Abstract
This study evaluated the daptomycin activity against two methicillin-resistant Staphylococcus epidermidis (MRSE) clinical isolates with different vancomycin susceptibilities: MRSE-375, with a vancomycin MIC of 2 μg/ml, and NRS6, a glycopeptide-intermediate S. epidermidis (GISE) strain with a vancomycin MIC of 8 μg/ml. The in vivo activity of daptomycin at two different doses (standard dose [SD-daptomycin], 6 mg/kg of body weight/day intravenously [i.v.]; high dose [HD-daptomycin], 10 mg/kg/day i.v.) was evaluated in a rabbit model of infective endocarditis and compared with that of a standard dose of vancomycin (SD-vancomycin; 1 g i.v. every 12 h) for 2 days. For the MRSE-375 strain, high-dose vancomycin (HD-vancomycin; 1 g i.v. every 6 h) was also studied. For MRSE-375, SD- and HD-daptomycin therapy sterilized significantly more vegetations than SD-vancomycin therapy (9/15 [60%] and 11/15 [73%] vegetations, respectively, versus 3/16 [19%] vegetations; P = 0.02 and P = 0.002, respectively). HD-daptomycin sterilized more vegetations than HD-vancomycin (11/15 [73%] versus 5/15 [33%] vegetations; P = 0.03) and was more effective than SD- and HD-vancomycin in reducing the density of bacteria in valve vegetations (0 log10 CFU/g vegetation [interquartile range {IQR}, 0 to 1 log10 CFU/g vegetation] versus 2 log10 CFU/g vegetation [IQR, 2 to 2 log10 CFU/g vegetation] and 2 log10 CFU/g vegetation [IQR, 0 to 2.8 log10 CFU/g vegetation]; P = 0.002 and P = 0.01, respectively). For the NRS6 strain, SD- and HD-daptomycin were significantly more effective than vancomycin in reducing the density of bacteria in valve vegetations (3.7 log10 CFU/g vegetation [IQR, 2 to 6 log10 CFU/g vegetation] versus 7.1 log10 CFU/g vegetation [IQR, 5.2 to 8.5 log10 CFU/g vegetation]; P = 0.02). In all treatment arms, isolates recovered from vegetations remained susceptible to daptomycin and vancomycin and had the same MICs. In conclusion, daptomycin at doses of 6 mg/kg/day or 10 mg/kg/day is more effective than vancomycin for the treatment of experimental endocarditis due to MRSE and GISE.
Though they were once considered innocuous skin commensals or culture contaminants, coagulase-negative staphylococci (CoNS) are now recognized as important pathogens (3). These organisms are the most common etiologic agents of bacteremia among hospitalized patients, and Staphylococcus epidermidis is isolated from 50% to 70% of catheter-related bloodstream infections (3, 28). Though these infections can be severe, CoNS bacteremia is infrequently life-threatening when it is treated promptly (3). However, treatment is complicated by resistance to multiple antibiotic agents. Methicillin resistance is seen in 70% to 80% of clinical isolates, and CoNS with reduced susceptibility to vancomycin have also been reported (13, 18).
CoNS, including Staphylococcus epidermidis, cause more than 10% of all cases of infective endocarditis (IE) and are the most common pathogens causing intracardiac prosthetic device infections (prosthetic valve endocarditis [PVE] and pacemaker and cardiac defibrillator lead endocarditis) (6, 9, 23). A recent multinational, prospective cohort study found that 16% of nonintravenous drug use-related cases of PVE were attributed to CoNS. The majority (82%) of these isolates were S. epidermidis, and 67% of these were methicillin-resistant S. epidermidis (MRSE) (6). CoNS are also increasingly identified as the cause of native valve endocarditis (NVE). Nearly 8% of all NVE cases not associated with intravenous drug use are caused by CoNS, predominantly S. epidermidis (7). Moreover, as many as 41% of NVE cases are caused by methicillin-resistant strains. These resistant strains are seen most commonly among health care-associated infections (7).
Antimicrobial treatment of MRSE NVE is based upon the administration of a glycopeptide agent, such as vancomycin, while gentamicin and rifampin are typically added to vancomycin for the management of PVE (1). Unfortunately, resistance to rifampin and gentamicin among MRSE strains, as well as the growing prevalence of glycopeptide-intermediate S. epidermidis (GISE), limits our therapeutic options and warrants the investigation of alternative agents (1, 2, 13, 16, 18).
Daptomycin is a cyclic lipopeptide antibiotic with activity against S. epidermidis and other CoNS, including MRSE and GISE (4, 16, 26). Daptomycin has demonstrated efficacy in the treatment of right-sided endocarditis caused by methicillin-susceptible and methicillin-resistant Staphylococcus aureus (MSSA and MRSA, respectively) and CoNS bacteremia and is a potential therapeutic option for IE caused by S. epidermidis (12, 27).
The aim of the present study was to evaluate the in vitro and in vivo activity of daptomycin against two MRSE clinical isolates with different vancomycin susceptibilities in experimental aortic valve endocarditis using a human-adapted pharmacokinetic (PK) model and to compare the activity of daptomycin at two different doses with the activity of vancomycin.
(This study was presented at the 10th International Symposium on Modern Concepts in Endocarditis and Cardiovascular Infections, 17 to 20 April 2009, Naples, Italy [abstr. ISCNA-62] and at the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy, 12 to 15 September 2009, San Francisco, CA [abstr. 1882].)
MATERIALS AND METHODS
Bacterial isolates.
Two clinical isolates of MRSE were selected for the in vitro and in vivo studies. MRSE-375 was isolated from a patient with endocarditis at Hospital Clínic University in Barcelona, Spain. NRS6, a GISE strain, was obtained through the Network of Antimicrobial Resistance in S. aureus (NARSA) program, supported under NIAID/NIH contract HHSN272200700055C. The isolates were stored at −80°C in skim milk.
Antibiotics.
Daptomycin powder was supplied by Cubist Pharmaceuticals, Inc. (Lexington, MA). Vancomycin was purchased from Sigma (St. Louis, MO).
Susceptibility testing.
Daptomycin and vancomycin MICs and minimum bactericidal concentrations (MBCs) were determined using the microdilution method in liquid medium, cation-adjusted Mueller-Hinton broth (CAMHB; Oxoid Ltd., Hampshire, England), according to the procedures of the Clinical and Laboratory Standards Institute (CLSI; formerly NCCLS) (24). Daptomycin susceptibility testing was performed in Mueller-Hinton broth adjusted to 50 μg/ml of calcium according to the standard methodology. S. aureus ATCC 29213 was used as the test control strain.
Time-kill studies.
The time-kill methodology was used to test the activities of daptomycin and vancomycin against the MRSE-375 and NRS6 strains, according to previously described criteria (10). After an overnight culture, a final inoculum between 5 × 105 and 1 × 106 CFU/ml was used. Prior to inoculation, each tube of fresh CAMHB was supplemented with daptomycin or vancomycin. Concentrations of 0.5, 1, and 2 times the MIC were chosen for testing. A tube without antibiotic was used as a growth control. Viability counts were performed at 0, 4, and 24 h, according to the recommendations of Isenberg (17). Drug carryover was addressed by dilution. Bactericidal activity was defined as at least a 3-log10 reduction in the numbers of CFU/ml at 24 h in comparison with the initial inoculum. Time-kill studies were performed in duplicate.
Study animals.
Experimental aortic valve endocarditis was induced in New Zealand White rabbits (body weight, 2 kg), which were obtained from San Bernardo Farm (Pamplona, Spain). The animals were housed in the animal facilities of the University of Barcelona School of Medicine, which is equipped with automatic air exchange with a high-efficiency particulate air (HEPA) filter and a circadian light cycle. They were nourished ad libitum. This study was approved by the Ethical Committee of Experimental Animal Studies of the University of Barcelona.
Human pharmacokinetic simulation studies.
In vivo experimental PK studies for these antibiotics have been described previously (20). The antibiotics were administered using a computer-controlled infusion pump system designed to reproduce human serum PKs in rabbits after an intravenous (i.v.) infusion. Animal antibiotic doses were chosen to simulate the human PK profile of standard-dose daptomycin (SD-daptomycin; 6 mg/kg of body weight/day i.v.), high-dose daptomycin (HD-daptomycin; 10 mg/kg/day i.v.), and standard-dose vancomycin (SD-vancomycin; 30 mg/kg i.v. divided into two doses; for an adult of 70 kg, 1 g i.v. every 12 h) (8, 20). In addition, a group of animals with MRSE experimental endocarditis was tested with high doses of vancomycin (HD-vancomycin) in order to achieve the optimal pharmacokinetic/pharmacodynamic (PK/PD) index (the ratio of the area under the serum drug concentration-versus-time curve [AUC] and the MIC [AUC/MIC]) of >400, which has been described for the treatment of serious infections due to MRSA (22, 25). For this HD-vancomycin treatment, the animals received 60 mg/kg i.v. vancomycin divided into four doses (equivalent to 1 g i.v. every 6 h for a 70-kg adult). Assuming an MIC for MRSE-375 of 2 μg/ml, this dose would simulate the human PK profile necessary to attain the aforementioned AUC/MIC ratio. The HD-vancomycin arm was not used as a comparator in rabbits infected with GISE, since the doses required to attain an AUC/MIC ratio of >400 and/or a trough concentration four times the MIC for this organism (8 μg/ml) cannot be achieved with conventional dosing and are not safe in humans (20, 22, 25). Daptomycin was administered over an infusion period of 15 min and vancomycin over an infusion period of 20 min.
Endocarditis model.
Experimental aortic valve IE was induced according to the method described by Garrison and Freedman (14). A catheter was inserted through the right carotid artery into the left ventricle, and the catheter used for antibiotic administration was placed into the inferior vena cava through the jugular vein (21). The infusion pump delivered 2 ml/h of 0.9% saline solution until the beginning of antimicrobial administration. Forty-eight hours after placement of the intracardiac catheter, all animals were infected via the marginal ear vein with 1 ml of saline solution containing about 1 × 109 CFU/ml of the MRSE-375 or NRS6 strain. One milliliter of blood was obtained 48 h after infection and immediately before the initiation of antimicrobial therapy to confirm the presence of bacteremia, which was interpreted to indicate IE. At the same time, control animals were anesthetized and killed, and vegetations were quantified for bacterial CFU. Antibiotics were administered via the computer-controlled infusion pump system for 48 h. The duration of treatment was deemed appropriate for this acute treatment model, which focused on the early efficacies of the different antibiotic treatments. After completion of the treatment, an additional 6 half-lives were allowed to elapse before the animals were killed. This provided time for viable bacteria remaining within the endocardial vegetations to grow.
Treatment groups.
For MRSE-375 experimental endocarditis, there was one control arm, two daptomycin arms (SD or HD), and two vancomycin arms (SD or HD). For GISE experimental endocarditis, there was one control arm, two daptomycin arms (SD or HD), and the SD-vancomycin arm. Each arm included 15 to 17 animals.
Analysis of endocardial vegetations.
After antibiotic treatment, rabbits were anesthetized and killed and aortic valve vegetations were removed and processed (21). The results were expressed as the number of log10 number of CFU/g of vegetation. The result was assigned a value of 2 if there was no growth on the quantitative plates but there was growth in the qualitative culture (the rest of the homogenate in tryptic soy broth). The result was assigned a value of 0 and the vegetation was considered sterile if there was no growth from the initial quantitative culture or from the homogenates cultured for a week.
PAP studies.
Isolates recovered from vegetations were frozen and stored at −80°C in skim milk. Before testing of the isolates, each isolate was subcultured twice on blood agar plates to ensure optimal growth.
The recovered bacteria were retested to measure the daptomycin and vancomycin MIC values for comparison to the pretreatment values.
Modified population analysis profile (PAP) studies were also performed. Fresh cultures were diluted in saline from 102 to 108 CFU/ml and were plated on brain heart infusion agar plates (Oxoid Ltd.) containing 0, 0.5, 1, 2, 4, and 6 mg/liter vancomycin or Mueller-Hinton agar plates (Oxoid Ltd.) containing 0, 0.25, 0.5, 1, 2, 4, 8, and 16 mg/liter daptomycin supplemented with 50 μg/ml of calcium (to achieve the physiological level of free calcium ion). The colonies were counted after 48 h of incubation at 37°C, and the viable count was plotted against the vancomycin or daptomycin concentration and compared with the initial strain count. S. aureus ATCC 700698 (Mu3) was used as a control strain (29).
Statistical analysis.
The results were expressed as the median and the interquartile range (IQR) interval of the number of log10 CFU per gram of vegetation. The Mann-Whitney rank sum test was used to compare the log10 CFU/g values between the different treatment groups. The Fisher exact test was used to compare the rate of sterilization of the vegetations and assess whether there were differences between treatment groups.
RESULTS
Susceptibility testing.
Strain MRSE-375 was susceptible to vancomycin and daptomycin (MICs, 2 μg/ml and 0.5 μg/ml, respectively; MBCs, 4 μg/ml and 1 μg/ml, respectively) and was resistant to gentamicin and rifampin (MICs, 128 μg/ml and 128 μg/ml, respectively), according to the CLSI standard MIC breakpoints (24). NRS6 was nonsusceptible to daptomycin (MIC, 2 μg/ml; MBC, 4 μg/ml), exhibited intermediate resistance to vancomycin (MIC, 8 μg/ml; MBC, 16 μg/ml), was resistant to gentamicin (MIC, 64 μg/ml), and was susceptible to rifampin (MIC, 0.008 μg/ml; MBC, >0.25 μg/ml).
In vitro time-kill experiments.
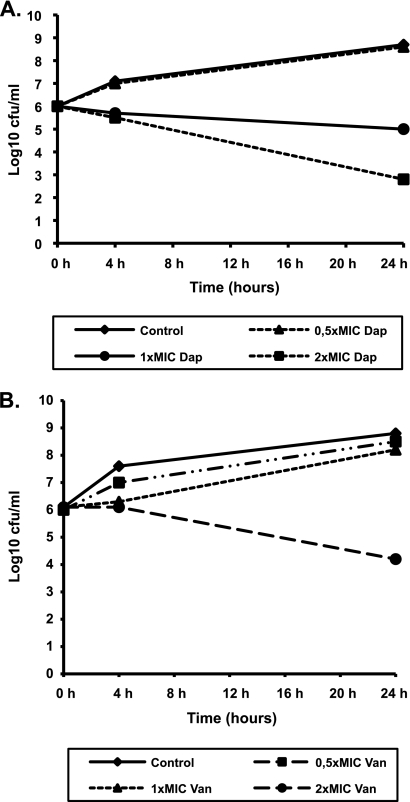
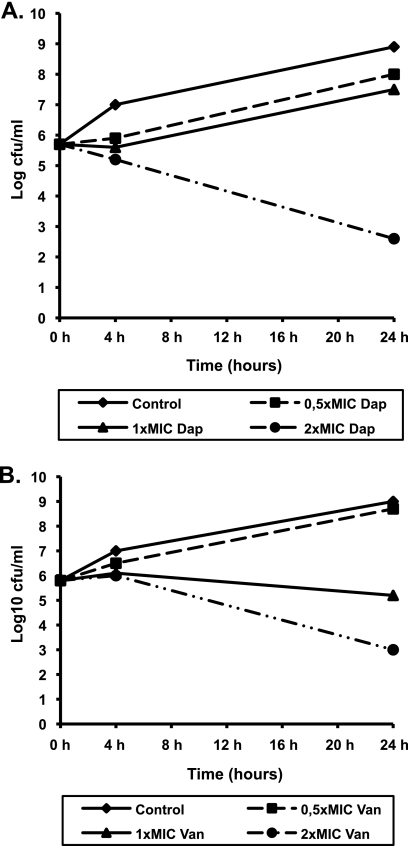
The in vitro activities of daptomycin and vancomycin against the selected strains are presented in Fig. 1 and 2. Daptomycin demonstrated bactericidal activity at two times the MIC, whereas vancomycin showed bacteriostatic activity at all concentrations tested. Though vancomycin produced a 2.8-log10 reduction in the numbers of NRS6 CFU at 24 h when it was tested at the MBC (16 μg/ml; two times the MIC), this did not meet the definition for bactericidal activity.
FIG. 1.
Results of time-kill experiments for MRSE-375 incubated with daptomycin (Dap) (A) or vancomycin (Van) (B). The MICs for daptomycin and vancomycin were 0.5 μg/ml and 2 μg/ml, respectively.
FIG. 2.
Results of time-kill experiments for GISE (NRS6 strain) incubated with daptomycin (Dap) (A) or vancomycin (Van) (B). The MICs for daptomycin and vancomycin were 2 μg/ml and 8 μg/ml, respectively.
Human pharmacokinetic simulation studies.
The values of the PK parameters for both SD- and HD-daptomycin obtained from the human-adapted model were similar to those seen in humans (20). The mean trough concentrations of HD-daptomycin were well above the daptomycin MICs of both the MRSE and the GISE strains (mean = 18.04 ± 2 μg/ml), and the mean maximum concentration (Cmax) was equal to 146 ± 19 μg/ml. The mean trough concentration of the HD-vancomycin arm was 20 ± 1.8 μg/ml.
Treatment of established endocarditis.
In the present study with a model of acute IE, our intention was to study the early response to antibiotic treatment, i.e., within the first 48 h. The relative effectiveness of the daptomycin and vancomycin regimens is shown in Table 1. All catheterized animals completed the 48-h antibiotic treatment. All control rabbits infected with either strain of S. epidermidis had infected aortic valve vegetations, and the median bacterial titer per gram of vegetation was ≥7.4 log10 CFU. Comparisons between treated groups revealed that after 48 h of treatment, HD-daptomycin was more active than SD-daptomycin in sterilizing the vegetations and reducing the median number of CFU. In fact, in four vegetations in the HD-daptomycin arm, bacteria grew only in the qualitative culture and were thus assigned a value of 2. Vegetations from the SD-daptomycin arm grew up to 6 to 7 log10 in three cases. Both HD- and SD-daptomycin were significantly more effective than SD-vancomycin against the MRSE-375 isolate (P = 0.02). In comparison with HD-vancomycin, the HD-daptomycin arm sterilized significantly more vegetations (P = 0.01) and reduced the density of bacteria in the vegetations (P = 0.03). For the NRS6 strain, the two daptomycin arms reduced the median number of CFU in the vegetations of the treated animals to a greater extent than SD-vancomycin. This difference was statistically significant (P = 0.02). SD-vancomycin did not reduce the number of CFU (P = 0.06) compared with the number in the control group.
TABLE 1.
Treatment of experimental endocarditis caused by MRSE-375 and GISE (NRS6) strains
| Strain and treatment group | No. of sterile vegetations/total no. (%) | Median (IQR) log10 CFU/g vegetation |
|---|---|---|
| MRSE-375 | ||
| Controla | 0/15 (0) | 7.4 (6-8.3) |
| SD-daptomycinb | 9/15 (60)c | 0 (0-4.1) |
| HD-daptomycind | 11/15 (73)e,f | 0 (0-1)e,g |
| SD-vancomycinh | 3/16 (19)c,e | 2 (2-2)e |
| HD-vancomycini | 5/15 (33)f | 2 (0-2.8)g |
| GISE (NRS6 strain) | ||
| Controla | 0/16 (0) | 8.4 (7.9-8.9)j |
| SD-daptomycinb | 1/17 (6) | 3.7 (2-6)c,k,l |
| HD-daptomycind | 0/16 (0) | 3.9 (2-5.8)c,k,l |
| SD-vancomycinh | 0/15 (0) | 7.1 (5.2-8.5)c,j,l |
The control animals were killed 48 h after the infection was started.
6 mg/kg/day i.v.
P = 0.02.
10 mg/kg/day i.v.
P = 0.002.
P = 0.03.
P = 0.01.
15 mg/kg i.v. every 12 h.
15 mg/kg i.v. every 6 h.
P = 0.06.
P = 0.09.
P = 0.02.
All isolates from the endocardial vegetations had the same daptomycin or vancomycin MIC as the initial infecting strain after daptomycin or vancomycin treatments. PAP studies did not show any subpopulations with increased resistance to either daptomycin or vancomycin.
DISCUSSION
Daptomycin is a cyclic lipopetide antibiotic with potent activity against Gram-positive organisms approved by both the U.S. Food and Drug Administration and the European Medicines Agency for the treatment of complicated skin and skin structure infections, as well as bacteremia and right-sided IE caused by S. aureus (8). Though CoNS such as MRSE are a common cause of nosocomial bloodstream infections, cardiac device infections, and PVE and an emerging cause of NVE, there is little published information regarding the activity of daptomycin against these pathogens (3, 5-7, 9, 28). In this study, the activity of daptomycin against MRSE and GISE experimental endocarditis was compared to that of vancomycin, the current standard therapy for MRSE NVE (1).
The time-kill experiments presented here revealed that daptomycin exhibited bactericidal activity against MRSE-375 when it was tested at two times the MIC, while vancomycin was bacteriostatic when it was tested at concentrations from 0.5 to 2 times the MIC. Rybak et al. found that both daptomycin and vancomycin showed bactericidal activity against MRSE in time-kill experiments when they were tested at concentrations four times the MIC (26). Moreover, these investigators utilized a strain of MRSE (R227) with lower MICs of daptomycin and vancomycin. As the number of clinical MRSE isolates with MICs of ≥2 μg/ml rises, it may be increasingly difficult to safely reach the serum vancomycin concentrations required to achieve this level of bactericidal activity (25). Daptomycin was also bactericidal against the GISE strain (NRS6) at all concentrations tested, while vancomycin again displayed bacteriostatic activity. LaPlante and Rybak compared the activity of daptomycin against a GISE strain (VA-5289) with an MIC of 8 μg/ml with that of vancomycin in time-kill experiments (19). They found that at concentrations two times the MIC, daptomycin was bactericidal against this organism, but vancomycin showed no activity. Daptomycin and vancomycin both exhibited bactericidal activity in experiments performed at four times the MIC, though daptomycin was significantly more active than vancomycin (P < 0.05). Daptomycin has been shown to exert its bactericidal effect in a concentration-dependent manner, and peak concentrations (Cmaxs) more than two to four times the MICs of these organisms can readily be achieved with standard daptomycin doses (8). Though vancomycin demonstrated bactericidal activity against GISE at four times the MIC (32 μg/ml), the corresponding serum drug concentration is not within the range of optimal trough concentrations set forth in a recently published consensus review (25).
This study is the first to evaluate the efficacy of daptomycin in the treatment of MRSE and GISE IE. In a rabbit model with human-adapted PKs, both standard-dose daptomycin and high-dose daptomycin were significantly more effective against MRSE-375 than vancomycin administered at a standard dose after 48 h of treatment (P = 0.02). Therapy with HD-vancomycin appeared to be slightly more active than therapy with SD-vancomycin against the MRSE-375 strain, with a greater percentage of vegetations being sterilized by HD-vancomycin, yet HD-vancomycin was less effective than HD-daptomycin (P = 0.03) and SD-daptomycin (P = 0.14). As in the vancomycin arms, therapy with HD-daptomycin appeared to be slightly more active than therapy with SD-daptomycin against the MRSE-375 strain, with a greater percentage of vegetations being sterilized and lower bacterial densities being achieved in the vegetations, though the difference between the two daptomycin treatment arms was not statistically significant. For the GISE strain (NRS6), SD-daptomycin and HD-daptomycin were significantly more effective than SD-vancomycin in reducing the density of bacteria within the valve vegetations (P = 0.02). No differences between the two daptomycin arms were found (P = 0.09). As described in the Materials and Methods section, the HD-vancomycin arm was not used as a comparator for rabbits infected with GISE, since the doses required to attain an AUC/MIC ratio of >400 and/or a trough concentration four times the MIC cannot be achieved with conventional dosing and are not safe in humans if the vancomycin MIC is >2 μg/ml (20, 22, 25). Though reduced susceptibility to daptomycin and vancomycin has developed during therapy for complicated S. aureus bacteremia, the MICs for both agents were unchanged after 2 days of treatment in this study (12). In addition, PAP studies performed with bacteria harvested from the vegetations did not reveal the development of heteroresistance to daptomycin or vancomycin for the MRSE strain or the development of more resistant subpopulations in the NRS6 strain. However, these data are limited by the short study period, and it is not clear if resistance would have emerged during a longer course of treatment.
Vancomycin monotherapy is the treatment of choice for MRSE NVE. In cases of MRSE PVE, the recommended therapy consists of vancomycin in combination with rifampin and gentamicin (triple antibiotic therapy) (1). However, triple antibiotic therapy cannot be effectively employed when the etiologic pathogen is resistant to rifampin or gentamicin (16). Only 18% of CoNS PVE cases identified in a multinational cohort were treated with this combination regimen, despite the fact that 68% of the CoNS strains were methicillin resistant (6). The present study did not compare daptomycin monotherapy to standard MRSE PVE triple antibiotic therapy. Nevertheless, these results may be extrapolated to those PVE cases where treatment is limited by rifampin and/or gentamicin resistance, pending the results of a specific investigation of daptomycin for the treatment MRSE PVE.
When the results described here are interpreted, it is important to consider the vancomycin MICs for the S. epidermidis strains used. For the MRSE-375 strain with a vancomycin MIC of 2 μg/ml, daptomycin was superior to SD-vancomycin but not to HD-vancomycin in the treatment of experimental endocarditis. As previously mentioned, the HD-vancomycin regimen was chosen in order to achieve an AUC/MIC ratio of >400, a target considered optimal for the treatment of MRSA respiratory infections (20, 22). However, HD-vancomycin regimens have been associated with an increased risk of nephrotoxicity compared to that from standard dosing, particularly when vancomycin is combined with another nephrotoxic agent (15). Concerns regarding a significant risk for vancomycin-induced ototoxicity among older patients treated with HD-vancomycin have also been raised (11). Furthermore, recent expert panel recommendations regarding vancomycin therapeutic drug monitoring have proposed a target AUC/MIC ratio of >400 (25). This target is not achievable with conventional dosing methods in patients with normal renal function if the vancomycin MIC is ≥2 μg/ml, and alternative therapies should be considered (25). The results of this study indicate that daptomycin is superior to vancomycin for the treatment of experimental IE caused by MRSE and GISE and provide support for the study of daptomycin as an alternative to vancomycin for the treatment of MRSE IE in humans.
Acknowledgments
This work was supported in part by a medical school grant from Cubist Pharmaceuticals, Inc. (Lexington, MA); grants from the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III (Madrid, Spain); the Spanish Network for the Research in Infectious Diseases (grant REIPI RD06/0008); CIBER de Respiratorio (grant CibeRes CB06/06/0028); Fondo de Investigaciones Sanitarias (FIS) (Madrid, Spain) grants FIS 05/0170, FIS 08/0268, and FISEC 08/00190; and a grant from the Fundación Máximo Soriano Jiménez (Barcelona, Spain). J. M. Miró received a research grant from the Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS; Barcelona, Spain).
We thank Michelle D'Aprile, agent of PharmaWrite, LLC, for providing editorial assistance, which was funded by Cubist Pharmaceuticals, Inc.
J. M. Miró has received honoraria for consultant and/or research grants from Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb (BMS), Cubist Pharmaceuticals, Inc., Novartis, GlaxoSmithKline (GSK), Gilead Sciences, Pfizer, Roche, and Theravance. No other author reports any potential conflict of interest.
Footnotes
Published ahead of print on 26 April 2010.
REFERENCES
- 1.Baddour, L. M., W. R. Wilson, A. S. Bayer, V. G. Fowler, Jr., A. F. Bolger, M. E. Levison, P. Ferrieri, M. A. Gerber, L. Y. Tani, M. H. Gewitz, D. C. Tong, J. M. Steckelberg, R. S. Baltimore, S. T. Shulman, J. C. Burns, D. A. Falace, J. W. Newburger, T. J. Pallasch, M. Takahashi, and K. A. Taubert. 2005. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 111:e394-e434. [DOI] [PubMed] [Google Scholar]
- 2.Biavasco, F., C. Vignaroli, and P. E. Varaldo. 2000. Glycopeptide resistance in coagulase-negative staphylococci. Eur. J. Clin. Microbiol. Infect. Dis. 19:403-417. [DOI] [PubMed] [Google Scholar]
- 3.Cervera, C., M. Almela, J. A. Martinez-Martinez, A. Moreno, and J. M. Miro. 2009. Risk factors and management of Gram-positive bacteraemia. Int. J. Antimicrob. Agents 34(Suppl. 4):S26-S30. [DOI] [PubMed] [Google Scholar]
- 4.Cha, R., and M. J. Rybak. 2003. Daptomycin against multiple drug-resistant Staphylococcus and Enterococcus isolates in an in vitro pharmacodynamic model with simulated endocardial vegetations. Diagn. Microbiol. Infect. Dis. 47:539-546. [DOI] [PubMed] [Google Scholar]
- 5.Chambers, S. T. 2005. Diagnosis and management of staphylococcal infections of pacemakers and cardiac defibrillators. Intern. Med. J. 35(Suppl. 2):S63-S71. [DOI] [PubMed] [Google Scholar]
- 6.Chu, V. H., J. M. Miro, B. Hoen, C. H. Cabell, P. A. Pappas, P. Jones, M. E. Stryjewski, I. Anguera, S. Braun, P. Munoz, P. Commerford, P. Tornos, J. Francis, M. Oyonarte, C. Selton-Suty, A. J. Morris, G. Habib, B. Almirante, D. J. Sexton, G. R. Corey, and V. G. Fowler, Jr. 2009. Coagulase-negative staphylococcal prosthetic valve endocarditis—a contemporary update based on the International Collaboration on Endocarditis: prospective cohort study. Heart 95:570-576. [DOI] [PubMed] [Google Scholar]
- 7.Chu, V. H., C. W. Woods, J. M. Miro, B. Hoen, C. H. Cabell, P. A. Pappas, J. Federspiel, E. Athan, M. E. Stryjewski, F. Nacinovich, F. Marco, D. P. Levine, T. S. Elliott, C. Q. Fortes, P. Tornos, D. L. Gordon, R. Utili, F. Delahaye, G. R. Corey, and V. G. Fowler, Jr. 2008. Emergence of coagulase-negative staphylococci as a cause of native valve endocarditis. Clin. Infect. Dis. 46:232-242. [DOI] [PubMed] [Google Scholar]
- 8.Cubist Pharmaceuticals. 2008. Cubicin (daptomycin for injection). Prescribing information. Cubist Pharmaceuticals, Inc., Lexington, MA.
- 9.del Rio, A., I. Anguera, J. M. Miro, L. Mont, V. G. Fowler, Jr., M. Azqueta, and C. A. Mestres. 2003. Surgical treatment of pacemaker and defibrillator lead endocarditis: the impact of electrode lead extraction on outcome. Chest 124:1451-1459. [DOI] [PubMed] [Google Scholar]
- 10.Eliopoulos, G., and R. Moellering. 1996. Antimicrobial combinations, p. 330-396. In V. Lorian. (ed.), Antibiotics in laboratory medicine. The Williams & Wilkins, Co., Baltimore, MD.
- 11.Forouzesh, A., P. A. Moise, and G. Sakoulas. 2009. Vancomycin ototoxicity: a reevaluation in an era of increasing doses. Antimicrob. Agents Chemother. 53:483-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowler, V. G., Jr., H. W. Boucher, G. R. Corey, E. Abrutyn, A. W. Karchmer, M. E. Rupp, D. P. Levine, H. F. Chambers, F. P. Tally, G. A. Vigliani, C. H. Cabell, A. S. Link, I. DeMeyer, S. G. Filler, M. Zervos, P. Cook, J. Parsonnet, J. M. Bernstein, C. S. Price, G. N. Forrest, G. Fatkenheuer, M. Gareca, S. J. Rehm, H. R. Brodt, A. Tice, and S. E. Cosgrove. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 355:653-665. [DOI] [PubMed] [Google Scholar]
- 13.Garrett, D. O., E. Jochimsen, K. Murfitt, B. Hill, S. McAllister, P. Nelson, R. V. Spera, R. K. Sall, F. C. Tenover, J. Johnston, B. Zimmer, and W. R. Jarvis. 1999. The emergence of decreased susceptibility to vancomycin in Staphylococcus epidermidis. Infect. Control Hosp. Epidemiol. 20:167-170. [DOI] [PubMed] [Google Scholar]
- 14.Garrison, P. K., and L. R. Freedman. 1970. Experimental endocarditis. I. Staphylococcal endocarditis in rabbits resulting from placement of a polyethylene catheter in the right side of the heart. Yale J. Biol. Med. 42:394-410. [PMC free article] [PubMed] [Google Scholar]
- 15.Hidayat, L. K., D. I. Hsu, R. Quist, K. A. Shriner, and A. Wong-Beringer. 2006. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch. Intern. Med. 166:2138-2144. [DOI] [PubMed] [Google Scholar]
- 16.Hope, R., D. M. Livermore, G. Brick, M. Lillie, and R. Reynolds. 2008. Non-susceptibility trends among staphylococci from bacteraemias in the UK and Ireland, 2001-06. J. Antimicrob. Chemother. 62(Suppl. 2):ii65-ii74. [DOI] [PubMed] [Google Scholar]
- 17.Isenberg, H. D. 2004. Clinical microbiology procedures handbook. ASM Press, Washington, DC.
- 18.John, J. F., and A. M. Harvin. 2007. History and evolution of antibiotic resistance in coagulase-negative staphylococci: susceptibility profiles of new anti-staphylococcal agents. Ther. Clin. Risk Manag. 3:1143-1152. [PMC free article] [PubMed] [Google Scholar]
- 19.LaPlante, K. L., and M. J. Rybak. 2004. Clinical glycopeptide-intermediate staphylococci tested against arbekacin, daptomycin, and tigecycline. Diagn. Microbiol. Infect. Dis. 50:125-130. [DOI] [PubMed] [Google Scholar]
- 20.Marco, F., C. G. de la Maria, Y. Armero, E. Amat, D. Soy, A. Moreno, A. del Rio, M. Almela, C. A. Mestres, J. M. Gatell, M. T. Jimenez de Anta, and J. M. Miro. 2008. Daptomycin is effective in treatment of experimental endocarditis due to methicillin-resistant and glycopeptide-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 52:2538-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miro, J. M., C. Garcia-de-la-Maria, Y. Armero, E. de-Lazzari, D. Soy, A. Moreno, A. del Rio, M. Almela, C. A. Mestres, J. M. Gatell, M. T. Jimenez-de-Anta, and F. Marco. 2007. Efficacy of telavancin in the treatment of experimental endocarditis due to glycopeptide-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 51:2373-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moise, P. A., A. Forrest, S. M. Bhavnani, M. C. Birmingham, and J. J. Schentag. 2000. Area under the inhibitory curve and a pneumonia scoring system for predicting outcomes of vancomycin therapy for respiratory infections by Staphylococcus aureus. Am. J. Health Syst. Pharm. 57(Suppl. 2):S4-S9. [DOI] [PubMed] [Google Scholar]
- 23.Murdoch, D. R., G. R. Corey, B. Hoen, J. M. Miro, V. G. Fowler, Jr., A. S. Bayer, A. W. Karchmer, L. Olaison, P. A. Pappas, P. Moreillon, S. T. Chambers, V. H. Chu, V. Falco, D. J. Holland, P. Jones, J. L. Klein, N. J. Raymond, K. M. Read, M. F. Tripodi, R. Utili, A. Wang, C. W. Woods, and C. H. Cabell. 2009. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 169:463-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NCCLS/CLSI. 2006. Performance standards for antimicrobial disk susceptibility tests; approved standard, 9th ed. M2-A9. NCCLS/CLSI, Wayne, PA.
- 25.Rybak, M., B. Lomaestro, J. C. Rotschafer, R. Moellering, Jr., W. Craig, M. Billeter, J. R. Dalovisio, and D. P. Levine. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 66:82-98. [DOI] [PubMed] [Google Scholar]
- 26.Rybak, M. J., E. Hershberger, T. Moldovan, and R. G. Grucz. 2000. In vitro activities of daptomycin, vancomycin, linezolid, and quinupristin-dalfopristin against staphylococci and enterococci, including vancomycin-intermediate and -resistant strains. Antimicrob. Agents Chemother. 44:1062-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakoulas, G., Y. Golan, K. C. Lamp, L. V. Friedrich, and R. Russo. 2007. Daptomycin in the treatment of bacteremia. Am. J. Med. 120:S21-S27. [DOI] [PubMed] [Google Scholar]
- 28.Wisplinghoff, H., T. Bischoff, S. M. Tallent, H. Seifert, R. P. Wenzel, and M. B. Edmond. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309-317. [DOI] [PubMed] [Google Scholar]
- 29.Wootton, M., R. A. Howe, R. Hillman, T. R. Walsh, P. M. Bennett, and A. P. MacGowan. 2001. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J. Antimicrob. Chemother. 47:399-403. [DOI] [PubMed] [Google Scholar]