Abstract
Ritonavir-boosted darunavir with efavirenz may be considered a nucleoside-sparing regimen for treatment-naïve HIV-infected patients. However, the pharmacokinetics of this combination administered once daily have not been studied. We conducted a three-period interaction study with healthy volunteers. The subjects were given darunavir at 900 mg with ritonavir at 100 mg once daily for 10 days. Efavirenz at 600 mg once daily was added for 14 days. Darunavir-ritonavir was then stopped and efavirenz alone was given for 14 days. At the end of each period, blood was taken predosing and for up to 24 h postdosing to measure the drug concentrations. We recruited seven males and five females ages 24 to 49 years and weighing 50 to 83 kg. The darunavir trough concentrations were reduced after efavirenz administration (geometric mean ratio [GMR], 0.43; 90% confidence interval [CI], 0.32 to 0.57]; P < 0.001). The mean darunavir trough concentrations were 1,180 ng/ml (standard deviation, 1,138 ng/ml) after efavirenz administration, but all darunavir trough concentrations were above the 50% effective concentration (EC50) of 55 ng/ml for the wild-type virus. For darunavir, the area under the concentration-time curve from 0 to 24 h (AUC0-24) (GMR, 0.86; 90% CI, 0.75 to 0.97; P = 0.05) and the half-life (GMR, 0.56; 90% CI, 0.49 to 0.65; P < 0.001) were also significantly reduced. The darunavir peak concentrations were not significantly changed (GMR, 0.92; 90% CI, 0.82 to 1.03; P = 0.23). The ritonavir trough concentrations (GMR, 0.46; 90% CI, 0.33 to 0.63; P = 0.001), AUC0-24 (GMR, 0.74; 90% CI, 0.64 to 0.86; P = 0.004), and half-life (GMR, 0.80; 90% CI, 0.75 to 0.86; P < 0.001) were also significantly reduced. The efavirenz half-life was significantly longer when it was coadministered with darunavir-ritonavir than when it was given alone (GMR, 1.66; 90% CI, 1.24 to 2.23; P = 0.01), but there were no differences in the efavirenz trough or peak concentration or AUC0-24 when it was coadministered with darunavir-ritonavir. Efavirenz reduced the trough concentrations of darunavir significantly, but the concentrations remained above the EC50 for the wild-type virus. This regimen should be evaluated with treatment-naïve patients with no preexisting resistance.
The treatment of HIV infection has been revolutionized by the advent of combination antiretroviral therapy (ARV), which suppresses viral replication and preserves immunological function. Currently recommended first-line combination therapy consists of two nucleoside reverse transcriptase inhibitors with either efavirenz or a ritonavir-boosted protease inhibitor (PI) (9).
Much interest in the study of nucleoside-sparing regimens has been generated, in view of the toxicities of nucleosides and the development of resistance. The pharmacokinetic interactions between efavirenz and PIs have been studied (11, 12). Efavirenz induces cytochrome P450 and reduces PI concentrations, necessitating dosage changes (2). The combination of lopinavir-ritonavir and efavirenz was compared with nucleoside-based therapies. This nucleoside-sparing regimen was found to cause more hyperlipidemia and was not as effective in patients with viral loads of >100,000 (17). It is also a twice-daily regimen and is not as convenient as current first-line therapies, which are mostly taken once daily.
Darunavir (TMC114) is a new-generation PI with activity against viruses resistant to other PIs (7). The FDA-approved dose of darunavir-ritonavir is 600/100 mg twice daily for treatment-experienced HIV-infected patients and 800/100 once daily for treatment-naïve patients (14). Darunavir is metabolized by cytochrome P450 3A4 (CYP3A4) and is used in combination with ritonavir, which is a potent inhibitor of CYP3A4. When darunavir is boosted with ritonavir, the darunavir half-life is 15 h, thus allowing once-daily administration to treatment-naïve patients.
Compared to lopinavir-ritonavir, darunavir-ritonavir is more effective in subjects with viral loads of >100,000 and causes less hyperlipidemia (15). Therefore, the combination of darunavir-ritonavir with efavirenz could be a novel nucleoside reverse transcriptase inhibitor-sparing regimen for treatment-naïve patients.
Efavirenz is known to induce CYP3A4 and reduce PI concentrations. When it is given with darunavir-ritonavir at 300/100 mg twice daily, efavirenz reduced the darunavir trough concentrations by 31% and the area under the concentration-time curve (AUC) by 13%. Darunavir-ritonavir inhibits cytochromes and increases the efavirenz trough concentrations by 17% and the efavirenz AUC by 21% (18). The pharmacokinetic interactions of this combination administered once daily have not been studied. Because of the longer dosage interval, it is possible that efavirenz may reduce the concentrations of darunavir below the threshold required for efficacy. Therefore, we conducted a pharmacokinetic study to characterize the interactions between darunavir-ritonavir and efavirenz administered once daily. We chose the 900-mg darunavir dose due to the availability of the 300-mg tablet formulation, which is easy to administer (three tablets once daily), at the time of the initiation of this study.
(Data from this study were presented at the 10th International Workshop on Clinical Pharmacology of HIV Therapy Conference, April 2009, Amsterdam, Netherlands.)
MATERIALS AND METHODS
Subjects.
Healthy men and women between 21 and 65 years of age were eligible for enrollment in the study. HIV-infected patients, individuals with a clinically significant medical condition, smokers, and individuals with a known history of alcohol and/or drug abuse were excluded from the study. Exclusion criteria also included recent participation in an investigational drug study and an inability to refrain from the use of prescription and nonprescription medications. The subjects agreed to participate in the studies by giving written informed consent prior to commencement of the study. The study protocol was reviewed and approved by the Domain Specific Review Board, National Healthcare Group. Written informed consent was obtained from all volunteers. The studies were conducted in accordance with the guidelines on good clinical practice and with the ethical standards for human experimentation established by the Declaration of Helsinki.
Study design.
This was an open-label single-sequence three-period pharmacokinetic study with healthy HIV-seronegative adults (Fig. 1). During period 1, the healthy volunteers received darunavir-ritonavir at 900/100 mg once daily for 10 days. Pharmacokinetic sampling for determination of the darunavir and ritonavir concentrations was performed on day 10. During period 2, efavirenz administered at 600 mg once daily was added to the darunavir-ritonavir regimen for 14 days. Pharmacokinetic sampling for determination of the concentrations of darunavir, ritonavir, and efavirenz was performed on days 24 and 25. During period 3, the darunavir-ritonavir was stopped and the healthy volunteers received efavirenz at 600 mg once daily only from days 25 to 38. Pharmacokinetic sampling for determination of the concentrations of efavirenz was performed on days 38 and 39.
FIG. 1.
Schematic of the dosing regimen and the times of collection of samples for pharmacokinetic (PK) analysis.
The sampling time points were predosing and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, and 24 h postdosing.
The safety of the volunteers was assessed by the use of laboratory tests, collection of a medical history, and a physical examination while they were inpatients and at the follow-up visit.
Bioanalytical methods.
The liquid chromatography-tandem mass spectrometry (LC/MS/MS) method used for the analysis of darunavir, ritonavir, and efavirenz was optimized on the basis of previously published methods (5, 6, 19). In brief, a protein precipitation solution (methanol-acetonitrile [1:1]) was added to the plasma sample with 100 nM internal standard [2,3-di-(2-pyridyl)-chinoxalin, 98%; 6,7-dimethyl-2,3-di(2-pyridyl) quinoxaline, 98%; Sigma]. The mixture was vortexed and centrifuged, and the supernatant was then injected into the LC/MS/MS system. High-pressure liquid chromatography (HPLC) was carried out on an Agilent Technologies (Palo Alto, CA) model 1200 liquid chromatography system with a binary pump. A Phenomenex Synergi (Torrance, CA) Fusion-RP 80A column (150 mm by 2 mm; particle size, 4 μm) was used for separation in a column oven at 35°C. The mobile phase consisted of solvent A (H2O with 0.05% formic acid [Fluka]) and solvent B (acetonitrile [Merck] with 0.05% formic acid). The chromatographic separation was performed at a flow rate of 0.4 ml/min in the gradient mode and with the amount of solvent B consistently increasing from 55% to 85%. The column was first equilibrated with 55% solvent B for 4 min, and the total run time was 7 min.
The HPLC effluent was analyzed with an API 3200 triple-quadrupole mass spectrometer (ABI-Sciex, Toronto, Ontario, Canada) equipped with a turbo ion spray source. All data were recorded and processed by using Analyst software (version 1.4.1; ABI-Sciex, Foster City, CA). The darunavir, ritonavir, efavirenz, and the internal standard in plasma were analyzed in the full-scan mode to generate the protonated precursor molecules [M + H]+; and the most abundant and characteristic product ions were selected for multiple-reaction-mode analysis. The ion pairs selected for the three analytes and the internal standard were 548 → 202 for darunavir, 721 → 197 for ritonavir, 316 → 168 for efavirenz, and 313 → 246 for the internal standard.
The linearities of the calibration curves were good (r2 > 0.999) within concentration ranges of 1 to 64 μM for darunavir, 0.78 nM to 64 μM for ritonavir, and 1 to 64 μM for efavirenz. The limits of detection for darunavir, ritonavir, and efavirenz were 20 nM, 7.8 nM, and 10 nM, respectively; and the limits of quantification for darunavir, ritonavir, and efavirenz were 40 nM, 10 nM, and 20 nM, respectively. The inter- and intra-assay variabilities were <15%, and the rates of accuracy were between 92.1 and 108%.
Pharmacokinetic and statistical analyses.
The areas under the plasma concentration-time curves from 0 to 24 h (AUC0-24) were calculated by use of the linear trapezoidal rule. The plasma clearance and volume of distribution were calculated by use of a noncompartmental model with the WinNonLin (version 5.2) program (Pharsight, Cary, NC). The elimination rate constant (kel) was determined by regression analysis of the latest time points. The half-life (t1/2) was calculated from the following equation: ln 2/kel.
The values of the pharmacokinetic parameters were compared before and after the addition of the other drug by calculating the geometric mean ratios (GMRs) and the 90% confidence intervals (CIs). Paired t tests were performed on the log-transformed parameters, and P values were computed. Statistical analyses were performed by use of the Stata (version 10) program (Stata Inc., College Station, TX).
RESULTS
Study population and safety.
We recruited seven males and five females ages 24 to 49 years and weighing 50 to 83 kg.
One female subject developed grade 3 hepatitis and had an alanine aminotransferase level of more than nine times the upper limit of normal during period 3 (treatment with efavirenz alone), but the grade 3 hepatitis resolved completely after 150 days. One female subject developed a grade 2 maculopapular rash on day 9 of darunavir-ritonavir treatment, but the rash resolved in 2 weeks. Three female subjects developed grade 1 papular rashes during period 3, but these resolved in 6 days. All subjects developed grade 1 neuropsychiatric symptoms, especially giddiness, while receiving efavirenz. Triglyceride levels increased 20% after darunavir-ritonavir treatment, 52% after darunavir-ritonavir-efavirenz treatment, and 38% after efavirenz treatment. There were no significant changes in low-density lipoprotein (LDL) or high-density lipoprotein (HDL) levels.
Pharmacokinetic data.
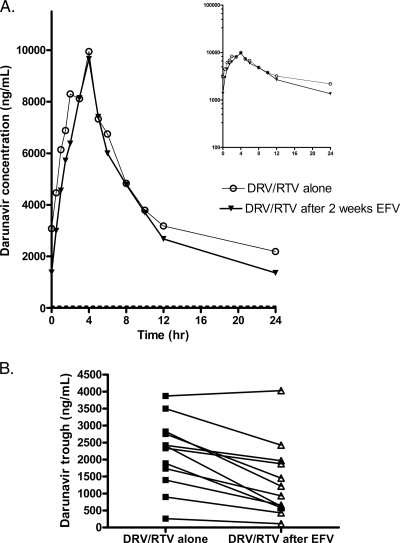
Efavirenz reduced the darunavir trough plasma concentration by 57% (GMR, 0.43; 90% CI, 0.32 to 0.57; P < 0.001) (Table 1; Fig. 2). The mean darunavir trough concentrations were 1,180 ng/ml (standard deviation [SD], 1,138 ng/ml) after efavirenz administration. All darunavir trough concentrations were above the 50% effective concentration (EC50) of 55 ng/ml for the wild-type virus (3). The darunavir AUC0-24 (GMR, 0.86; 90% CI, 0.75 to 0.97; P = 0.05) and half-life (GMR, 0.56; 90% CI, 0.49 to 0.65; P < 0.001) were also significantly reduced by efavirenz. The darunavir peak concentrations were not significantly changed (GMR, 0.92; 90% CI, 0.82 to 1.03; P = 0.23).
TABLE 1.
Pharmacokinetics of darunavir when 900 mg was given once daily with ritonavir at 100 mg once daily, before and after administration of efavirenz at 600 mg once dailya
| Parameter | DRV-RTV |
DRV-RTV + EFV |
LS means |
P value | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Ratio | 90% CI | ||
| Cmin (ng/ml) | 2,137 | 1,034 | 1,180 | 1,138 | 0.43 | 0.32-0.57 | 0.0003 |
| Cmax (ng/ml) | 10,967 | 3,320 | 10,027 | 2,552 | 0.92 | 0.82-1.03 | 0.23 |
| Tmax (h) | 4 | 2-6b | 4 | 2-5b | NA | 0.50 | |
| AUC (ng·h/ml) | 103,261 | 32,963 | 89,498 | 33,889 | 0.86 | 0.75-0.97 | 0.049 |
| t1/2 (h) | 15.3 | 7.4 | 8.5 | 4.6 | 0.56 | 0.49-0.65 | 0.00001 |
| CL/F (ml/h) | 9,657 | 3,466 | 11,392 | 4,127 | 1.17 | 1.03-1.33 | 0.047 |
Abbreviations: RTV, ritonavir; DRV, darunavir; EFV, efavirenz; NA, not available; LS, least-squares; Cmin, trough concentration in plasma; Cmax, maximum concentration in plasma; Tmax, time to the maximum concentration in plasma; AUC, area under the concentration-time curve; t1/2, half-life; CL/F, clearance.
Instead of SDs, the values for the time to the maximum concentration in plasma are ranges.
FIG. 2.
(A) Arithmetic mean darunavir (DRV) plasma concentrations at steady state after the administration of 900 mg darunavir with 100 mg ritonavir (RTV) once daily with or without 2 weeks of treatment with 600 mg efavirenz (EFV) once daily in healthy male and female subjects (inset, semilogarithmic scale). The EC50 of 55 ng/ml is indicated by the dashed line. (B) Individual darunavir 24-h trough concentrations before and after efavirenz treatment.
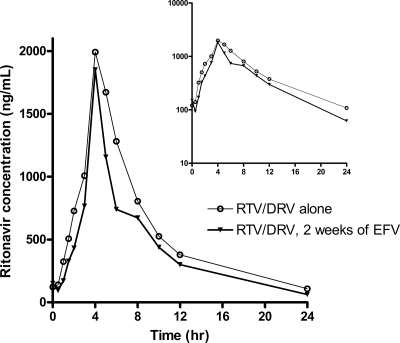
Ritonavir trough concentrations were 39 ng/ml (GMR, 0.46; 90% CI, 0.33 to 0.63; P = 0.001), and the ritonavir AUC0-24 (GMR, 0.74; 90% CI, 0.64 to 0.86; P = 0.004) and half-life (GMR, 0.80; 90% CI, 0.75 to 0.86; P < 0.001) were also significantly reduced by efavirenz administration (Table 2; Fig. 3).
TABLE 2.
Pharmacokinetics of ritonavir when 100 mg was given once daily with darunavir at 900 mg once daily, before and after administration of efavirenz at 600 mg once dailya
| Parameter | RTV-DRV |
RTV-DRV + EFV |
LS means |
P value | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Ratio | 90% CI | ||
| Cmin (ng/ml) | 81 | 41 | 39 | 22 | 0.46 | 0.33-0.63 | 0.001 |
| Cmax (ng/ml) | 2,272 | 1,354 | 1,914 | 1,054 | 0.82 | 0.65-1.03 | 0.15 |
| Tmax (h) | 4 | 2-6b | 4 | 1.5-5b | NA | 0.58 | |
| AUC (ng·h/ml) | 13,575 | 5,032 | 10,173 | 4,395 | 0.74 | 0.64-0.86 | 0.004 |
| t1/2 (h) | 6.1 | 1.6 | 4.8 | 0.7 | 0.80 | 0.75-0.86 | 0.00017 |
| CL/F (ml/h) | 9,453 | 7,514 | 12,495 | 7,430 | 1.35 | 1.17-1.57 | 0.003 |
Abbreviations: RTV, ritonavir; DRV, darunavir; EFV, efavirenz; NA, not available; LS, least-squares; Cmin, trough concentration in plasma; Cmax, maximum concentration in plasma; Tmax, time to the maximum concentration in plasma; AUC, area under the concentration-time curve; t1/2, half-life; CL/F, clearance.
Instead of SDs, the values for the time to the maximum concentration in plasma are ranges.
FIG. 3.
Arithmetic mean plasma ritonavir concentrations at steady state after the administration of 100 mg ritonavir once daily with 900 mg darunavir with or without 2 weeks of treatment with 600 mg efavirenz once daily in healthy male and female subjects (inset, semilogarithmic scale).
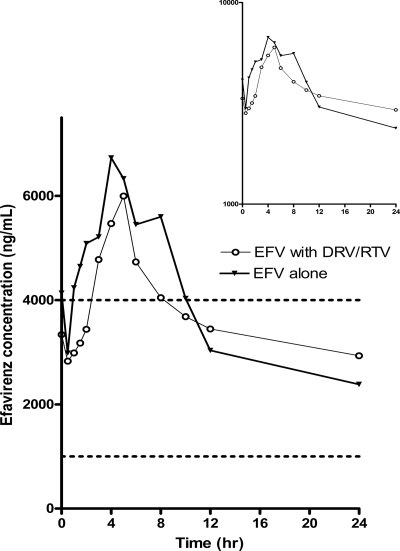
The half-life of efavirenz was significantly longer when it was coadministered with darunavir-ritonavir than when it was given alone (GMR, 1.66; 90% CI, 1.24 to 2.23; P = 0.01); but there were no differences in trough concentrations (GMR, 1.01; 90% CI, 0.81 to 1.25; P = 0.96), peak concentrations (GMR, 0.80; 90% CI, 0.63 to 1.02; P = 0.13), or AUC0-24 (GMR, 0.91; 90% CI, 0.75 to 1.11; P = 0.41) whether efavirenz was administered with or without darunavir-ritonavir (Table 3; Fig. 4).
TABLE 3.
Pharmacokinetics of efavirenz when 600 mg was given once daily, with and without darunavir at 900 mg once daily and ritonavir at 100 once dailya
| Parameter | EFV |
EFV + DRV-RTV |
LS means |
P value | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Ratio | 90% CI | ||
| Cmin (ng/ml) | 2,361 | 1,545 | 2,393 | 1,619 | 1.01 | 0.81-1.25 | 0.96 |
| Cmax (ng/ml) | 8,018 | 3,190 | 6,387 | 2,274 | 0.80 | 0.63-1.02 | 0.13 |
| Tmax (h) | 4 | 1-5b | 4 | 2-5b | NA | 0.55 | |
| AUC (ng·h/ml) | 91,648 | 37,362 | 87,808 | 50,563 | 0.91 | 0.75-1.11 | 0.41 |
| t1/2 (h) | 26.3 | 27.9 | 38.2 | 29.2 | 1.66 | 1.24-2.23 | 0.01 |
| CL/F (ml/h) | 7,407 | 2,464 | 8,348 | 3,235 | 1.09 | 0.90-1.33 | 0.43 |
Abbreviations: RTV, ritonavir; DRV, darunavir; EFV, efavirenz; NA, not available; LS, least-squares; Cmin, trough concentration in plasma; Cmax, maximum concentration in plasma; Tmax, time to the maximum concentration in plasma; AUC, area under the concentration-time curve; t1/2, half-life; CL/F, clearance.
Instead of SDs, the values for the time to the maximum concentration in plasma are ranges.
FIG. 4.
Arithmetic mean plasma efavirenz concentrations at steady state after the administration of 600 mg of efavirenz with or without 2 weeks of treatment with 900 mg darunavir once daily and 100 mg ritonavir once daily in healthy male and female subjects (inset, semilogarithmic scale). The therapeutic range of 1,000 to 4,000 ng/ml is indicated by the dashed lines.
DISCUSSION
Two weeks of efavirenz treatment reduced the trough concentrations obtained by once-daily darunavir treatment significantly more than it did during the twice-daily regimen studied previously (18). This result is not surprising, given that efavirenz induces CYP3A4 (10). In addition, we have recently shown that efavirenz induces the transporter function on peripheral blood mononuclear cells, and this induction of transporters could also contribute to the reduction of darunavir concentrations (12a).
Efavirenz also significantly reduced the ritonavir concentrations. The reduction of the boosting effect of ritonavir due to the lower concentrations may contribute to the reduction of darunavir concentrations, especially during the later part of the dosing interval.
Despite the significant reduction in the darunavir concentrations, all trough concentrations remained above the EC50 for darunavir for wild-type virus (3). All trough concentrations were also above 1.5 times the EC50 of wild-type virus, which would equate to an inhibitory quotient of more than 1.5. An inhibitory quotient of 1.5 or above predicted antiviral efficacy in a previous study (16). Therefore, this regimen should provide sufficient efficacy in treatment-naïve patients with no preexisting resistance.
In addition, it has been shown in the ARTEMIS study that the administration of darunavir-ritonavir once daily is known to be more effective than the administration of lopinavir-ritonavir in situations in which patients have high viral loads (15), and therefore, we predict that this regimen would be more potent than the lopinavir-ritonavir-efavirenz regimen, even though it is given once daily.
However, we note that there is considerable variability in the trough darunavir concentrations (coefficient of variation, 96%). Therefore, it is possible that some patients may not achieve plasma concentrations that are 1.5 times above the EC50. In addition, the concentrations at tissue sites may be even lower due to protein binding and transporter activity. Therefore, the clinical efficacy and durability of this regimen need to be validated with a larger sample of treatment-naïve patients.
The half-life of darunavir was reduced to 8.5 h after the administration of efavirenz. Thus, there is also an increased risk that the concentrations will be at subtherapeutic levels if a single dose is missed. Therefore, use of this once-daily regimen would best be restricted to patients with good adherence to the treatment regimen, in order to reduce the risk of failure and resistance.
The currently recommended dose of darunavir for treatment-naïve patients is 800 mg once daily, with ritonavir being given at 100 mg once daily. If it is assumed that the pharmacokinetics of darunavir are dose proportional at doses ranging from 800 to 900 mg, we expect that the 800-mg dose of darunavir will still yield trough concentrations above the EC50. Therefore, the regimen should still be easily administered by use of the 400-mg formulation when the 300-mg formulation is phased out. The 150-mg formulation is now also approved by FDA, and we can also use a darunavir dose of 950 mg.
Darunavir-ritonavir increased the half-life of efavirenz, as expected from the inhibition of CYPs 2B6 and 3A4. In contrast to the findings of previous studies, which showed that darunavir-ritonavir increased the trough concentrations and AUCs of efavirenz, it had no significant effect on the values of the other pharmacokinetic parameters for efavirenz. The carryover effects from the darunavir-ritonavir treatment may account for the lack of this interaction, as we did not have a washout period. Although this was recognized as a limitation of our study design, the concentrations of darunavir-ritonavir would be negligible 2 weeks after the cessation of the study, and any carryover effects would be indirect due to persistent enzyme effects. Therefore, we believe that this possibility did not affect the results in a significant way.
The reduced clearance of efavirenz may have contributed to the high rates of toxicity seen in this study. The concentrations of efavirenz achieved in our subjects were mostly above the therapeutic range of 1,000 to 4,000 ng/ml reported in another study (13). Increased efavirenz concentrations have been associated with central nervous system symptoms in other Asian populations (8).
While increased toxicities may also be expected in HIV-infected patients, neuropsychiatric adverse events as a result of efavirenz treatment tend to resolve over time (4). Efavirenz also induces its own metabolism, and this can also result in reduced concentrations over time (20). Therefore, these toxicities are likely to be tolerable in most treated patients. In addition, we administered the efavirenz in the morning, and this may have increased the perception of the neuropsychiatric adverse events. In HIV-infected patients, efavirenz would most likely be prescribed at night, and this tends to reduce the perceived severity of side effects.
While the study participants were fasting and receiving the darunavir-ritonavir-efavirenz combination, triglyceride levels increased significantly but the LDL cholesterol levels did not increase, in contrast to the findings obtained with the lopinavir-ritonavir-efavirenz combination (17). Analysis of the 96 weeks of data from the ARTEMIS study showed that the median increase in triglyceride levels was lower for darunavir (12%) than lopinavir (50%). A lower increase was also seen for total cholesterol (15% and 23%, respectively) (1). Therefore, this regimen could have less potential for long-term cardiovascular risk.
In conclusion, this study provides evidence of the feasibility of using ritonavir-boosted darunavir with efavirenz as a novel once-daily nucleoside-sparing regimen. This treatment option should be more effective and less toxic than lopinavir-ritonavir with efavirenz. The values of the pharmacokinetic parameters show that a wide margin of safety is maintained and that trough concentrations remain above the EC50. However, the clinical significance of this interaction needs to be evaluated in treatment-naïve patients.
Acknowledgments
We thank the healthy volunteers, May Low, and the Clinical Trials Unit staff for the conduct of this study.
Funding for this study was provided by the National Healthcare Group CEO Special Research Fund.
C.F. has served on scientific advisory boards for Bristol-Myers Squibb and Tibotec and has received honoraria for lectures sponsored in part by Abbott Laboratories. P.P. has served on the scientific advisory board for Tibotec.
Footnotes
Published ahead of print on 12 April 2010.
REFERENCES
- 1.Baraldi, E., J. Morales-Ramírez, S. Schneider, A. Stoehr, A. Orani, C. Vanden Abeele, and L. Lavreys. 2009. Effects of once-daily darunavir/ritonavir versus lopinavir/ritonavir on lipid parameters and anthropometrics in treatment-naïve HIV-1-infected ARTEMIS patients at week 96, abstr. MOPEB034. Abstr. 5th IAS Conf. HIV Pathog., Treatment, Prevention.
- 2.Bergshoeff, A. S., P. L. Fraaij, J. Ndagijimana, G. Verweel, N. G. Hartwig, T. Niehues, R. De Groot, and D. M. Burger. 2005. Increased dose of lopinavir/ritonavir compensates for efavirenz-induced drug-drug interaction in HIV-1-infected children. J. Acquir. Immune Defic. Syndr. 39:63-68. [DOI] [PubMed] [Google Scholar]
- 3.Boffito, M., D. Miralles, and A. Hill. 2008. Pharmacokinetics, efficacy, and safety of darunavir/ritonavir 800/100 mg once-daily in treatment-naive and -experienced patients. HIV Clin. Trials 9:418-427. [DOI] [PubMed] [Google Scholar]
- 4.Clifford, D. B., S. Evans, Y. Yang, E. P. Acosta, K. Goodkin, K. Tashima, D. Simpson, D. Dorfman, H. Ribaudo, and R. M. Gulick. 2005. Impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals. Ann. Intern. Med. 143:714-721. [DOI] [PubMed] [Google Scholar]
- 5.Colombo, S., A. Beguin, A. Telenti, J. Biollaz, T. Buclin, B. Rochat, and L. A. Decosterd. 2005. Intracellular measurements of anti-HIV drugs indinavir, amprenavir, saquinavir, ritonavir, nelfinavir, lopinavir, atazanavir, efavirenz and nevirapine in peripheral blood mononuclear cells by liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 819:259-276. [DOI] [PubMed] [Google Scholar]
- 6.D'Avolio, A., M. Siccardi, M. Sciandra, L. Baietto, S. Bonora, L. Trentini, and G. Di Perri. 2007. HPLC-MS method for the simultaneous quantification of the new HIV protease inhibitor darunavir, and 11 other antiretroviral agents in plasma of HIV-infected patients. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 859:234-240. [DOI] [PubMed] [Google Scholar]
- 7.De Meyer, S., H. Azijn, D. Surleraux, D. Jochmans, A. Tahri, R. Pauwels, P. Wigerinck, and M. P. de Bethune. 2005. TMC114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor-resistant viruses, including a broad range of clinical isolates. Antimicrob. Agents Chemother. 49:2314-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gatanaga, H., T. Hayashida, K. Tsuchiya, M. Yoshino, T. Kuwahara, H. Tsukada, K. Fujimoto, I. Sato, M. Ueda, M. Horiba, M. Hamaguchi, M. Yamamoto, N. Takata, A. Kimura, T. Koike, F. Gejyo, S. Matsushita, T. Shirasaka, S. Kimura, and S. Oka. 2007. Successful efavirenz dose reduction in HIV type 1-infected individuals with cytochrome P450 2B6 *6 and *26. Clin. Infect. Dis. 45:1230-1237. [DOI] [PubMed] [Google Scholar]
- 9.Hammer, S. M., J. J. Eron, Jr., P. Reiss, R. T. Schooley, M. A. Thompson, S. Walmsley, P. Cahn, M. A. Fischl, J. M. Gatell, M. S. Hirsch, D. M. Jacobsen, J. S. Montaner, D. D. Richman, P. G. Yeni, and P. A. Volberding. 2008. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society—USA panel. JAMA 300:555-570. [DOI] [PubMed] [Google Scholar]
- 10.Hariparsad, N., S. C. Nallani, R. S. Sane, D. J. Buckley, A. R. Buckley, and P. B. Desai. 2004. Induction of CYP3A4 by efavirenz in primary human hepatocytes: comparison with rifampin and phenobarbital. J. Clin. Pharmacol. 44:1273-1281. [DOI] [PubMed] [Google Scholar]
- 11.Hsu, A., J. Isaacson, S. Brun, B. Bernstein, W. Lam, R. Bertz, C. Foit, K. Rynkiewicz, B. Richards, M. King, R. Rode, D. J. Kempf, G. R. Granneman, and E. Sun. 2003. Pharmacokinetic-pharmacodynamic analysis of lopinavir-ritonavir in combination with efavirenz and two nucleoside reverse transcriptase inhibitors in extensively pretreated human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 47:350-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee, L. S., A. Panchalingam, M. C. Yap, and N. I. Paton. 2004. Pharmacokinetics of indinavir at 800, 600, and 400 milligrams administered with ritonavir at 100 milligrams and efavirenz in ethnic Chinese patients infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 48:4476-4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Lee, L. S., G. H. Soon, P. Shen, E. L. Yong, C. Flexner, and P. Pham. 2010. Darunavir/ritonavir and efavirenz exert differential effects on MRP1 transporter expression and function in healthy volunteers. Antivir. Ther. 15:275-279. [DOI] [PubMed]
- 13.Marzolini, C., A. Telenti, L. A. Decosterd, G. Greub, J. Biollaz, and T. Buclin. 2001. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS 15:71-75. [DOI] [PubMed] [Google Scholar]
- 14.McKeage, K., C. M. Perry, and S. J. Keam. 2009. Darunavir: a review of its use in the management of HIV infection in adults. Drugs 69:477-503. [DOI] [PubMed] [Google Scholar]
- 15.Mills, A. M., M. Nelson, D. Jayaweera, K. Ruxrungtham, I. Cassetti, P. M. Girard, C. Workman, I. Dierynck, V. Sekar, C. V. Abeele, and L. Lavreys. 2009. Once-daily darunavir/ritonavir vs. lopinavir/ritonavir in treatment-naive, HIV-1-infected patients: 96-week analysis. AIDS 23:1679-1688. [DOI] [PubMed] [Google Scholar]
- 16.Molto, J., J. R. Santos, N. Perez-Alvarez, S. Cedeno, C. Miranda, S. Khoo, L. Else, J. M. Llibre, M. Valle, and B. Clotet. 2008. Darunavir inhibitory quotient predicts the 48-week virological response to darunavir-based salvage therapy in human immunodeficiency virus-infected protease inhibitor-experienced patients. Antimicrob. Agents Chemother. 52:3928-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riddler, S. A., R. Haubrich, A. G. DiRienzo, L. Peeples, W. G. Powderly, K. L. Klingman, K. W. Garren, T. George, J. F. Rooney, B. Brizz, U. G. Lalloo, R. L. Murphy, S. Swindells, D. Havlir, and J. W. Mellors. 2008. Class-sparing regimens for initial treatment of HIV-1 infection. N. Engl. J. Med. 358:2095-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekar, V. J., M. De Pauw, K. Marien, M. Peeters, E. Lefebvre, and R. M. Hoetelmans. 2007. Pharmacokinetic interaction between TMC114/r and efavirenz in healthy volunteers. Antivir. Ther. 12:509-514. [PubMed] [Google Scholar]
- 19.ter Heine, R., C. G. Alderden-Los, H. Rosing, M. J. Hillebrand, E. C. van Gorp, A. D. Huitema, and J. H. Beijnen. 2007. Fast and simultaneous determination of darunavir and eleven other antiretroviral drugs for therapeutic drug monitoring: method development and validation for the determination of all currently approved HIV protease inhibitors and non-nucleoside reverse transcriptase inhibitors in human plasma by liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 21:2505-2514. [DOI] [PubMed] [Google Scholar]
- 20.Zhu, M., S. Kaul, P. Nandy, D. M. Grasela, and M. Pfister. 2009. Model-based approach to characterize efavirenz autoinduction and concurrent enzyme induction with carbamazepine. Antimicrob. Agents Chemother. 53:2346-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]