Abstract
Coagulase-negative species of Staphylococcus are often associated with opportunistic hospital-acquired infections that arise from the colonization of indwelling catheters. Here we show that the antiparasitic drug nitazoxanide (NTZ) and its active metabolite, tizoxanide (TIZ), are inhibitory to the growth of Staphylococcus epidermidis and other staphylococci, including methicillin-resistant Staphylococcus aureus strains, under aerobic and microaerobic conditions (MICs, 8 to 16 μg/ml). At sub-MIC levels, NTZ and TIZ also inhibited biofilm production under static conditions by strains of S. epidermidis and Staphylococcus haemolyticus with a 50% inhibitory concentration of ∼2.5 μg/ml (8 μM). The 5-nitro group was required for biological activity, and a hydrophilic derivative of NTZ (AMIX) also inhibited biofilm formation. NTZ did not disperse the existing biofilm but did block further accumulation. Sub-MICs of NTZ had no effect on primary attachment to surfaces at either 4 or 37°C. The inhibitory action of NTZ and TIZ, but not vancomycin, on biofilm production could be reversed by the addition of zinc salts (2.5 to 40 μM) but not other metals, suggesting that NTZ might target the zinc-dependent accumulation-associated protein (Aap) that mediates accumulation on surfaces. However, neither NTZ nor TIZ formed chelation complexes with zinc salts, based on spectrophotometric and nuclear magnetic resonance analyses, and addition of excess zinc to NTZ-grown bacteria (apo-Aap) did not restore the accumulation phenotype. Our studies suggest that sub-MIC levels of NTZ may affect the assembly or function of cell structures associated with the biofilm phenotype.
Coagulase-negative staphylococci (CoNS) have emerged as important opportunistic hospital-acquired pathogens that are the leading cause of catheter and indwelling device-associated infections (27, 31, 36). The ability of CoNS, including the archetypal species, Staphylococcus epidermidis, to cause disease depends on their ability to adhere to polymer surfaces, where they form thick, multilayered, cellular agglomerations known as biofilms (31, 36). The principal component of the S. epidermidis biofilm is poly-β-1,6-N-acetyl-d-glucosamine (PNAG; also known as polysaccharide intercellular adhesion [PIA]), synthesized by the products of the ica genes (7, 17, 18). Recently, the cell-surface-expressed accumulation-associated protein Aap has been shown to mediate PNAG-independent biofilm formation in some strains of S. epidermidis (4, 30), suggesting that proteinaceous matrix components are important for biofilm formation by certain strains. Biofilm contributes to persistence by limiting the efficacies of antibiotics and host immune responses (2, 11, 29, 35). Bloodstream and urinary tract infections were ranked as the 2nd and 3rd most common causes of health care-associated deaths in the United States in 2002, respectively (35). More than 5 million central venous catheters are inserted annually in the United States, and of the more than 200,000 health care-acquired bloodstream infections that occur annually, most are due to central venous catheters (14, 23, 35). These infections lead to increased morbidity, mortality, lengths of hospitalization, and total health care costs.
Many drugs and compounds have been tested as biofilm inhibitors, and some (e.g., silver, minocycline, rifampin, platinum, nitrofurantoin, chlorhexidine, and sulfadiazine) are used to coat catheters (1, 10, 13, 20). Several randomized trials have shown the benefits of using antibiotic(s)-impregnated catheters in hospitalized patients to reduce colonization and catheter-related bloodstream infections (CRBSIs) by comparison of, for example, chlorhexidine-silver sulfadiazine-impregnated catheters with nonimpregnated catheters, rifampin-minocycline-coated catheters compared with noncoated catheters, and rifampin-minocycline-impregnated catheters compared with chlorhexidine-silver sulfadiazine-impregnated catheters (1, 20).
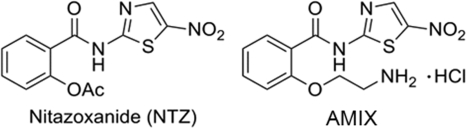
Nitazoxanide (NTZ) is a 5-nitrothiazole therapeutic (Fig. 1) that is used to treat a wide variety of parasitic and anaerobic bacterial infections (6) and that is FDA approved for the treatment of Cryptosporidium parvum and Giardia intestinalis infections in adults and children (8). The drug also shows efficacy against Clostridium difficile infections (22, 26). Mechanistic studies have shown that NTZ is a potent inhibitor of pyruvate:ferredoxin oxidoreductase (PFOR) (9) and is therefore active against all organisms (anaerobic bacteria and parasites) expressing this enzyme (28). Mechanistic studies revealed that the anionic form of the drug is biologically active, and a proton abstraction mechanism has been proposed (9). Such a generic mechanism might account for the wide range of biological targets reported for this drug (9, 24, 25, 33).
FIG. 1.
Chemical structures of NTZ and AMIX.
One of the original communications on the spectrum of action of NTZ indicated that the drug was active against Staphylococcus aureus only under anaerobic conditions and that the active metabolite of NTZ, tizoxanide (TIZ), was not active against staphylococci (6). In the present study, we explored the inhibitory nature of these inhibitors against strains of S. aureus and S. epidermidis. Our studies show that NTZ, as well as TIZ, is inhibitory to the aerobic growth of staphylococcal species, including methicillin-resistant S. aureus (MRSA) strains (MICs, 8 to 16 μg/ml). At sub-MIC levels, NTZ blocks biofilm formation by S. epidermidis, and this inhibition can be reversed by zinc salts in a dose-dependent manner. While the function of the accumulation-associated protein (Aap) in biofilm formation is zinc dependent (4), the findings of our studies rule out simple chelation as a mechanism of drug action. Rather, NTZ appears to affect the assembly or function of surface components associated with biofilm production.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. Unless otherwise specified, the strains were grown in Trypticase soy broth or agar (TSB and TSA, respectively) at 37°C and with shaking for liquid cultures. All strains were stored at −80°C in 15% glycerol.
TABLE 1.
Strains and primers used in this study
| Strain or primer specificity and primer | Genotype, phenotype, or sequencea | Reference or source |
|---|---|---|
| Strains | ||
| Staphylococcus epidermidis | ||
| 9142 | Wild-type PIA-producing strain | 19 |
| CAV1005 | Native valve endocarditis clinical isolate | 37 |
| 5179 | CSF shunt infection isolate; biofilm forming- and PNAG-negative strain, icaA::IS257 | 30 |
| 5179-R1 | Strain 5179 revertant that produces a proteinaceous biofilm dependent on a truncated 140-kDa isoform of Aap, PNAG negative, icaA::IS257 | 30 |
| ICS1 | CRBSI | This study |
| ICS2 | CRBSI | This study |
| ICS3 | CRBSI | This study |
| ICS4 | CRBSI | This study |
| ICS5 | CRBSI | This study |
| Staphylococcus aureus NCTC 8325 | Reference strain | 12 |
| Staphylococcus hominis subsp. novobiosepticus F13532 | Blood culture isolate | 2 |
| Staphylococcus haemolyticus S33208 | Blood culture isolate | This study |
| Primer (5′ → 3′) | ||
| serp0107 | ||
| Serp0107F | TTGAGCTTGTCATTGGTTCG | 16 |
| Serp0107R | TGTAGAGGTTGCACGTCGAG | 16 |
| tuf | ||
| TStaG422 | GGCCGTGTTGAACGTGGTCAAATCA | 21 |
| TStag765 | TIACCATTTCAGTACCTTCTGGTAA | 21 |
Identification of S. epidermidis isolates from catheter-related bloodstream infections.
Infection-causing strains (ICSs) of S. epidermidis were obtained from individuals with a long-term intravenous catheter and a CRBSI, defined by more than one blood culture and a concomitant catheter tip culture with CoNS. The medical records for these patients were reviewed to confirm that the patient exhibited clinical manifestations of a systemic inflammatory response syndrome, the absence of another focus of infection, and the resolution of systemic inflammatory response syndrome within 48 h of catheter removal.
Species identification was performed by one of two methods: either by using the API Staph system (bioMérieux, Durham, NC), according to the manufacturer's instructions, or by genetic testing. The latter was performed by a combination of two methods: PCR identification of the S. epidermidis-specific gene serp0107 (16) and PCR-restriction fragment length polymorphism (RFLP) analysis of the Staphylococcus tuf gene (15). Genomic DNA was prepared by suspending an individual bacterial colony in 20 μl of lysis buffer containing 0.25% SDS and 0.05 N NaOH, incubating the suspension at 95°C for 5 min, and then adding 180 μl of sterile distilled H2O. After centrifugation at 16,100 × g for 1 min to sediment the debris, the supernatant was used as the template DNA for PCR. Samples were kept at −20°C for long-term storage. PCRs were carried out with a Mastercycler gradient thermal cycler (Eppendorf, Westbury, NY). PCR amplification of serp0107 was performed as described previously (16) using the primers listed in Table 1. The presence of a single 581-bp product on 1% agarose gels by electrophoresis was consistent with a species identification of S. epidermidis. To confirm the identification, RFLP analysis of the Staphylococcus tuf gene PCR product was performed, using the method described by Kontos and colleagues (15). Briefly, PCR amplification of the tuf gene yielded a 370-bp amplicon that was confirmed by gel electrophoresis on a 2% agarose gel (15). The product was extracted using a QIAquick gel extraction kit (Qiagen, Valencia, CA), according to the manufacturer's instructions, and subjected to BstZ17I, BseNI, and MseI restriction enzyme digestion. The resulting fragment(s) was resolved by electrophoresis through a 3% agarose gel containing ethidium bromide at 50 V for 45 min and visualized under UV light. Digestion of the tuf gene amplicon of S. epidermidis produces two fragments (243 and 127 bp) using BstZ17I, three fragments (246, 86, and 38 bp) using BseNI, and no restriction using MseI (a single 370-bp product). The presence of these fragments positively identified the strain as S. epidermidis. For both the serp0107 amplification and the tuf RFLP analysis, S. epidermidis strains ATCC 12228 and 9142 served as positive controls, while Staphylococcus haemolyticus F16942, S. aureus 8325, and water alone served as negative controls. S. epidermidis ICSs were identified when (i) the case met the clinical definition of a CRBSI, (ii) concomitant blood and catheter culture isolates were both positively identified as S. epidermidis, and (iii) the blood and catheter isolates had congruent antibiotic susceptibility patterns.
The study protocol was approved by the Institutional Review Board of the University of Virginia Health System.
Determination of MIC.
Testing for the MIC of NTZ was done in sterile round-bottom 96-well microtiter polystyrene plates (Corning Inc., Corning, NY) by microdilution. TIZ (the deacetylated form of NTZ), denitro-TIZ (TIZ without the 5-nitro group), and AMIX (a water-soluble ethylamine derivative of NTZ; Fig. 1) were also tested. The bacteria were grown overnight and suspended in fresh TSB to an optical density at 600 nm (OD600) of 0.01, and 100 μl was dispensed into wells, with the first well containing 200 μl. NTZ and other antibiotics (with a dimethyl sulfoxide [DMSO] control) were added to well 1 and serially diluted from 32 μg/ml. All compounds were tested in triplicate, and the plates were read visually or with a plate reader (Molecular Devices) at 8, 12, and 24 h. The MIC was determined as the concentration in the first well in which no visible bacterial growth was noted relative to the growth of the controls. The effects of the accumulated biofilm were corrected for by transfer of 100-μl aliquots to another microplate. Drug effects on aerobic growth were determined in 125-ml flasks containing 25 ml of TSB medium, and following inoculation (detailed above), the flasks were shaken on a gyratory shaker at 200 rpm at 37°C. The final concentrations of NTZ were 0, 10, and 25 μg/ml. Samples were removed at hourly intervals and diluted, and the turbidity was determined at 600 nm.
Biofilm determinations.
The staphylococcal strains were cultured overnight in TSB at 37°C with shaking and diluted in fresh medium to an OD600 of 0.01, and 100 μl was dispensed into sterile flat-bottom polystyrene 96-well microtiter plates (Costar 3596; Corning Inc.). NTZ, TIZ, denitro-NTZ, and AMIX were tested at different concentrations (0, 5, 10, 15, 20, and 25 μg/ml) with DMSO as a control, as described for MIC testing. Each compound and dilution was tested in triplicate. The plates were incubated overnight at 37°C in a humidified incubator without shaking, and both bacterial growth (turbidity) and biofilm accumulation were determined at 16 h.
Following recording of the turbidities, the culture medium was aspirated and the wells were washed three times with distilled water, blotted on paper towels, and fixed with 75% ethanol for 10 min. To visualize biofilm material, 0.5% crystal violet was added to each well, and after 5 min the crystal violet was removed and the wells were washed three times with distilled water. The plates were read using a microplate reader at 570 nm (Molecular Devices). The dye was then extracted by adding 200 μl of 95% ethanol, and the absorbance was again read at 570 nm. All assays were performed in triplicate, and the mean and standard deviation (SD) were determined. The ethanol-solubilized crystal violet was generally more reliable, and determinations obtained with that material are reported throughout.
Catheter adherence model.
An overnight culture of S. epidermidis 9142 was used to inoculate fresh TSB medium to a final OD600 of 0.01 in a volume of 3 ml in 15-ml screw-cap tubes. Sterile sheets of polyurethane were divided into 1-cm-square pieces and placed into tubes containing different concentrations of NTZ or controls containing DMSO. The tubes were incubated for 24 h with shaking. Each catheter portion was washed three times with sterile PBS, suspended in 4 ml sterile PBS, and sonicated for 10 min in a sonic water bath. Ten microliters of the sonicated fluid was diluted in fresh PBS and plated onto TSA plates, and the plates were incubated at 37°C overnight. The bacteria were enumerated (triplicate plates), and the mean and standard deviation were determined and reported as the numbers of CFU/ml. Viability effects were corrected for by plating the supernatants from the experiment with 3 ml for total bacterial counts.
NTZ effect on biofilm dispersal.
The effect of NTZ on biofilm accumulation or the dispersal of existing biofilms was determined in microtiter dishes in which S. epidermidis 9142 was allowed to grow for 8 h prior to drug treatment. In this experiment, duplicate flat-bottom plates were inoculated with 200 μl of a bacterial suspension diluted in fresh TSB to an OD600 of 0.01 and incubated statically at 37°C. The first three rows of each plate contained no drug, while the subsequent rows contained NTZ (2.5, 5, 10, and 15 μg/ml). At 8 h, one plate was developed for biofilm determination, as described above, while the medium in the replicate plate was aspirated and replaced with fresh TSB, with the first three rows thus containing NTZ at 5, 10, and 15 μg/ml and the remaining wells containing the same concentration of NTZ. These plates were developed at 24 h, and the relative amount of biofilm for each set was compared.
Direct attachment assay.
To determine if NTZ directly inhibited attachment of the bacteria to polystyrene, S. epidermidis strain 9142 was grown overnight in TSB medium with shaking in 125-ml flasks in the presence or absence of 15 μg/ml NTZ. In some experiments, the overnight growth was diluted to an OD600 of 0.1 in fresh TSB medium and grown exponentially (shaking or static and in the presence or absence of 15 μg/ml NTZ) for 3 h. Under each condition, the bacteria were harvested by centrifugation and suspended in phosphate-buffered saline (PBS) to an OD600 of 0.01, and 200 μl was added to six-well polystyrene microtiter plates (Nulcon; Nunc AIS, Denmark). After 1 h of incubation at 4 and 37°C, the plates were washed with PBS (five times with 5 ml each time) to remove nonadherent bacteria, and the adherent bacteria were visualized microscopically (directly or following staining with crystal violet) with an Axiovert 200 inverted microscope (Carl Zeiss Inc., Thornwood, NY). The number of adherent bacteria per field (multiple fields) was video recorded and analyzed using Image Pro Plus software (Media Cybernetics, Bethesda, MD). The means and SDs were computed from the values for at least three wells and three multiple fields and were analyzed statistically (t test).
Effect of zinc on action of NTZ.
To assess whether ZnSO4 or ZnCl2 could overcome the inhibitory action of NTZ on biofilm production, standard biofilm assays were established at an NTZ concentration of 12.5 μg/ml, which is sufficient to abolish biofilm formation by S. epidermidis strain 9142 (∼2× 50% inhibitory concentration [IC50]). A 40 μM ZnSO4 solution was serially diluted in wells containing NTZ (in triplicate), and biofilm production was assessed at 24 h with crystal violet. As a control, ZnSO4 was added to untreated bacteria. Additional controls included testing of 40 μM CaCl2 and MgCl2 in the same format. The inhibitory effect of EDTA on biofilm production and its reversibility by Zn2+ were also assessed as described for NTZ. The effect of Zn2+ on the MICs of NTZ and AMIX was evaluated by microdilution in TSB with a fixed concentration of ZnCl2 at 20 μM (in triplicate). The turbidities were measured at 8 and 24 h, and means and standard deviations are reported.
Spectrophotometric chelation assay.
To test the direct binding of Zn2+ (Cl22− or SO42−) by NTZ in solution, we tested a range of concentrations of both NTZ and TIZ and scanned the solutions spectrophotometrically using a Cary-14 spectrophotometer (OLIS Instruments Co., Bogart, GA). The absolute spectra were recorded over a UV/visible range from 220 nm to 700 nm. Spectral shifts in the 418-nm range would be attributable to changes in resonance within the thiazole ring that would result if Zn2+ were coordinated by NTZ (9).
1H NMR.
Chelation experiments were also performed utilizing NTZ and TIZ in DMSO-d6 (0.5 ml) with ZnCl2 using a 300-MHz Varian MercuryPlus spectrometer. Specifically, three ratios of NTZ to Zn2+ were investigated (1:1, 1:2, 2:1), where the amount of NTZ was held constant (4 mg, 13 μmol) while the amount of ZnCl2 was varied (1.8 mg, 13 μmol, or 3.5 mg, 26 μmol) or the amounts of both NTZ (8 mg, 26 μmol) and ZnCl2 (1.8 mg, 13 μmol) were held constant. Three ratios of TIZ to Zn2+ were also investigated (1:1, 1:2, 2:1), where the amount of TIZ was held constant (4 mg, 15 μmol) while the amount of ZnCl2 was varied (2.1 mg, 15 μmol, or 4.1 mg, 30 μmol) or the amounts of both TIZ (8 mg, 30 μmol) and ZnCl2 (2.1 mg, 15 μmol) were held constant. The 1H nuclear magnetic resonance (NMR) spectra were then obtained after mixing of the components for 5 min, 24 h, 48 h, and 72 h at room temperature. Any chelation event would be evidenced by shifting or broadening of the thiazole and benzene protons as well as loss of the amide proton.
RESULTS
MIC testing of S. aureus and S. epidermidis strains.
The inhibitory effect of NTZ on the staphylococcal strains was first investigated by determining the MIC. Our studies showed that most strains tested were susceptible to NTZ and TIZ and had MICs of 8 to 16 μg/ml (Fig. 2 A), indicating that these strains are generally more susceptible to this drug than was previously reported (6). In contrast to previous studies indicating that the drug was active under anaerobic conditions (6), we found that NTZ and the active metabolite, TIZ, inhibited the growth of S. epidermidis in liquid culture under vigorous aeration in a dose-dependent manner, with significant inhibition occurring at 25 μg/ml (Fig. 2B). At lower drug concentrations (10 μg/ml), there was little effect on exponential growth rates. Under static (microaerobic) growth conditions in liquid culture, biofilm accumulation on the bottom of the flasks was completely ablated by 10 μg/ml of NTZ, while the final bacterial turbidities were only slightly decreased compared with those for the drug-free controls (data not presented). These observations suggest that subinhibitory concentrations of NTZ may also affect the production of biofilm.
FIG. 2.
NTZ inhibition of S. epidermidis growth and biofilm production. (A) MIC testing. Biofilm-forming strain 9142 (▪) and non-biofilm-forming strains 5179 (⧫) and CAV1005 (•) were tested for their susceptibilities to NTZ by microdilution, and bacterial growth was measured by measurement of the turbidity, as described in the text. (B) Aerobic growth. Bacteria were grown in TSB medium with shaking at 37°C in the presence of no NTZ (⧫), 10 μg/ml NTZ (▪), or 25 μg/ml NTZ ▴). (C) Biofilm inhibition by NTZ. Staphylococcus strains were grown in the presence of different concentrations of NTZ in 96-well polystyrene plates and subjected to crystal violet staining after 24 h, as described in the text. The biofilm-positive strains (see the key) used were S. epidermidis 9142 and S. haemolyticus S33208, and the biofilm-negative strains were S. hominis F13532 and S. epidermidis CAV1005. The IC50 for biofilm-producing strains was 2.5 μg/ml.
NTZ inhibits biofilm production at sub-MIC levels.
To further explore the effects of the drug at sub-MICs on biofilm accumulation, we screened several CoNS species for biofilm production. As seen in Fig. 2C, NTZ inhibited biofilm production by S. epidermidis strain 9142 and S. haemolyticus strain 33208 in a dose-dependent manner, as determined by crystal violet staining. Biofilm inhibition was not due to growth inhibition (Fig. 2A), although bacterial growth was decreased at 8- and 16-μg/ml drug concentrations. The IC50 was determined to be 1 to 3 μg/ml (∼3 to 10 μM). Two additional strains tested (Fig. 2C) were non-biofilm-producing strains. While the results are not depicted, the deacetylated form of NTZ (TIZ) gave results equivalent to those for NTZ, while the denitro form of the drug did not inhibit biofilm production and exhibited an MIC of >32 μg/ml (data not presented).
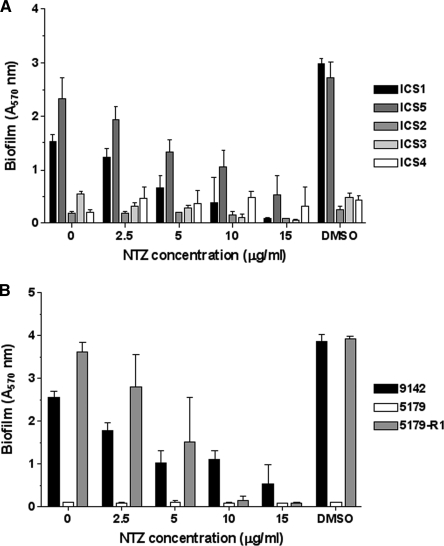
Screening NTZ activity against S. epidermidis strains.
To determine if the antibiofilm activity of NTZ at sub-MICs was more generalized, we screened a collection of clinical S. epidermidis isolates obtained from patients with CRBSIs (see Table 1 for strain details). As seen in Fig. 3 A, NTZ inhibited biofilm formation by all clinical isolates that produced a biofilm in the absence of the drug in a dose-dependent manner. Furthermore, NTZ inhibited biofilm formation by S. epidermidis strain 5179-R1 (Fig. 3B), which generates a PNAG-independent, protein-rich biofilm that is dependent on the production of a proteolytically processed derivative of Aap (30). For all strains tested, the IC50 ranged from 1 to 5 μg/ml and the MIC was 16 μg/ml.
FIG. 3.
Screening for antibiofilm activity. (A) Clinical isolates confirmed to be S. epidermidis were tested for biofilm production and for concentration-dependent biofilm inhibition by NTZ at the indicated concentrations, with DMSO serving as an additional control. Strains ICS1 and ICS5 were biofilm positive. (B) Concentration-dependent inhibition of biofilm production of S. epidermidis strain 9142, icaA mutant strain 5179, and revertant strain 5179-R1, which produces a truncated Aap. The data presented represent the means and standard deviations of three replicates.
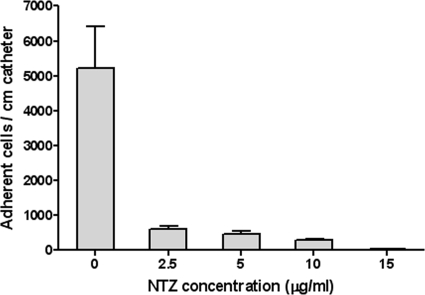
NTZ inhibits bacterial accumulation on catheters.
Several studies have indicated that bacterial attachment and biofilm accumulation are distinct but interrelated activities (7). To test whether NTZ interfered with attachment, biofilm-producing strains of S. epidermidis were scored for attachment to plastic squares, derived from catheter material, in the presence or absence of NTZ. As seen in Fig. 4, NTZ inhibited the attachment of S. epidermidis strain 9142 in a dose-dependent manner and within the IC50 range. Since the attachment to catheter pieces was conducted over a 24-h period under permissive growth conditions, it is not possible to distinguish between primary attachment and attachment and subsequent accumulation.
FIG. 4.
Effect of NTZ on attachment to catheter material. One-centimeter-square polyurethane pieces were incubated with the indicated concentrations of NTZ and S. epidermidis strain 9142, and the adherent bacteria were enumerated at 24 h. Means and standard deviations were determined from triplicates.
NTZ does not inhibit primary attachment to plastic.
To address primary attachment to plastic more directly, bacteria were collected following overnight culture in TSB medium and were tested either directly or following 3 h of outgrowth under shaking or static conditions. Adherent bacteria were enumerated after 1 h of incubation at 4 and 37°C. Since temperature was not a variable, the results depicted in Table 2 are those obtained at 37°C. Bacterial attachment was not appreciably affected by the presence of NTZ during primary attachment (P = 0.157). Similarly, inclusion of 15 μg/ml NTZ during outgrowth for 3 h under static or shaking conditions did not significantly alter the attachment efficiency. While there was a slight effect by NTZ under static conditions, the result was not considered significant, as static growth conditions or a slight NTZ effect might have contributed to the count. Since zinc contributes to improved accumulation via Aaps, we tested whether 10 μM zinc sulfate or zinc chloride might improve the attachment of the bacteria in the presence of NTZ. Zinc salts (the results for the sulfate are shown) had no effect on attachment (P = 0.49). Generally, addition of zinc back to the putative apo-Aap should result in bacterial clumping, but this was not observed in these experiments. These studies indicate that NTZ does not interfere with primary attachment, which appears to be independent of the mechanisms associated with biofilm formation.
TABLE 2.
Primary attachment to plastica
| Time of assessment and growth condition | Microscopic count (mean ± SD) | P |
|---|---|---|
| Primary attachment | ||
| −NTZ | 314 ± 10 | |
| +NTZ | 300 ± 16 | 0.157 |
| After 3 h growth (shaken) | ||
| −NTZ | 423 ± 36 | |
| +NTZ | 342 ± 40 | 0.263 |
| After 3 h growth (static) | ||
| −NTZ | 324 ± 95 | 0.062 |
| +NTZ | 243 ± 76 | |
| +NTZ + 10 μM Zn2+ | 310 ± 7 | 0.49 |
S. epidermidis strain 9142 was grown overnight in TSB with shaking, diluted to an OD600 of 0.01 in fresh medium, and grown for 3 h (with or without shaking). The bacteria were suspended in PBS to an OD600 of 0.1, and 1 ml was added to six-well plates, as described in the text. NTZ was added to the wells at 15 μg/ml, and ZnSO4 was added at 10 μM. −NTZ, no NTZ added; +NTZ, NTZ added. The adherent bacteria were enumerated microscopically, and mean and SD values were computed from multiple fields of triplicates. Data were analyzed by a one-tail t test. None of the listed P values was considered significant.
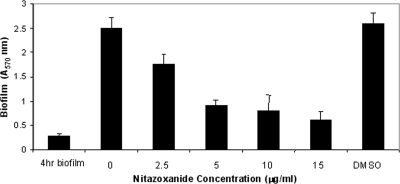
Does NTZ affect biofilm production by colonized bacteria?
We next investigated whether NTZ was inhibitory to biofilm production by established S. epidermidis communities. If NTZ is a specific inhibitor of accumulation, the drug should inhibit further deposition of the biofilm in established communities. To test this hypothesis, we allowed S. epidermidis bacteria to establish a biofilm in the absence of drug for 4 h, and following washings to remove nonadherent bacteria, NTZ (over a range of concentrations) was added in fresh TSB medium for an additional 12 h. As seen in Fig. 5, the amount of biofilm produced at 4 h was ca. 0.5 absorbance units by the crystal violet assay; and by the end of the assay, the value for the biofilm accumulated in the non-drug-treated well had increased to 3 absorbance units. While the amount of biofilm also increased in the NTZ-treated wells, there was a drug-dependent effect on the further accumulation of biofilm material. However, drug treatment did not eliminate the preformed biofilm, indicating that NTZ was not dispersive.
FIG. 5.
Biofilm dispersal by NTZ. S. epidermidis strain 9142 bacteria were grown under biofilm-producing conditions for 4 h. Following washing of the bacteria, fresh medium and the indicated concentrations of NTZ were added for an additional 16 h, and biofilm accumulation was determined with crystal violet. NTZ inhibited further biofilm formation in a concentration-dependent manner but did not disperse the existing biofilm.
Reversal of NTZ inhibition by zinc.
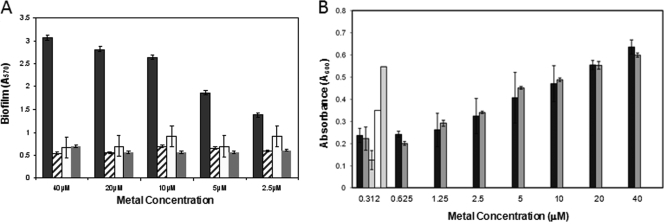
Recent studies have shown that the surface proteins Aap of S. epidermidis and SasG of S. aureus promote the intercellular adhesion of staphylococcal bacteria and that metal chelators like EDTA can inhibit biofilm production (4, 5, 13). Studies by Conrady et al. (4) have shown that Aaps contain G5-domain repeats that mediate the associative accumulation of bacteria onto surfaces in a Zn2+-dependent manner. These domains bind Zn2+, which is hypothesized to enable Zn2+ zipper protein interactions to entwine protein ribbons in adherent bacterial aggregates on surfaces. To test whether NTZ affects the function of the Aaps of S. epidermidis, we added increasing concentrations of ZnSO4 or ZnCl2 to the biofilm assays. As seen in Fig. 6 A, the addition of Zn2+ to the standard biofilm assay reversed the inhibitory action of NTZ in a dose-dependent manner. While the result is not depicted, EDTA also inhibited biofilm production, and similar to the action of NTZ, the action could be reversed by zinc (4, 13). The effects of Zn2+ were specific, as addition of other metals (calcium and magnesium) did not reverse the inhibition (Fig. 6A). While NTZ seems to achieve the same phenotype as treatment with EDTA, we could not distinguish between whether NTZ acted directly as a Zn2+ chelator or NTZ was bound to the G5 domains.
FIG. 6.
Effect of zinc on bacterial growth and biofilm production. (A) Biofilm. Zinc sulfate (black bars), calcium chloride (hatched bars), and magnesium chloride (white bars) were added at the indicated concentrations to TSB containing a fixed concentration of NTZ (12.5 μg/ml; gray bars). Zinc chloride, but not the other metals, reversed the biofilm-inhibitory effect of NTZ, with 50% reversal occurring at ∼5 μM. (B) Growth. Effect of the following metals or conditions on bacterial growth at a fixed concentration of NTZ (12.5 μg/ml): ZnSO4 (black bars), ZnCl2 (dark gray bars), no metal (light gray bar), DMSO control (white bar), and no NTZ (dark gray bar).
NTZ and TIZ do not chelate Zn2+.
To address whether Zn2+ is directly chelated by NTZ (TIZ) and alters the potency of the drug, we scanned putative NTZ/TIZ-Zn2+ coordination complexes for any spectral changes that might result from changes in ring resonance due to bound ligand. On the basis of the results of NMR studies, the amino thiazole moiety of NTZ in the anionic form simultaneously exists in several resonance states, which contributes to the absorption maxima at 418 nm (9). Metal coordination would be predicted to alter resonance, and this should manifest in a spectral shift. Spectral scans performed at different concentrations of NTZ and TIZ with Zn2+ did not reveal any changes in the absorption spectrum (data not presented). To further explore NTZ-metal interactions, putative NTZ and TIZ complexes with Zn2+ were analyzed by 1H NMR spectroscopy in DMSO-d6. No chelation with ZnCl2 was evident from any ratios tested, even after the 72-h time point, with NTZ. All spectra remained identical and unchanged throughout the course of the experiment. Tizoxanide also displayed no chelation with ZnCl2 under the experimental conditions used. Taken together, these studies indicate that these drugs did not chelate zinc under the assay conditions used.
Effect of Zn2+ on bacterial growth.
To test the possibility that the action of Zn2+ on ablation of the biofilm by NTZ might result from reversal of the growth-inhibitory effect of the drug, the bacterial turbidities at 16 h were determined at a fixed concentration of NTZ of 12.5 μg/ml. As seen in Fig. 6B, Zn2+ enhances the growth of S. epidermidis strain 9142 in a dose-dependent manner. MIC testing at a fixed concentration of Zn2+ (10 μM) showed a shift in the MIC from 16 μg/ml to 32 μg/ml (data not presented). We also noted that Zn2+ supplementation of the TSB medium improved bacterial growth, as indicated by slightly higher final turbidities at 16 h (A600 of 0.65 for controls to 0.82 with Zn2+). To test whether enhanced growth might lead to breakthrough, we repeated the MIC experiments over a shorter period. Zn2+ had no effect on the inhibitory action of NTZ up to 10 h, suggesting that zinc might affect other bacterial systems unrelated to biofilm production that promote breakthrough growth, thus contributing to biofilm production. It should be noted that similar studies with sub-MIC levels of vancomycin showed no effect by zinc (data not presented).
Hydrophobicity effects.
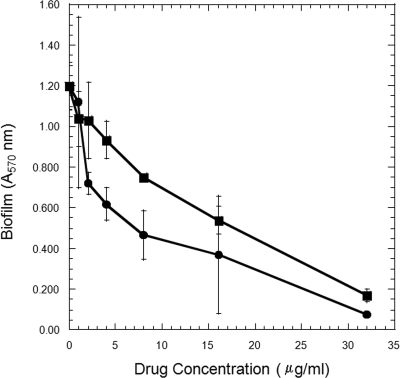
NTZ is hydrophobic and sparingly soluble in water, and its deacylated form (TIZ) is 97.5% bound to plasma proteins (34). It is possible, therefore, that the action of the drug is nonspecific and results from binding onto surface proteins and ablating their action. To test this possibility, we synthesized a more soluble derivative of NTZ (AMIX) by adding an ethylamine R group to the benzene ring of TIZ. The drug retained bioactivity with an MIC of 16 to 32 μg/ml. This derivative was soluble at 400 μg/ml in water (NTZ concentration < 32 μg/ml) and, as seen in Fig. 7, exhibited a slightly higher IC50 than NTZ (8 and 4 μg/ml, respectively, in this experiment). The 2-fold difference in biofilm inhibition was less than the 15-fold difference in solubility. Taken together, these studies suggest that the action of NTZ in blocking accumulation and biofilm production is most likely specific.
FIG. 7.
Comparative antibiofilm activities of NTZ and AMIX. The inhibition of biofilm production by S. epidermidis strain 9142 by NTZ (•) and water-soluble AMIX (▪) was concentration dependent. All assays were performed in triplicate, with means and standard deviations being presented.
DISCUSSION
We have determined that the antiparasitic drug nitazoxanide and its active metabolite, tizoxanide, are inhibitory to the in vitro growth of various staphylococcal species, including MRSA strains. This contrasts with an earlier report that TIZ was not active against staphylococci and that the action of NTZ was limited to anaerobic growth conditions (6). In the current study, we demonstrated that NTZ inhibited the growth of S. epidermidis strain 9142 in highly aerated broth cultures at 25 μg/ml but that little inhibition was observed at concentrations at or below 10 μg/ml. We found by MIC tests NTZ to be slightly more active than TIZ, which might be attributed to slight differences in solubility, while denitro-NTZ was biologically inert. Our studies further demonstrate that at sub-MICs (1 to 5 μg/ml), NTZ and TIZ inhibited biofilm production in a static microtiter plate assay. Under static conditions in flasks, NTZ at 10 μg/ml ablated biofilm production, with only a slight decrease in the final bacterial turbidity being noted, and there was no discernible difference in primary attachment to plastic. In comparison with untreated bacteria, NTZ-treated bacteria did not clump and appeared to be more dispersed by microscopic examination. The antibiofilm activity of NTZ could be reversed by zinc salts (IC50 = 5 μM) but not by Ca2+ or Mg2+ and was growth dependent. The zinc effect seems to be unique to NTZ and TIZ, as zinc had no effect on the sub-MIC action of vancomycin on growth or biofilm formation. The possibility that zinc inactivated NTZ or formed chelates could not be demonstrated by optical spectrophotometry or by NMR.
One possible candidate for the action of NTZ is the zinc-dependent Aap, which has been extensively studied and which was recently shown to mediate the intercellular adhesion of S. epidermidis on surfaces (4). In this regard, purified Aap monomers associated into aggregates in the presence of Zn2+, and this association was mediated by G5 domains through the formation of zinc zippers (4). The Aap target was initially considered because NTZ inhibited biofilm production by S. epidermidis strain 5179-R1, which produces only a proteinaceous biofilm composed of Aap (30). However, we found no evidence for the chelation of Zn2+ by NTZ, as is the case for the antibiofilm action of EDTA and other metal chelators or by low pH (4, 13). Similarly, NTZ does not appear to demetal Aap surface proteins, since experiments in which zinc is added back to NTZ-grown bacteria did not restore the spontaneous aggregation (clumping) of bacteria in solution that is attributed to Aap (4). While the absence of Aap function might suggest that NTZ affects aap gene expression or a later step in the assembly of Aap, we could not rule out the possibility that these hydrophobic drugs might bind nonspecifically to surface components.
To address the possibility of nonspecific binding to surface proteins, polysaccharides, or teichoic acids through hydrophobic or ionic interactions (36), we tested a more hydrophilic derivative of NTZ (AMIX) and found that it had inhibitory antibiofilm action similar to that of NTZ. The findings of these studies lead us to suggest that NTZ affects either the regulation of biofilm gene expression (aap and ica) or some step in function required for the phenotype of biofilm formation. The regulatory view is supported by the fact that accumulation mediated by Aap and the polymerization and secretion of polysaccharide biofilm material by the Ica pathway are distinctly different. The possibility that a secretion step is targeted by NTZ is supported by previous studies that sub-MIC levels of NTZ affected the secretion of VacA cytotoxin by Helicobacter pylori (38). In Escherichia coli, NTZ reportedly ablates the assembly of fimbrial adhesins (AafA and type I) (32). Studies are in progress to assess the effects of NTZ on gene expression and on the elaboration of surface proteins.
Coating of catheters with antimicrobial agents has been shown to delay or prevent microbial colonization and to extend their useful life span (1, 3, 13, 20). In simulated catheter experiments under growth-promoting conditions, we showed that NTZ blocked the attachment or, more likely, the accumulation of bacteria onto catheter surfaces but did not display any biofilm-dispersive activity. The prevention of further accumulation is consistent with the postulate that the drug affects biofilm production in general. While NTZ has poor pharmacological properties, it is likely that less hydrophobic derivatives, such as AMIX, might prove more efficacious in treating systemic infections. AMIX is less toxic (than NTZ) to staphylococci by MIC testing, while it retains antibiofilm activity. Moreover, the inhibitory action of AMIX was not reversed by Zn2+ salts, raising the possibility that the noted effects of Zn2+ on NTZ potency might be related to drug uptake or efflux activities, where differences in hydrophobicity would likely manifest.
The primary attachment to and accumulation of microorganisms on surfaces is an essential step in the infection process, and conceptually, targeting these requisite virulence determinants might provide an additional strategy for limiting or reducing the severity of infections. Our findings extend early observations of Dubreuil et al. by demonstrating that NTZ and its derivatives are more potent against staphylococcal species, including MRSA strains under aerobic as well as microaerobic conditions, than was previously considered (6). Since we have synthesized derivatives of NTZ in which the MIC and IC50 are the same (∼1 μg/ml) and assuming that the target is unchanged, we suggest that NTZ must target an essential function where the measured differences between MIC and IC50 can be explained by the relative affinities of the drug for its target. Since the interactions of staphylococci with surfaces are complex and involve many factors and surface materials, the effects of NTZ on the phenotype of biofilm formation may not be direct.
Acknowledgments
We thank Gina Devasahayam for assistance with the microscopic enumeration studies and to Michelle Warthan and Xia Wang for technical assistance.
This work was supported by U01 grant AI075520 from the National Institute of Allergy and Infectious Diseases to P.S.H. F.T.-N. was supported by ID Training Grant 5T32AI007046-33.
Footnotes
Published ahead of print on 19 April 2010.
REFERENCES
- 1.Aslam, S., B. W. Trautner, V. Ramanathan, and R. O. Darouiche. 2007. Combination of tigecycline and N-acetylcysteine reduces biofilm-embedded bacteria on vascular catheters. Antimicrob. Agents Chemother. 51:1556-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Begun, J., J. M. Gaiani, H. Rohde, D. Mack, S. B. Calderwood, F. M. Ausubel, and C. D. Sifri. 2007. Staphylococcal biofilm exopolysaccharide protects against Caenorhabditis elegans immune defenses. PLoS Pathog. 3:e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cerca, N., S. Martins, S. Sillankorva, K. K. Jefferson, G. B. Pier, R. Oliverira, and J. Azeredo. 2005. Effects of growth in the presence of subinhibitory concentrations of dicloxacillin on Staphylococcus epidermidis and Staphylococcus haemolyticus biofilms. Appl. Environ. Microbiol. 71:8677-8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conrady, D. G., C. C. Brescia, K. Horii, A. A. Weiss, D. J. Hassett, and A. B. Herr. 2008. A zinc-dependent adhesion module is responsible for intercellular adhesion in staphylococcal biofilms. Proc. Natl. Acad. Sci. U. S. A. 105:19456-19461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corrigan, R. M., D. Rigby, P. Handley, and T. J. Foster. 2007. The role of Staphylococcus aureus surface protein SasG in adherence and biofilm formation. Microbiology 153:2435-2446. [DOI] [PubMed] [Google Scholar]
- 6.Dubreuil, L., I. Houcke, Y. Mouton, and J. F. Rossignol. 1996. In vitro evaluation of activities of nitazoxanide and tizoxanide against anaerobes and aerobic organisms. Antimicrob. Agents Chemother. 40:2266-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heilmann, C., O. Schweitzer, C. Gerke, N. Vanittanakom, D. Mack, and F. Gotz. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20:1083-1091. [DOI] [PubMed] [Google Scholar]
- 8.Hemphill, A., J. Mueller, and M. Esposito. 2006. Nitazoxanide, a broad-spectrum thiazolide anti-infective agent for the treatment of gastrointestinal infections. Expert Opin. Pharmacother. 7:953-964. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman, P. S., G. Sisson, M. A. Croxen, K. Welch, W. D. Harman, N. Cremades, and M. G. Morash. 2007. Antiparasitic drug nitazoxanide inhibits the pyruvate oxidoreductases of Helicobacter pylori, selected anaerobic bacteria and parasites, and Campylobacter jejuni. Antimicrob. Agents Chemother. 51:868-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huigens, R. W., III, S. A. Rogers, A. T. Steinhauer, and C. Melander. 2009. Inhibition of Acinetobacter baumannii, Staphylococcus aureus and Pseudomonas aeruginosa biofilm formation with a class of TAGE-triazole conjugates. Org. Biomol. Chem. 7:794-802. [DOI] [PubMed] [Google Scholar]
- 11.Hussain, M., M. Herrmann, C. von Eiff, F. Perdreau-Remington, and G. Peters. 1997. A 140-kilodalton extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces. Infect. Immun. 65:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iandolo, J. J. 2000. Genetic and physical map of the chromosome of Staphylococcus aureus 8325, p. 317-325. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. A. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, DC.
- 13.Juda, M., K. Paprota, D. Jałoza, A. Malm, P. Rybojad, and K. Goździuk. 2008. EDTA as a potential agent preventing formation of Staphylococcus epidermidis biofilm on polychloride vinyl biomaterials. Ann. Agric. Environ. Med. 15:237-241. [PubMed] [Google Scholar]
- 14.Klevens, R. M., J. R. Edwards, C. L. Richards, Jr., T. C. Horan, R. P. Gaynes, D. A. Pollock, and D. M. Cardo. 2007. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 122:160-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kontos, F., E. Petinaki, I. Spiliopoulou, M. Maniati, and A. N. Maniatis. 2003. Evaluation of a novel method based on PCR restriction fragment length polymorphism analysis of the tuf gene for the identification of Staphylococcus species. J. Microbiol. Methods 55:465-469. [DOI] [PubMed] [Google Scholar]
- 16.Liu, D., E. Swiatlo, F. W. Austin, and M. L. Lawrence. 2006. Use of a putative transcriptional regulator gene as target for specific identification of Staphylococcus epidermidis. Lett. Appl. Microbiol. 43:325-330. [DOI] [PubMed] [Google Scholar]
- 17.Mack, D., W. Fischer, A. Krokotsch, K. Leopold, R. Hartmann, H. Egge, and R. Laufs. 1996. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol. 178:175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mack, D., M. Nedelmann, A. Krokotsch, A. Schwarzkopf, J. Heesemann, and R. Laufs. 1994. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infect. Immun. 62:3244-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mack, D., N. Siemssen, and R. Laufs. 1992. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect. Immun. 60:2048-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maki, D. G., S. M. Stolz, S. Wheeler, and L. A. Mermel. 1997. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann. Intern. Med. 127:257-266. [DOI] [PubMed] [Google Scholar]
- 21.Martineau, F., F. J. Picard, D. Ke, S. Paradis, P. H. Roy, M. Ouellette, and M. G. Bergeron. 2001. Development of a PCR assay for identification of staphylococci at genus and species levels. J. Clin. Microbiol. 39:2541-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McVay, C. S., and R. D. Rolfe. 2000. In vitro and in vivo activities of nitazoxanide against Clostridium difficile. Antimicrob. Agents Chemother. 44:2254-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mermel, L. A. 2000. Prevention of intravascular catheter-related infections. Ann. Intern. Med. 132:391-402. [DOI] [PubMed] [Google Scholar]
- 24.Müller, J., D. Sidler, U. Nachbur, J. Wastling, T. Brunner, and A. Hemphill. 2008. Thiazolides inhibit growth and induce glutathione-S-transferase Pi (GSTP1)-dependent cell death in human colon cancer cells. Int. J. Cancer 123:1797-1806. [DOI] [PubMed] [Google Scholar]
- 25.Müller, J., J. Wastling, S. Sanderson, N. Müller, and A. Hemphill. 2007. A novel Giardia lamblia nitroreductase, GlNR1, interacts with nitazoxanide and other thiazolides. Antimicrob. Agents Chemother. 51:1979-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musher, D. M., N. Logan, A. M. Bressler, D. P. Johnson, and J. F. Rossignol. 2009. Nitazoxanide versus vancomycin in Clostridium difficile infection: a randomized, double-blind study. Clin. Infect. Dis. 48:41-46. [DOI] [PubMed] [Google Scholar]
- 27.National Nosocomial Infections Surveillance. 2004. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1992 through June 2004, issued October 2004. Am. J. Infect. Control 32:470-485. [DOI] [PubMed] [Google Scholar]
- 28.Pankuch, G. A., and P. C. Appelbaum. 2006. Activities of tizoxanide and nitazoxanide compared to those of five other thiazolides and three other agents against anaerobic species. Antimicrob. Agents Chemother. 50:1112-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parsek, M. R., and P. K. Singh. 2003. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 57:677-701. [DOI] [PubMed] [Google Scholar]
- 30.Rohde, H., C. Burdelski, K. Bartscht, M. Hussain, F. Buck, M. A. Horstkotte, J. K. Knobloch, C. Heilmann, M. Herrmann, and D. Mack. 2005. Induction of Staphylococcus epidermidis biofilm formation via proteolytic processing of the accumulation-associated protein by staphylococcal and host proteases. Mol. Microbiol. 55:1883-1895. [DOI] [PubMed] [Google Scholar]
- 31.Rupp, M. E., J. S. Ulphani, P. D. Fey, and D. Mack. 1999. Characterization of Staphylococcus epidermidis polysaccharide intercellular adhesin/hemagglutinin in the pathogenesis of intravascular catheter-associated infection in a rat model. Infect. Immun. 67:2656-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shamir, E. R., M. Warthan, S. P. Brown, J. P. Nataro, R. L. Guerrant, and P. S. Hoffman. 2010. Nitazoxanide inhibits biofilm production and hemagglutination by enteroaggregative Escherichia coli strains by blocking assembly of AafA fimbriae. Antimicrob. Agents Chemother. 54:1526-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sisson, G., A. Goodwin, A. Raudonikiene, N. J. Hughes, A. K. Mukhopadhyay, D. E. Berg, and P. S. Hoffman. 2002. Enzymes associated with reductive activation and action of nitazoxanide, nitrofurans, and metronidazole in Helicobacter pylori. Antimicrob. Agents Chemother. 46:2116-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stockis, A., X. Deroubaix, R. Lins, B. Jeanbaptiste, P. Calderon, and J. F. Rossignol. 1996. Pharmacokinetics of nitazoxanide after single oral dose administration in 6 healthy volunteers. Int. J. Clin. Pharmacol. Ther. 34:349-351. [PubMed] [Google Scholar]
- 35.von Eiff, C., G. Peters, and C. Heilmann. 2002. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect. Dis. 2:677-685. [DOI] [PubMed] [Google Scholar]
- 36.Vuong, C., and M. Otto. 2002. Staphylococcus epidermidis infections. Microbes Infect. 4:481-489. [DOI] [PubMed] [Google Scholar]
- 37.Winchester, D. E., M. J. Lipinski, M. E. Cupp, and C. D. Sifri. 2008. Not so innocuous. Am. J. Med. 121:855-857. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto, Y., A. Hakki, H. Friedman, S. Okubo, T. Shimamura, P. S. Hoffman, and J. F. Rossignol. 1999. Nitazoxanide, a nitrothiazolide antiparasitic drug, is an anti-Helicobacter pylori agent with anti-vacuolating toxin activity. Chemotherapy 45:303-312. [DOI] [PubMed] [Google Scholar]