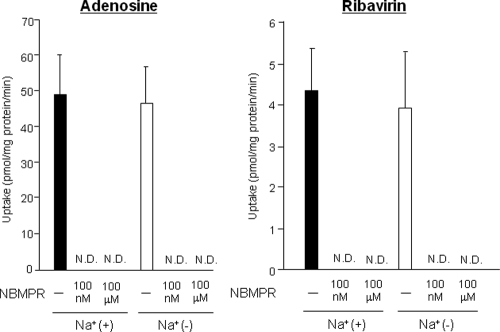
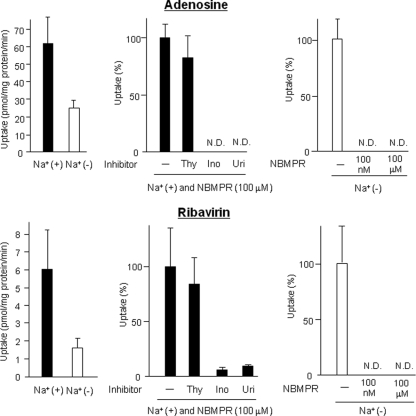
Adenosine (0.5 μM) uptake and ribavirin (1 μM) uptake into rodent hepatocytes were analyzed. Adenosine is an endogenous substrate for ENT1, ENT2, CNT1, CNT2, and CNT3 (7). Ribavirin, a synthetic nucleoside analog used for anti-hepatitis C therapy, has been reported to be a substrate for ENT1, ENT2, CNT2, and CNT3 (11). Na+-dependent nucleoside uptake activity was hardly detected with either substrate in mouse hepatocytes (Fig. 2). On the other hand, rat hepatocytes showed strong Na+-dependent uptake activity, and the removal of Na+ from the medium decreased adenosine and ribavirin uptake levels to 39% and 27% of the levels obtained with Na+-plus KHB, respectively (Fig. 3). Na+-dependent adenosine and ribavirin uptake activities in rat hepatocytes, which were obtained with Na+-plus KHB containing 100 μM NBMPR (inhibition of both rat ENT1 [rENT1] and rENT2), were slightly affected by the addition of thymidine (substrate of both rCNT1 and rCNT3) (Fig. 3). However, they were completely or mostly abolished by inosine (substrate of both rCNT2 and rCNT3) or uridine (substrate of all NTs). In both mouse and rat hepatocytes, Na+-independent nucleoside uptake activities completely disappeared in the presence of either 100 nM NBMPR (inhibition of ENT1 only) or 100 μM NBMPR (inhibition of both ENT1 and ENT2) in Na+-free KHB (Fig. 2 and 3). The profiles of ribavirin uptake by mouse hepatocytes and rat hepatocytes were not changed by intracellular ATP reduction (see Fig. S2 in the supplemental material).
FIG. 2.
Effects of Na+ and NBMPR on adenosine and ribavirin uptake by mouse hepatocytes. The effects of Na+ and NBMPR on adenosine and ribavirin uptake by mouse hepatocytes were analyzed in Na+-plus KHB (black bars) and in Na+-free KHB (white bars) with or without NBMPR (100 nM or 100 μM). Adenosine and ribavirin concentrations were set at 0.5 μM and 1 μM, respectively. Each value was calculated by subtracting the activity obtained at 4°C from that obtained at 37°C at the same adenosine concentration and ribavirin concentration. Each value represents the mean plus standard deviation (SD) (error bar) of the uptake level from five separate experiments, each performed in duplicate. N.D., not detected.
FIG. 3.
Characterization of Na+-dependent and Na+-independent adenosine and ribavirin uptake by rat hepatocytes. (Left) The effects of the removal of Na+ from the transport medium on adenosine and ribavirin uptake by rat hepatocytes were analyzed. The hepatocytes were suspended in Na+-plus KHB (black bars) or Na+-free KHB (white bars). (Middle) The effects of thymidine (Thy) (1 mM), inosine (Ino) (1 mM), and uridine (Uri) (1 mM) on Na+-dependent adenosine and ribavirin uptake by rat hepatocytes were analyzed in Na+-plus KHB in the presence of 100 μM NBMPR. N.D., not detected. (Right) The effects of NBMPR (100 nM and 100 μM) on Na+-independent adenosine and ribavirin uptake by rat hepatocytes were analyzed in Na+-free KHB. Adenosine and ribavirin concentrations were set at 0.5 μM and 1 μM, respectively. Each value was calculated by subtracting the activity obtained at 4°C from that obtained at 37°C at the same adenosine concentration and ribavirin concentration. Each value is the mean plus SD (error bar) of transport activity (right panels) or the mean plus SD of relative uptake level (as a percentage) of the level obtained in the absence of an inhibitor (middle panels and left panels) for five separate experiments, each performed in duplicate.
Results of direct comparison of nucleoside uptake properties of mouse and rat hepatocytes in the same experimental system showed differential contribution of Na+-dependent activity to nucleoside uptake by the cells. This differential contribution may not be restricted to adenosine and ribavirin, since the same conclusion was obtained from similar experiments using uridine, a substrate for all rodent CNTs identified (T. Furihata et al., unpublished observations). Significant Na+-dependent nucleoside uptake activity in rat hepatocytes has also been reported (2, 6), supporting our results. rCNT2 is thought to be the transporter mainly responsible for Na+-dependent activity in rat hepatocytes on the basis of the results of inhibition experiments and RT-PCR analysis.
Here, we should point out the possibility that the lack of expression of mCNT2 is limited to hepatocytes. The results of our preliminary experiments showed that mCNT2 mRNA was detected in total liver cDNA and cDNA from a mixture of nonhepatocyte cells residing in the liver (see Fig. S3 in the supplemental material). It should also be pointed out that our results do not exclude the possibility that mCNT2 expression in hepatocytes is induced under a specific condition, such as hepatic regeneration.
Errasti-Murugarren et al. (3) have recently suggested that nucleosides transported by CNTs were more readily metabolized to nucleobases than were those transported by ENTs, which preferentially proceeded to phosphorylation or remained unmetabolized. Therefore, the main nucleoside metabolism pathway directed by the nucleoside transport process might be different in mouse and rat hepatocytes. It has been shown that there are some differences in the clearance of nucleoside analogs in plasma in the mouse and rat (1, 8). Although the relationship of these pharmacokinetic differences with our results has not been established, it would be intriguing if the different nucleoside transport pathways are related to species-dependent pharmacokinetics of nucleoside analogs.
The major reason for the species difference in CNT2 activity may be different levels of expression of its mRNA. The regulatory mechanisms for rodent SLC28A2 gene transcription have not been fully clarified; therefore, we characterized the structures and DNA sequences of the 5′ regions of SLC28A2 genes and their 3′ untranslated regions (see Fig. S4 in the supplemental material). Since they were found to be very similar, molecular mechanisms causing the species difference seem to be complex.
In conclusion, to our knowledge, the present study is the first study to show that CNT2 greatly contributes to nucleoside uptake in rat hepatocytes but not in mouse hepatocytes. Recently, we reported results showing that only trace levels of CNT2 mRNA are expressed in human hepatocytes (4) and that the profile of ribavirin uptake by human hepatocytes is similar to that by mouse hepatocytes. Therefore, the mouse might be a better model for humans in terms of the nucleoside uptake system in hepatocytes. Although several issues, such as the role of CNT2 expressed in nonhepatocyte liver cells in hepatic extraction of nucleoside analogs and the difference in nucleoside metabolism among species, remain to be elucidated, our findings provide an important step for clarification of species difference in nucleoside handling, which would be essential information for understanding preclinical data for the development of new nucleoside analogs as well as similarities and differences in pharmacokinetics of the analogs between rodents and humans.
Supplementary Material
Acknowledgments
This work was supported by a grant (20790128) from the Ministry of Education, Sciences, Sports and Culture of Japan.
Footnotes
Published ahead of print on 26 April 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Arnold, S. T., H. N. Jayaram, G. R. Harper, C. L. Litterst, L. Malspeis, J. J. DeSouza, A. E. Staubus, G. S. Ahluwalia, Y. A. Wilson, and D. A. Cooney. 1984. The disposition and metabolism of tiazofurin in rodents, rabbits, and dogs. Drug Metab. Dispos. 12:165-173. [PubMed] [Google Scholar]
- 2.del Santo, B., R. Valdes, J. Mata, A. Felipe, F. J. Casado, and M. Pastor-Anglada. 1998. Differential expression and regulation of nucleoside transport systems in rat liver parenchymal and hepatoma cells. Hepatology 28:1504-1511. [DOI] [PubMed] [Google Scholar]
- 3.Errasti-Murugarren, E., M. Pastor-Anglada, and F. J. Casado. 2007. Role of CNT3 in the transepithelial flux of nucleosides and nucleoside-derived drugs. J. Physiol. 582:1249-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukuchi, Y., T. Furihata, M. Hashizume, M. Iikura, and K. Chiba. 2010. Characterization of ribavirin uptake system in human hepatocytes. J. Hepatol. 52:486-492. [DOI] [PubMed] [Google Scholar]
- 5.Furihata, T., M. Hosokawa, M. Masuda, T. Satoh, and K. Chiba. 2006. Hepatocyte nuclear factor-4alpha plays pivotal roles in the regulation of mouse carboxylesterase 2 gene transcription in mouse liver. Arch. Biochem. Biophys. 447:107-117. [DOI] [PubMed] [Google Scholar]
- 6.Holstege, A., H. Gengenbacher, L. Jehle, and J. Hoppmann. 1991. Facilitated diffusion and Na+-dependent transport of purine and pyrimidine nucleosides in rat liver. Hepatology 14:373-380. [PubMed] [Google Scholar]
- 7.Kong, W., K. Engel, and J. Wang. 2004. Mammalian nucleoside transporters. Curr. Drug Metab. 5:63-84. [DOI] [PubMed] [Google Scholar]
- 8.Kong, X. B., P. Vidal, W. P. Tong, J. Chiang, C. A. Gloff, and T. C. Chou. 1992. Preclinical pharmacology and pharmacokinetics of the anti-hepatitis virus agent 2′-fluoro-5-ethyl-1-beta-d-arabinofuranosyluracil in mice and rats. Antimicrob. Agents Chemother. 36:1472-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seglen, P. O. 1976. Preparation of isolated rat liver cells. Methods Cell Biol. 13:29-83. [DOI] [PubMed] [Google Scholar]
- 10.Wang, J., S. Su, M. J. Dresser, M. E. Schaner, C. B. Washington, and K. M. Giacomini. 1997. Na+-dependent purine nucleoside transporter from human kidney: cloning and functional characterization. Am. J. Physiol. Renal Physiol. 273:1058-1065. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto, T., K. Kuniki, Y. Takekuma, T. Hirano, K. Iseki, and M. Sugawara. 2007. Ribavirin uptake by cultured human choriocarcinoma (BeWo) cells and Xenopus laevis oocytes expressing recombinant plasma membrane human nucleoside transporters. Eur. J. Pharmacol. 557:1-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.