Abstract
Peramivir is a neuraminidase (NA) inhibitor (NAI) under development that must be administered by the systemic route. The prophylactic activity of intramuscular (IM) peramivir was evaluated with mice infected with wild-type (WT) and oseltamivir-resistant (H274Y NA mutant) recombinant influenza A/WSN/33 (H1N1) viruses. Treatment regimens consisted of IM injections starting 1 h before viral challenge that were single (45 mg/kg or 90 mg/kg) or multiple (45 mg/kg daily for 5 days). All peramivir regimens prevented mortality and weight loss while significantly reducing lung viral titers (LVT) in mice infected with the WT virus. For animals infected with the H274Y mutant, the multiple-dose regimen completely prevented mortality and was associated with significant reduction in weight loss and LVT compared to untreated animals. In contrast, both single-treatment regimens reduced mortality and weight loss but did not significantly reduce LVT. Although further experiments using different influenza A/H1N1 virus strains and other animal models are needed, our results suggest that 5-day IM peramivir therapy may be considered a prophylactic alternative to control influenza infections caused by oseltamivir-resistant viruses with the H274Y mutation.
Neuraminidase (NA) inhibitors (NAIs) constitute one of the most valuable options for the control of influenza epidemics and pandemics. Two NAIs, inhaled zanamivir and oral oseltamivir, have been approved for the treatment and prevention of influenza infections in many countries (16). In addition, other NAIs are at different stages of development. Peramivir, which is a cyclopentane analogue compound, has shown potent in vitro activity against influenza A and B viruses (4). By the use of NAI assays, we previously demonstrated that peramivir 50% inhibitory concentration (IC50) values for Canadian clinical influenza A/H3N2, A/H1N1, and B viruses were lower than those of zanamivir and oseltamivir (10). In other studies, mean IC50 values of clinical influenza A/H1N1 viruses from untreated individuals against peramivir were also lower than those against oseltamivir and zanamivir (14, 15). Furthermore, on-site dissociation studies demonstrated that peramivir remained tightly bound to the NA enzyme with a half-time for the substrate conversion of >24 h compared to 1.25 h for both zanamivir and oseltamivir (5).
In controlled trials of prophylaxis and treatment, oral peramivir was associated with reduced viral titers but no significant decrease in time to relief of symptoms, a feature that could be attributed to a low oral bioavailability in humans (6). The bioavailability of peramivir may be improved by using intravenous (IV) or intramuscular (IM) injections. Indeed, comparison of single IM versus oral peramivir with the same dose (10 mg/kg), administered 4 h prior to a lethal influenza A/WSN/33 (H1N1) virus challenge, demonstrated that the IM route was associated with a higher survival rate in mice than that of the oral route (100% versus 50%) (5). Also, a single IV injection of 3 mg/kg of peramivir provided a significant therapeutic effect that was superior to that of oral oseltamivir in a lethal mouse model of influenza A and B virus infections (18). The emergence and rapid dissemination of the seasonal A/Brisbane/59/2007 (H1N1) virus containing the NA mutation H274Y in N2 numbering (H275Y in N1 numbering), which is associated with a high level of resistance to oseltamivir and moderate cross-resistance to peramivir in vitro (9), are a major clinical concern. The aim of the present study was to evaluate the prophylactic efficacy of IM injections of peramivir in mice infected with a recombinant influenza A/WSN/33 (H1N1) virus containing or not containing the H274Y NA mutation, which has been associated with 427- and 48-fold increases in oseltamivir and peramivir IC50 values, respectively, in NAI assays (1).
MATERIALS AND METHODS
Six- to 8-week-old male BALB/c mice (Charles River, Lasalle, QC, Canada) were randomized on the basis of their weight (20 to 25 g), housed four per cage, and kept under conditions which prevented cage-to-cage infections. Peramivir (Biocryst, Birmingham, AL) was dissolved to 50 mg/ml in saline buffered to pH 3.0 and administered intramuscularly (i.e., by hip injection) 1 h prior to viral challenge. Groups of 12 mice received either a single dose of 45 mg/kg, a single dose of 90 mg/kg, or multiple doses which consisted of 45 mg/kg once daily for 5 days. Control groups included 12 untreated and infected mice, 6 uninfected mice that received multiple 45-mg/kg doses, and 6 uninfected mice that received phosphate-buffered saline (PBS). The mice were inoculated intranasally, under isoflurane anesthesia, with 8 × 103 PFU of the cell-grown recombinant wild-type (WT) or H274Y NA mutant virus in 30 μl of PBS. Mice were monitored daily for body weight loss, and mortality was recorded over a period of 14 days. For determination of lung viral titers (LVT), subgroups of 4 mice were sacrificed on day 4, and then their lungs were removed aseptically and homogenized in 2 ml of sterile PBS containing antibiotics. Lung homogenates were then centrifuged at 600 × g for 10 min, and supernatants were titrated in Madin-Darby bovine kidney (MDBK) cells by using a standard plaque assay.
A one-way analysis of variance was done to compare mean weight loss values between different treatment regimens, whereas LVT were compared by using the two-tailed Student unpaired t test.
RESULTS
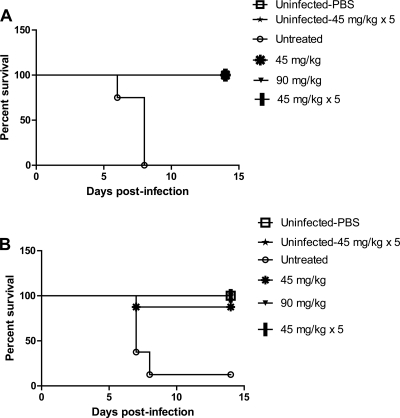
Intranasal inoculation of untreated mice with 8 × 103 PFU of the recombinant WT or the H274Y NA mutant virus resulted in mortality rates of 100% (8/8) and 87.5% (7/8), respectively. For the WT virus group, 2/8 mice died on day 6 postinfection (PI), and the remaining 6 mice died on day 7 PI, with a mean day to death of 6.75 (Fig. 1 A). For the H274Y mutant virus group, 5/8 mice died on day 7 PI, 2 died on day 8 PI, and the last mouse survived, although it appeared ill and its body weight loss reached 15.2% on day 7 PI (Fig. 1B). The resulting mean day to death for the H274Y mutant virus group was 7.28. There was no death recorded for all peramivir-treated groups infected with the WT virus, whereas only one animal died in the single-45-mg/kg-dose group following infection with the H274Y NA mutant virus. No mortality was recorded for uninfected mice that received PBS or the multiple-45-mg/kg-dose peramivir regimen.
FIG. 1.
Impact of IM peramivir regimens on survival in mice infected with 8 × 103 PFU of the recombinant WT or H274Y neuraminidase mutant influenza A/H1N1 viruses. Peramivir regimen treatments were single (45 mg/kg or 90 mg/kg) or multiple (45 mg/kg once daily for 5 days). The survival curves for mice infected with the WT (A) and the H274Y mutant (B) are shown.
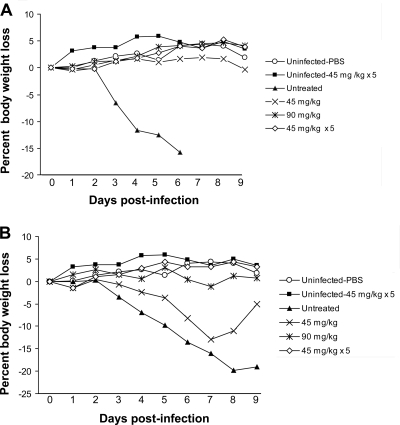
Infection with the WT and H274Y NA mutant viruses resulted in significant body weight loss in untreated animals, with mean weight losses on day 6 PI of 15.8% and 13.6%, respectively. In sharp contrast, there was no weight loss in all peramivir-treated animals infected with the WT virus (Fig. 2 A). Similarly, there was no significant weight loss in mice infected with the H274Y NA mutant virus that received the single 90-mg/kg-dose and the multiple 45-mg/kg-dose peramivir regimens (Fig. 2B). In contrast, there was a significant weight loss on day 6 (i.e., 8.2%, P < 0.05 compared to uninfected mice receiving PBS) in the single-45-mg/kg-dose peramivir regimen. Notably, no weight loss was noted for uninfected mice that received multiple 45-mg/kg peramivir doses.
FIG. 2.
Impact of IM peramivir regimens on body weight loss in mice infected with 8 × 103 PFU of the recombinant WT (A) or H274Y neuraminidase mutant (B) influenza A/H1N1 viruses. Peramivir regimen treatments were single (45 mg/kg or 90 mg/kg) or multiple (45 mg/kg once daily for 5 days). Uninfected mice that received PBS or multiple doses (45 mg/kg once daily for 5 days) as a peramivir regimen were used as controls.
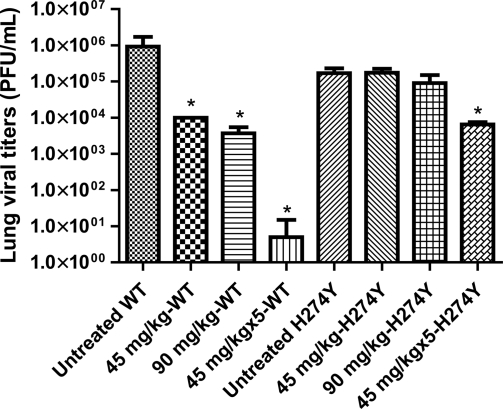
All peramivir regimens were associated with a significant reduction in LVT compared to untreated animals, with a clear dose response in mice infected with the WT virus (Fig. 3). The highest reduction in LVT (approximately 5 log10) was observed with the group that received the multiple-dose (45 mg/kg × 5 days) peramivir regimen. In mice infected with the H274Y NA mutant virus, the multiple-dose regimen was also associated with a significant, albeit smaller, reduction in LVT (approximately 1.5 log10, P < 0.05) compared to the untreated group. However, there was no significant reduction in LVT in the two single-dose peramivir groups. Sequence analysis of the hemagglutinin (HA) and NA genes of viruses collected from lung samples confirmed the expected genotypes with no additional NA or HA mutations being detected compared to the challenge viruses.
FIG. 3.
Impact of IM peramivir regimens on lung viral titers in mice infected with 8 × 103 PFU of the recombinant WT or H274Y neuraminidase mutant influenza A/H1N1 viruses. Peramivir regimen treatments were single (45 mg/kg or 90 mg/kg) or multiple (45 mg/kg once daily for 5 days). Lungs were removed from subgroups of 4 mice that were sacrificed on day 4 postinfection. Viral titers were determined by plaque assays in triplicate experiments. Mean lung viral titers with standard deviations are shown. Asterisks denote significant reduction in viral titers (P < 0.05) compared to the corresponding WT- or H274Y NA mutant-untreated groups.
DISCUSSION
Despite the fact that immunization programs remain the primary means for the control of influenza infections, antiviral therapy may provide valuable additional benefits for both therapeutic and prophylactic purposes (2). This is particularly true when a vaccine is not available (pandemics), when antigenic mismatches occur (epidemics), or for severely immunocompromised individuals not responding to immunization. However, the long-term usefulness of anti-influenza agents may be compromised by the development and transmission of drug-resistant viruses. This has been a particularly important problem in recent years, with the global dissemination of seasonal A/H3N2 viruses resistant to M2 inhibitors (11) and seasonal A/H1N1 viruses resistant to oseltamivir (13). In addition, occasional cases of oseltamivir resistance have also been reported for avian A/H5N1 viruses and for the new pandemic A/H1N1 virus of swine origin (8, 12). In influenza viruses of the N1 subtype, the most frequent NA change conferring resistance to oseltamivir occurs at codon 274 (H→Y), and such a mutation is associated with a lower level of resistance to peramivir but susceptibility to zanamivir (3). Although resistance to zanamivir in clinical influenza virus isolates has remained marginal so far (16), the use of this drug has been limited by the need for an inhaler device and the risk of bronchospasms. These facts highlight the importance of developing other anti-influenza agents that remain active against oseltamivir-resistant viruses.
In this study, we investigated the in vivo prophylactic efficacy of peramivir against the recombinant influenza A/WSN/33 (H1N1) virus containing or not containing the H274Y NA mutation. We hypothesized that, due to an improved pharmacodynamic profile conferred by IM injections, peramivir would retain an inhibitory effect not only against the WT influenza A/H1N1 virus but also against the H274Y mutant associated with resistance to oseltamivir.
Based on mortality rates, body weight losses, and LVT, our results confirm the efficacy of a single 45- or 90-mg/kg-dose as well as multiple (45-mg/kg × 5)-dose peramivir regimens against the WT A/H1N1 virus. Also, based on body surface area conversion calculations, the 45-mg/kg and 90-mg/kg doses in mice are equivalent to approximately 300-mg and 600-mg doses in humans, which are the doses evaluated in clinical trials. Interestingly, our results further demonstrate the efficacy of IM peramivir using the 5-day regimen against the H274Y mutant despite in vitro data showing moderate levels of resistance to this drug. This could be explained by the pharmacokinetic (PK) properties of IM peramivir. In a mouse PK study conducted with IM peramivir at a single dose of 30 mg/kg (Y. S. Babu, Biocryst, personal communication), the drug was rapidly absorbed with drug levels reaching their maximum concentration by 30 min. The peramivir plasma concentration at 30 min (17,675 ng/ml) was 4,149 times above the IC50 value (4.26 ng/ml) for the H274Y mutant evaluated in this study. The plasma concentration (29.3 ng/ml) was still 6.9 times higher than the IC50 value after 24 h. One would expect even higher drug levels with the 45-mg/kg and 90-mg/kg doses that were used in this mouse efficacy study. Thus, the rapid absorption and the high drug levels achieved with IM peramivir proved to be efficacious against the H274Y mutant virus used in this mouse model. In a similar mouse model, we recently demonstrated that prophylactic and therapeutic oral oseltamivir regimens did not prevent mortality and weight loss following infection with the same recombinant H274Y mutant virus (7). Unlike what we observed in the peramivir-treated groups infected with the WT virus, reduction in LVT did not closely match reduction in weight loss in the case of mice infected with the H274Y NA mutant virus. However, as the WT and mutant viruses may differ in their replication profiles, the determination of LVT at additional time points would have been needed to clarify this discrepancy. Thus, additional studies are needed to understand the differences in viral dynamics between the WT and mutant viruses.
Previous toxicology studies using different animal models (ferrets, mice, rats, dogs, and primates) showed that peramivir was well tolerated following oral, IV, and IM administrations (5, 6, 17, 18). Accordingly, in this study, we did not observe any obvious signs of drug-related toxicity in the control uninfected group that received multiple 45-mg/kg IM doses of peramivir.
In conclusion, based on our lethal influenza A/WSN/33 (H1N1) virus model, IM peramivir may be considered a possible alternative for selected prophylactic indications in at-risk patients and in the case of institutional outbreaks in which an H274Y NA mutant could be involved. Whether IM peramivir would also be efficacious in treatment of such mutants still requires further experiments. In addition, our findings remain to be confirmed by testing other human influenza virus strains, such as recent seasonal A/H1N1 strains containing the H274Y NA mutation. However, other animal models may be required for such investigation, as human influenza viruses are generally not pathogenic in mice. Finally, human trials are needed to confirm whether the efficacy of IM peramivir against the H274Y mutant shown in our mouse model could be extended to humans.
Acknowledgments
This work was supported by a research grant from Biocryst.
Footnotes
Published ahead of print on 19 April 2010.
REFERENCES
- 1.Abed, Y., N. Goyette, and G. Boivin. 2004. A reverse genetics study of resistance to neuraminidase inhibitors in an influenza A/H1N1 virus. Antivir. Ther. 9:577-581. [PubMed] [Google Scholar]
- 2.Abed, Y., and G. Boivin. 2006. Treatment of respiratory virus infections. Antiviral Res. 70:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoki, F. Y., G. Boivin, and N. Roberts. 2007. Influenza virus susceptibility and resistance to oseltamivir. Antivir. Ther. 12:603-616. [PubMed] [Google Scholar]
- 4.Babu, Y. S., P. Chand, S. Bantia, P. Kotian, A. Dehghani, Y. El-Kattan, T. H. Lin, T. L. Hutchison, A. J. Elliott, C. D. Parker, S. L. Ananth, L. L. Horn, G. W. Laver, and J. A. Montgomery. 2000. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J. Med. Chem. 43:3482-3486. [DOI] [PubMed] [Google Scholar]
- 5.Bantia, S., C. S. Arnold, C. D. Parker, R. Upshaw, and P. Chand. 2006. Anti-influenza virus activity of peramivir in mice with single intramuscular injection. Antiviral Res. 69:39-45. [DOI] [PubMed] [Google Scholar]
- 6.Barroso, L., J. Treanor, L. Gubareva, and F. G. Hayden. 2005. Efficacy and tolerability of the oral neuraminidase inhibitor peramivir in experimental human influenza: randomized, controlled trials for prophylaxis and treatment. Antivir. Ther. 10:901-910. [PubMed] [Google Scholar]
- 7.Baz, M., Y. Abed, B. Nehmé, and G. Boivin. 2009. Activity of the oral neuraminidase inhibitor A-322278 against the oseltamivir-resistant H274Y (A/H1N1) influenza virus mutant in mice. Antimicrob. Agents Chemother. 53:791-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baz, M., Y. Abed, J. Papenburg, X. Bouhy, M. E. Hamelin, and G. Boivin. 2009. Emergence of oseltamivir-resistant pandemic H1N1 virus during prophylaxis. N. Engl. J. Med. 23:2296-2297. [DOI] [PubMed] [Google Scholar]
- 9.Baz, M., Y. Abed, P. Simon, M. E. Hamelin, and G. Boivin. 2010. Impact of the neuraminidase mutation H274Y conferring resistance to oseltamivir on the replicative capacity and virulence of different influenza A/H1N1 viruses. J. Infect. Dis. 201:740-745. [DOI] [PubMed] [Google Scholar]
- 10.Boivin, G., and N. Goyette. 2002. Susceptibility of recent Canadian influenza A and B virus isolates to different neuraminidase inhibitors. Antiviral Res. 54:143-147. [DOI] [PubMed] [Google Scholar]
- 11.Bright, R. A., D. K. Shay, B. Shun, N. J. Cox, and A. L. Klimov. 2006. Adamantane resistance among influenza A viruses isolated early during the 2005-2006 influenza season in the United States. JAMA 295:891-894. [DOI] [PubMed] [Google Scholar]
- 12.de Jong, M. D., T. T. Tran, H. K. Truong, M. H. Vo, G. J. Smith, V. C. Nguyen, T. Q. Phan, Q. H. Do, Y. Guan, J. S. Peiris, T. H. Tran, and J. Farrar. 2005. Oseltamivir resistance during treatment of influenza A(H5N1) infection. N. Engl. J. Med. 353:2667-2672. [DOI] [PubMed] [Google Scholar]
- 13.Dharan, N. J., L. V. Gubareva, and J. J. Meyer. 2009. Infections with oseltamivir resistant influenza A/H1N1 virus in the United States. JAMA 301:1034-1041. [DOI] [PubMed] [Google Scholar]
- 14.Gubareva, L. V., R. G. Webster, and F. G. Hayden. 2001. Comparison of the activities of zanamivir, oseltamivir, and RWJ-270201 against clinical isolates of influenza virus and neuraminidase inhibitor-resistant variants. Antimicrob. Agents Chemother. 45:3403-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurt, A. C., J. Ernest, Y. M. Deng, P. Lanello, T. G. Besselaer, C. Birch, P. Buchy, M. Chittaganpitch, S. C. Chiu, D. Dwyner, A. Guigon, B. Harrower, L. P. Kei, T. Kok, C. Lin, K. McPhie, A. Mohd, R. Olveda, T. Panayotou, W. Rawlinson, L. Scott, D. Smith, H. D'Souza, N. Komadina, R. Shaw, A. Kelso, and I. G. Barr. 2009. Emergence and spread of oseltamivir-resistant A(H1N1) influenza viruses in Oceania, South East Asia and South Africa. Antiviral Res. 83:90-93. [DOI] [PubMed] [Google Scholar]
- 16.Moscona, A. 2005. Neuraminidase inhibitors for influenza. N. Engl. J. Med. 353:1363-1373. [DOI] [PubMed] [Google Scholar]
- 17.Sidwell, R. W., D. F. Smee, J. H. Hufman, D. L. Barnard, K. W. Bailey, J. D. Morrey, and Y. S. Babu. 2001. In vivo influenza virus-inhibitory effects of the cyclopentane neuraminidase inhibitor RWJ-270201. Antimicrob. Agents Chemother. 45:749-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida, R., M. Kodama, M. Kobayashi, M. Kitano, A. Sato, and Y. Yamano. 2009. Therapeutic effect of peramivir (S-021812, BCX-1812) after single intravenous treatment of mice infected with influenza A and B viruses, abstr. V-1065, p. 67. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.