Abstract
Ampicillin-resistant Enterococcus faecium (ARE) and vancomycin-resistant E. faecium (VRE) are important nosocomial pathogens. We quantified effects of probiotics and antibiotics on intestinal acquisition of ARE colonization in patients hospitalized in two non-intensive care unit (non-ICU) wards with high ARE prevalence. In a prospective cohort study with crossover design, all patients with a length of stay of >48 h were offered a multispecies probiotic product twice daily until discharge (4.5 months, intervention period) or not (4.5 months, control period). Perianal ARE carriage was determined <48 h after admission, twice weekly, and <48 h before discharge. The first isolates were genotyped by multiple-locus variable-number tandem repeat analysis (MLVA). Risk factors for acquisition were determined by Cox proportional hazards modeling, with special emphasis on ecological postantibiotic effects and delays between actual acquisition and culture positivity. Of 530 patients included, 94 (18%) were ARE colonized on admission. Of the remaining 436 noncolonized patients, 92 acquired ARE colonization: 28 (25%) of 110 probiotic users and 64 (20%) of 326 control patients (χ2 test, P = 0.325). In all, 661 ARE strains were isolated from 186 patients, of which 186 were genotyped. In both wards, two MLVA types (MTs; MT1 and MT159) were responsible for >80% of acquisitions. Both MTs were genetically different from the probiotic E. faecium strain. Antibiotics to which ARE is resistant (hazard ratio [HR], 7.73 [95% confidence interval (CI), 4.52 to 13.22]), an ecological postantibiotic effect (HR, 7.11 [95% CI, 3.10 to 16.30]), and age (HR, 1.01 [95% CI, 0.99 to 1.02]) were associated with ARE acquisition. The HR of probiotics was 1.43 (95% CI, 0.88 to 2.34). In a setting with high selective antibiotic pressure, probiotics failed to prevent acquisition of multiresistant enterococci.
Enterococci have emerged from a commensal of the digestive tract to the third most common nosocomial pathogen in U.S. hospitals (13). Acquired antibiotic resistance seriously limits therapeutic options when infections occur (19). In the United States, ampicillin-resistant Enterococcus faecium (ARE) emerged as a nosocomial pathogen in the 1980s, and the nationwide epidemic of vancomycin-resistant E. faecium (VRE) followed in the 1990s (7, 19). Nowadays, VRE nosocomial outbreaks have been encountered on all continents (16). In the University Medical Center Utrecht (UMCU), the proportion of ARE infections among all enterococcal infections increased from 2% in 1994 to 50% in 2008, with the prevalence of intestinal carriage being as high as 50% in several wards, mimicking VRE epidemiology in U.S. hospitals (4, 24).
Molecular epidemiological analyses revealed the existence of a polyclonal subpopulation of E. faecium responsible for the majority of nosocomial infections and hospital outbreaks worldwide. These so-called hospital-acquired E. faecium clones, previously designated clonal complex 17, are characterized by ampicillin and ciprofloxacin resistance and are enriched with >100 specific genes, including genes encoding antibiotic resistance and (putative) virulence factors (16, 17).
In humans, intestinal colonization with hospital-acquired bacteria, such as E. faecium, is thought to be facilitated by disruption of the commensal microbiota of the gastrointestinal tract, which is assumed to protect against overgrowth with opportunistic pathogens. Antibiotics are considered the key factor in this process (4, 9, 24). Other important variables for acquisition of enterococci include colonization pressure, environmental contamination, and length of stay (3, 11).
It has been hypothesized that probiotics help to maintain the integrity of the intestinal flora and that they augment restoration of integrity after disruption, for instance in patients with antibiotic-induced diarrhea (8, 12). In one study, probiotics were associated with increased clearance of intestinal colonization with VRE (18). Yet, the role of probiotics in preventing the spread of multiresistant bacteria has not been elucidated.
In this study we aimed to quantify the effects of probiotics and antibiotics on acquisition of ARE colonization in patients admitted to two non-intensive care unit (non-ICU) hospital wards with a previously documented, high prevalence of intestinal ARE carriage (4, 24). As the effects of probiotics and antibiotics will not occur (or disappear) instantaneously after administration (or discontinuation) and since carriage will not be detectable immediately after acquisition, we explicitly addressed the potential delays in these events through time-dependent sensitivity analyses.
MATERIALS AND METHODS
Setting.
The study was executed in two non-ICU wards with documented high prevalences of intestinal ARE colonization: the gastroenterology/nephrology ward (24 beds) and geriatric ward (15 beds) (4). The UMCU is a tertiary care center with 1,042 beds.
Design.
We undertook a prospective cohort study with a crossover design to study the effects of probiotics on acquisition of ARE colonization, both on a patient level as well as on a ward level. The latter is relevant, as the chance for an individual patient to acquire ARE colonization depends on the number of other colonized patients present in the ward (i.e., colonization pressure) (3). As a consequence, modulation of the risk of acquisition in one patient will also influence the risk of acquisition in other patients: it will induce a so-called ecologic effect. There were no changes in hygiene and infection prevention policies or alterations in antibiotic policies and practices in antibiotic use throughout the study.
Patients.
During 267 days between May 2007 and January 2008 (gastroenterology/nephrology ward) and 295 days between June 2007 and March 2008 (geriatric ward), all patients with an expected length of stay (LOS) of >48 h were screened for ARE colonization by obtaining perianal swabs within 48 h after admission, within 48 h before discharge, and twice weekly. For both wards, the study consisted of two periods of 4.5 months: a control period without intervention and an intervention period in which all patients with an expected LOS of >48 h were offered probiotics twice daily during their entire stay on the wards. Dysphagia was the only exclusion criterion for taking probiotics.
Probiotics.
We used a multispecies probiotic powder (Ecologic AAD; Winclove Bio Industries B.V., Amsterdam, Netherlands) containing 10 species (Bifidobacterium bifidum, Bifidobacterium lactis [2×], Enterococcus faecium, Lactobacillus acidophilus [2×], Lactobacillus paracasei, Lactobacillus plantarum, Lactobacillus rhamnosus, and Lactobacillus salivarius), each at 108 CFU/g, with a total concentration of 109 CFU/g. This compilation of probiotics was specially selected according to documented in vitro inhibition of growth and biofilm formation in hospital-associated ARE (15). Moreover, the strains in this product all harbored the intrinsic capacity to survive, in vitro, bile, digestive enzymes (pancreatin and pepsin), and a low pH (2.5), enabling them to survive passage of the first part of the digestive tract alive (14). Antagonistic effects on the growth of the combination of probiotic strains in vitro were excluded. The susceptibility of the probiotic strains was known for ampicillin, ciprofloxacin, erythromycin, vancomycin, tetracycline, trimethoprim, cefotiam, and clindamycin. For vancomycin, ciprofloxacin, and cefotiam, 6 of 10 probiotic strains were susceptible, for tetracycline 7 of 10, for trimethoprim 8 of 10, for clindamycin 9 of 10, and for ampicillin and erythromycin 10 of 10. The same probiotic mixture has been associated with enhanced recovery of the intestinal flora after amoxicillin use in healthy volunteers, without observation of side effects (14). The multilocus sequence type (MLST) and virulence gene profile of the probiotic E. faecium strain (susceptible to amoxicillin) was genetically distinct from previously studied hospital-acquired E. faecium clones (16). Before intake, nursing staff dissolved a sachet containing 5 g of the probiotic powder in 100 ml water. The dissolved probiotics were distributed twice daily during the morning and evening medication rounds. Intake was recorded on special study forms. Probiotics were given at least 2 h before or after oral antibiotics.
Ethics approval.
As perianal screening for ARE (and VRE) carriage is part of the regular infection control surveillance policy in our hospital and probiotics were considered harmless food supplements, the Institutional Review Board waived the need for written informed consent. Only verbal consent was required.
Clinical trial registration.
This trial was registered under ISRCT number ISRCTN58761709 in the Nederlands Trial Register (NTR).
Microbiology and genotyping.
Perianal swabs were analyzed as described previously (4). Resistance to amoxicillin was confirmed by Rosco tablets (Rosco Diagnostics A/S, Taastrup, Denmark). From all patients colonized with ARE during admission, the first ARE isolate was genotyped using multiple-locus variable-number tandem repeat analysis (MLVA) as previously described (23).
Statistical analysis.
Acquisition of ARE, among patients not colonized with ARE on admission, was the primary outcome. Acquisition was assumed to happen exactly between the last negative and the first positive swab. Patients who did not acquire ARE colonization were censored at discharge (when the last negative culture was obtained <48 h before) or 1 day after the last screening sample. Once colonized, patients were no longer considered at risk for acquisition.
The data were analyzed with a Cox proportional hazards model with the intervention, probiotics, incorporated as a dichotomous time-dependent covariate. In a second analysis this variable was replaced by study period (intervention or control) to study the ecologic effect of probiotics. The following variables were designated potential confounders: age and ward as time-constant covariates and colonization pressure, isolation, and use of antibiotics as time-dependent covariates. Colonization pressure was calculated per day for each patient at risk.
Colonization on admission was defined as ARE carriage documented <48 h after admission. If the first culture was taken >48 h after admission and yielded ARE, the patient was considered to be colonized from the midpoint between the day of admission and the first positive swab. There were no infection control measures for ARE during the study periods, and general hygiene and infection control policies did not change.
Antibiotics active in the intestinal compartment were distinguished into two groups, based on their effect (or lack of effect) on ARE (Table 1) (20). Here, we considered piperacillin-tazobactam to be intestinally active against ARE, based on its ability to achieve high biliary concentrations in human bile exceeding the MICs for most multiresistant enterococci (5, 21). A third antibiotic group was designed to account for an “ecological postantibiotic” effect of 3 days, as restoration of the indigenous flora takes time after antibiotic discontinuation. Sensitivity analyses were done with 0 and 5 days, and statistical interaction between antibiotics and probiotics was considered.
TABLE 1.
Antibiotic use by patients at risk for ARE acquisition
| Antibiotic | No. of patients | No. of days |
|---|---|---|
| Antibiotics active in the intestinal compartment | ||
| Oral | ||
| Amoxicillin | 3 | 12 |
| Amoxicillin-clavulanic acid | 38 | 204 |
| Azithromycin | 2 | 6 |
| Ciprofloxacin | 41 | 279 |
| Clarithromycin | 1 | 5 |
| Clindamycin | 2 | 13 |
| Cotrimoxazol | 42 | 361 |
| Erythromycin | 3 | 24 |
| Norfloxacin | 4 | 45 |
| Tetracycline | 2 | 20 |
| Trimethoprim | 5 | 26 |
| Vancomycina | 1 | 6 |
| Flucloxacillin | 2 | 7 |
| Intraperitoneal | ||
| Cefazolin | 1 | 3 |
| Cephalothin | 1 | 2 |
| Intravenous | ||
| Amoxicillin | 3 | 10 |
| Amoxicillin-clavulanic acid | 48 | 237 |
| Cefazolin | 4 | 5 |
| Cefotaxime | 1 | 7 |
| Ceftazidime | 1 | 8 |
| Ceftriaxone | 61 | 403 |
| Ciprofloxacin | 6 | 11 |
| Erythromycin | 2 | 6 |
| Meropenem | 5 | 36 |
| Metronidazole | 2 | 2 |
| Piperacillina | 1 | 2 |
| Piperacillin-tazobactama | 4 | 21 |
| Antibiotics without activity in the intestinal compartment | ||
| Intraperitoneal | ||
| Vancomycin | 1 | 5 |
| Intravenous | ||
| Cefuroxime | 7 | 7 |
| Gentamicin | 20 | 35 |
| Vancomycin | 2 | 5 |
| Benzylpenicillin | 1 | 29 |
| Flucloxacillin | 5 | 27 |
Antibiotics active against ARE in the intestinal compartment.
Allowing covariates to change in time might lead to biased estimates, as the relationship between determinants and outcome is longitudinal (6). Moreover, there will be a delay between actual acquisition and reaching the detection limit of the culture method. Therefore, risks of acquisition (or, more correctly, of culture positivity) will be influenced by the values of covariates on 1 or more days before that time point. This was investigated by fitting a full model (with all covariates) to different values of lagged time (0 to 3 days) for the time-dependent covariates, which were compared using Akaike's information criterion (AIC; in this case the AIC is equivalent to the likelihood, as the numbers of parameters are similar in all models), and the model with the best fit was selected for further analyses (1).
The Cox model was fitted with the survival and spline packages in R 2.8.0, using the Efron method for ties. We used AIC to determine the model with the best balance of parsimony and fit of the data (i.e., the model with the lowest AIC value). The proportional hazards assumption was assessed by formal tests and graphically, using scaled Schoenfeld residuals.
In a sample size calculation we determined that, assuming equal proportions of patients in both the probiotic and control groups, at least 66 acquisitions were required to detect a hazard ratio (HR) of 0.5 at a 5% significance level (two sided) with a power of 80% (22). Based on previous admission data (on average 850 and 250 admissions per year with a length of stay of >48 h on the gastroenterology/nephrology and hematology wards, respectively), an assumed inclusion rate of 70%, and a formerly documented acquisition rate of 15%, we set the length of the study to at least two times 3.5 months (4).
RESULTS AND DISCUSSION
Patient characteristics and acquisition rates.
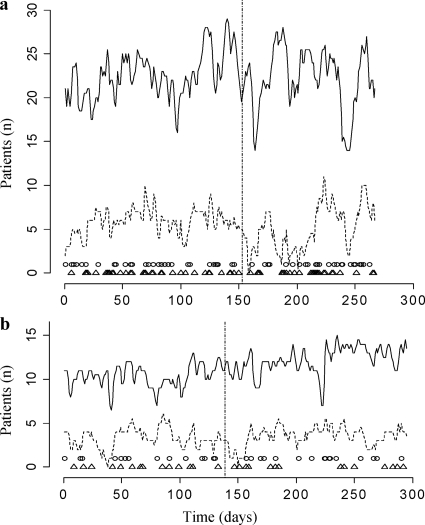
During the study, 971 and 273 patients, of whom 598 and 228 had a length of stay of >48 h, were admitted to the gastroenterology/nephrology and geriatric wards, respectively. Colonization status on admission was determined for 383 (64%) and 147 (64%) of all patients with a length of stay of >48 h, and daily colonization status was, on average, known for 71% of the patients in both wards. In all, 2,087 cultures were obtained. Based on length of stay, there was no reason to assume that patients with an admission swab taken (n = 530; median length of stay [interquartile range {IQR}], 8 [8.25]) were different from those without (n = 296; median length of stay [IQR], 7 [7]). Ninety-four (18%) of 530 patients were colonized with ARE on admission, leaving 436 patients at risk for acquisition. From 62 (14%) of the patients at risk, no discharge swab was taken. Of the 436 patients at risk, 110 (25%) used probiotics during, on average, 58% (range of 10 to 100%) of their days at risk. Eighty-one percent of the users started with probiotics within 4 days after admission, and 83% stopped taking probiotics in the last 48 h before the end of follow-up (e.g., discharge, acquisition, or last culture). Intake of probiotics was interrupted for 15 patients for, in total, 26 days (1 to 6 days per patient). Acquisition of ARE colonization occurred in 92 (21%) patients at risk: in 28 (25%) of 110 patients taking probiotics and in 64 (20%) of 326 control patients (χ2 test, P = 0.325). Incidence densities were 27.0 and 20.4 per 1,000 patient days at risk in the probiotic and control groups, respectively. Besides normal fluctuations in both the number of colonized patients and the total number of patients, no structural changes in the ARE prevalence between periods were noted (Fig. 1). In total, 661 ARE strains were isolated from the 186 patients that were colonized during admission, of which all 186 first isolates were genotyped using MLVA. In both wards two MLVA types (MTs) were found most frequently among patients colonized on admission: MT1 in 24% and 23% and MT159 in 56% and 52% of the colonized admissions in the control periods and intervention periods, respectively. MT159 was responsible for 71% (n = 35) and for 79% (n = 34) of ARE acquisitions in the control and intervention periods, respectively, whereas only 10% and 5% of acquisitions in these periods were with MT1. Therefore, cross-transmission between patients appeared to be responsible for >80% of ARE acquisitions. Based on their MLVA profiles, all these strains belonged to the known circulating nosocomial reservoir of E. faecium and were genetically different from the probiotic E. faecium strain.
FIG. 1.
ARE colonization in the two study wards. (a) Gastroenterology/nephrology ward. (b) Geriatric ward. Solid line represents the total number of patients with a length of stay of >48 h in the ward. Dashed line represents the number of ARE-colonized patients in the ward. Vertical dashed line separates the two study periods of each ward. Open triangles indicate days of acquisition of ARE colonization. Closed circles indicate admission of ARE-colonized patients.
Risk factors for acquisition.
Patient characteristics are listed in Table 2. First, a Cox proportional hazards model was fitted for different values of lagged time (0, 1, 2, and 3 days between actual acquisition and culture positivity) and identified a delay of 1 day as the best fit. Therefore, this delay has been used for all time-dependent covariates in further analyses. This means that the value of a time-dependent covariate on a certain day, for example, the use of antibiotics, influences the chance of a positive culture result on the day after.
TABLE 2.
Baseline characteristics
| Characteristica | Probiotics |
P valueb | |
|---|---|---|---|
| Yes | No | ||
| Patients (n = 436) | 110 | 326 | |
| Median length of stay (days) (IQR) | 9 (8.25) | 8.5 (8.25) | 0.25c |
| Loss to follow-up (no. of patients) (%) | 17 (15) | 45 (14) | 0.67 |
| Median age (yr) (IQR)a | 69 (52-78) | 62 (48-75) | 0.06c |
| Specialty (no. of patients) (%) | |||
| Nephrology | 32 (29) | 132 (40) | 0.001 |
| Gastroenterology | 35 (32) | 123 (38) | |
| Geriatrics | 43 (39) | 71 (22) | |
| Antibiotic use (no. of patients) (%) | 47 (43) | 162 (50) | 0.21 |
| Anti-ARE antibiotics | 1 (1) | 4 (1) | 0.99d |
| Other antibiotics | 47 (43) | 160 (50) | 0.25 |
| Isolation for other reasons (n) (%) | 7 (6) | 22 (7) | 0.89 |
| Patient days (n = 4,168) | 1,037 | 3,131 | |
| Antibiotic use (days) (%) | 301 (29) | 856 (27) | 0.29 |
| Anti-ARE antibiotics | 2 (0) | 27 (1) | 0.03d |
| Other antibiotics | 299 (29) | 829 (26) | 0.14 |
| Ecological postantibiotic effect of 3 days | 37 (4) | 84 (3) | 0.14 |
| Isolation (days) (%) | 57 (5) | 76 (2) | <0.001 |
IQR, interquartile range.
Chi-square test.
Mann-Whitney test.
Fisher's exact test
The hazard ratio for probiotics for ARE acquisition was 1.43 (95% CI, 0.88 to 2.34) (P = 0.15) in the crude (unadjusted) Cox proportional model. A model with, besides probiotics, age and antibiotics as covariates had the best fit, based on the AIC. Yet, after adjustment for potential confounders, the HR for probiotics remained 1.43 (95% CI, 0.87 to 2.35; P = 0.16) (Table 3). Interaction between probiotics and the different categories of antibiotics were not statistically significant. Acquisition of ARE was associated with the use of antibiotics to which ARE is resistant (HR, 7.73 [95% CI, 4.52 to 13.22]; P < 0.001) and an ecological postantibiotic effect during the 3 days after discontinuation of antibiotic treatment (HR, 7.11 [95% CI, 3.10 to 16.30]; P < 0.001), whereas a trend was seen for increasing age (HR, 1.01 [95% CI, 0.99 to 1.02]; P = 0.06) (Table 3).
TABLE 3.
Hazard ratios for ARE acquisition
| Covariate | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Probiotics | 1.43 | 0.87-2.35 | 0.16 |
| Antibiotics | |||
| Anti-ARE antibiotics | 3.74 | 0.49-28.52 | 0.2 |
| Other antibiotics | 7.73 | 4.52-13.22 | <0.001 |
| Ecological postantibiotic effect of 3 days | 7.11 | 3.10-16.30 | <0.001 |
| Age (per year) | 1.01 | 0.99-1.02 | 0.06 |
Sensitivity analyses for the assumption that acquisition occurred exactly between the last negative and the first positive swab did not change the results. Naturally, when the day of acquisition is changed, the value for the delay of the time-dependent covariates might also change; this value will increase when the day of acquisition is moved forward and will decrease when the time at risk for acquisition is shortened. Therefore, we determined the value for the delay for different assumptions for acquisition data, before recalculating hazard ratios for probiotics. The hazard ratio of ARE acquisition for taking probiotics was 1.60 (95% CI, 0.98 to 2.60) (P = 0.06) when acquisition was assumed to occur 1 day after the last negative swab (using a delay of 0 days for time-dependent covariates) and 1.35 (95% CI, 0.81 to 2.25) (P = 0.26) when assuming acquisition occurred 1 day before the first positive culture (using a delay of 1 day).
Likewise, discarding the 3-day ecological postantibiotic effect (HR for probiotics, 1.48 [95% CI, 0.91 to 2.43]; P = 0.12) or extending it to 5 days (HR for probiotics, 1.51 [95% CI, 0.92 to 2.47]; P = 0.11) did not alter conclusions. Although the assumption of an ecological postantibiotic effect of 3 days was based on common sense rather than on available evidence, these analyses support the presence of such an effect, as the model with an ecological postantibiotic effect of 3 days had the best fit based on the AIC.
Finally, we explored the possibility that probiotics need time to achieve a beneficial effect by ignoring the first 2 days of probiotic intake in all patients. Introduction of this threshold effect for probiotics did not change the interpretation of results (HR, 1.56 [95% CI, 0.90 to 2.69]; P = 0.11).
In order to study a potential ecologic effect of probiotics, we replaced the determinant probiotics by study period. Again, a delay of 1 day provided the best fit of the time-dependent covariates. The model with the best balance between parsimony and fit was the one with only age and (all three categories of) antibiotics incorporated as covariates. The hazard ratio of ARE acquisition when admitted in the probiotic period was 1.31 (95% CI, 0.866 to 1.98).
Comments.
In a setting with a high prevalence of intestinal carriage with multiresistant enterococci, daily intake of probiotics did not reduce ARE acquisition rates nor did it change the enterococcal ecology on a ward level. Exposure to antibiotics for which ARE is resistant, though, increased the hazard of acquisition, both during treatment and during (at least) the 3 days after their discontinuation.
We previously demonstrated that ARE epidemiology in our hospital closely mimics the epidemiology of VRE in American hospitals (4, 10). Moreover, vancomycin is the only difference in antibiotic susceptibility between ARE and VRE. Since only one patient in our study received oral vancomycin (during a total of 5 days), we consider our findings on the effects of probiotics and antibiotics generalizable to settings where VRE, instead of ARE, is endemic.
The multispecies probiotic powder that was used in the present study was selected for its ability to inhibit in vitro growth and biofilm formation of hospital-acquired E. faecium, in combination with its capacity to reach the intestinal tract in active form (14). Moreover, it contained an E. faecium strain to enhance competition with ARE for an intestinal niche. Although this strain is susceptible to ampicillin and, therefore, would not be identified in the selective microbial procedure used in our study, we confirmed with MLVA that all isolated ARE strains were genetically different from the probiotic E. faecium strain. In our setting, where both the intestinal flora and ingested probiotic strains are under high selective pressure of antibiotics, the hypothesized preventive effect could not be demonstrated. In another study, though, successful eradication of VRE colonization was reported for patients randomized to receive L. rhamnosus GG yogurt (n = 14) instead of normal yogurt (n = 13) during 3 weeks (18). However, study size and absence of detailed information on baseline characteristics and antibiotic use call for prudence in interpretation.
In general, probiotics are considered harmless food supplements, and the probiotic mixture evaluated in this study had been used with healthy volunteers without any side effects (14). Despite the recently reported adverse events of probiotic administration via a nasojejunal tube in patients with severe acute pancreatitis, there was no evidence that probiotic use, given orally in addition to a normal diet, was unsafe in our population of patients who were not critically ill (2).
Up until now, few studies have evaluated the usefulness of probiotics as a measure to control nosocomial spread of multiresistant pathogens. The strengths of our study include its size and the detailed microbiological analysis with extensive genotyping. However, our study also suffers from some limitations. Although all patients with a length of stay of >48 h were offered probiotics on a daily base, some refused. This might be related to the fact that intestinal colonization is asymptomatic and that, therefore, the potential benefits of probiotics remained intangible for patients. Nevertheless, with 92 acquisitions we still would have been able to demonstrate the effect at which we originally aimed. Moreover, it is unlikely that an increased sample size would have led to a positive effect of probiotics, as the estimate of the hazard points in the opposite direction. Yet, unwillingness to take probiotics, although not related to specific patient groups, introduces the risk of selection bias. This was reflected by some differences in baseline characteristics, for which we adjusted in multivariate analysis. Although we cannot rule out residual confounding, we expect it to have minimal impact, as we adjusted for most important risk factors (4, 9, 24). Furthermore, since cultures were taken twice per week, the exact moment of ARE acquisition was not known and was therefore assumed to occur exactly between the last negative and first positive culture. Sensitivity analyses of the timing of acquisition, however, did not change conclusions. Finally, microbiological culture techniques are imperfect in detecting colonization immediately after acquisition, since time is needed to reach the detection limit of culture methods. This delay between actual acquisition and culture positivity will be important when estimating the effects of time-dependent risk factors. We, therefore, performed time-dependent sensitivity analyses to take this uncertainty about the exact time point of acquisition into account.
In conclusion, the results of this study indicate that there is no role for probiotics in the prevention of colonization with multiresistant enterococci, such as ARE and VRE, in nosocomial settings where they are endemic and selective antibiotic pressure is high.
Acknowledgments
We thank the medical and nursing staff of the gastroenterology/nephrology and geriatric wards and L. Beks for their assistance with data collection and R. Besamusca, R. van Doorn, H. Dekker, and C. Schapendonk of the Department of Medical Microbiology for performing the microbiological cultures and genotyping.
This work was supported by a grant from the European Union Sixth Framework Program under contract LSHE-CT-2007-037410 (ACE project). M. Bonten was supported by NWO-VICI 918.76.611.
We have no financial conflicts to disclose.
Footnotes
Published ahead of print on 19 April 2010.
REFERENCES
- 1.Akaike, H. 1974. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 19:716-723. [Google Scholar]
- 2.Besselink, M. G., H. C. van Santvoort, E. Buskens, M. A. Boermeester, H. van Goor, H. M. Timmerman, V. B. Nieuwenhuijs, T. L. Bollen, B. van Ramshorst, B. J. Witteman, C. Rosman, R. J. Ploeg, M. A. Brink, A. F. Schaapherder, C. H. Dejong, P. J. Wahab, C. J. van Laarhoven, E. van der Harst, C. H. van Eijck, M. A. Cuesta, L. M. Akkermans, and H. G. Gooszen. 2008. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 371:651-659. [DOI] [PubMed] [Google Scholar]
- 3.Bonten, M. J., S. Slaughter, A. W. Ambergen, M. K. Hayden, J. van Voorhis, C. Nathan, and R. A. Weinstein. 1998. The role of “colonization pressure” in the spread of vancomycin-resistant enterococci: an important infection control variable. Arch. Intern. Med. 158:1127-1132. [DOI] [PubMed] [Google Scholar]
- 4.de Regt, M. J., L. E. van der Wagen, J. Top, H. E. Blok, T. E. Hopmans, A. W. Dekker, R. J. Hene, P. D. Siersema, R. J. Willems, and M. J. Bonten. 2008. High acquisition and environmental contamination rates of CC17 ampicillin-resistant Enterococcus faecium in a Dutch hospital. J. Antimicrob. Chemother. 62:1401-1406. [DOI] [PubMed] [Google Scholar]
- 5.Donskey, C. J., J. A. Hanrahan, R. A. Hutton, and L. B. Rice. 2000. Effect of parenteral antibiotic administration on the establishment of colonization with vancomycin-resistant Enterococcus faecium in the mouse gastrointestinal tract. J. Infect. Dis. 181:1830-1833. [DOI] [PubMed] [Google Scholar]
- 6.Fisher, L. D., and D. Y. Lin. 1999. Time-dependent covariates in the Cox proportional-hazards regression model. Annu. Rev. Public Health 20:145-157. [DOI] [PubMed] [Google Scholar]
- 7.Grayson, M. L., G. M. Eliopoulos, C. B. Wennersten, K. L. Ruoff, P. C. De Girolami, M. J. Ferraro, and R. C. Moellering, Jr. 1991. Increasing resistance to beta-lactam antibiotics among clinical isolates of Enterococcus faecium: a 22-year review at one institution. Antimicrob. Agents Chemother. 35:2180-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guarino, A., A. Lo Vecchio, and R. B. Canani. 2009. Probiotics as prevention and treatment for diarrhea. Curr. Opin. Gastroenterol. 25:18-23. [DOI] [PubMed] [Google Scholar]
- 9.Harbarth, S., S. Cosgrove, and Y. Carmeli. 2002. Effects of antibiotics on nosocomial epidemiology of vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 46:1619-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayden, M. K. 2000. Insights into the epidemiology and control of infection with vancomycin-resistant enterococci. Clin. Infect. Dis. 31:1058-1065. [DOI] [PubMed] [Google Scholar]
- 11.Hayden, M. K., M. J. Bonten, D. W. Blom, E. A. Lyle, D. A. van de Vijver, and R. A. Weinstein. 2006. Reduction in acquisition of vancomycin-resistant enterococcus after enforcement of routine environmental cleaning measures. Clin. Infect. Dis. 42:1552-1560. [DOI] [PubMed] [Google Scholar]
- 12.Hickson, M., A. L. D'Souza, N. Muthu, T. R. Rogers, S. Want, C. Rajkumar, and C. J. Bulpitt. 2007. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ 335:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hidron, A. I., J. R. Edwards, J. Patel, T. C. Horan, D. M. Sievert, D. A. Pollock, and S. K. Fridkin. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect. Control Hosp. Epidemiol. 29:996-1011. [DOI] [PubMed] [Google Scholar]
- 14.Koning, C. J., D. M. Jonkers, E. E. Stobberingh, L. Mulder, F. M. Rombouts, and R. W. Stockbrugger. 2008. The effect of a multispecies probiotic on the intestinal microbiota and bowel movements in healthy volunteers taking the antibiotic amoxycillin. Am. J. Gastroenterol. 103:178-189. [DOI] [PubMed] [Google Scholar]
- 15.Kuipers, M. I. 2006. Selected probiotic bacteria disrupt biofilm development of vancomycin-resistant Enterococcus faecium, abstr. 20-21. Biofilms II Int. Conf., Leipzig, Germany, 23 to 24 March 2006.
- 16.Leavis, H. L., M. J. Bonten, and R. J. Willems. 2006. Identification of high-risk enterococcal clonal complexes: global dispersion and antibiotic resistance. Curr. Opin. Microbiol. 9:454-460. [DOI] [PubMed] [Google Scholar]
- 17.Leavis, H. L., R. J. Willems, W. J. van Wamel, F. H. Schuren, M. P. Caspers, and M. J. Bonten. 2007. Insertion sequence-driven diversification creates a globally dispersed emerging multiresistant subspecies of E. faecium. PLoS Pathog. 3:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manley, K. J., M. B. Fraenkel, B. C. Mayall, and D. A. Power. 2007. Probiotic treatment of vancomycin-resistant enterococci: a randomised controlled trial. Med. J. Aust. 186:454-457. [DOI] [PubMed] [Google Scholar]
- 19.Murray, B. E. 2000. Vancomycin-resistant enterococcal infections. N. Engl. J. Med. 342:710-721. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan, A., C. Edlund, and C. E. Nord. 2001. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect. Dis. 1:101-114. [DOI] [PubMed] [Google Scholar]
- 21.Taylor, E. W., V. Poxon, J. Alexander-Williams, and D. Jackson. 1983. Biliary excretion of piperacillin. J. Int. Med. Res. 11:28-31. [DOI] [PubMed] [Google Scholar]
- 22.Therneau, T. M., and P. M. Grambsch. 2000. Modeling survival data: extending the Cox model. Springer, New York, NY.
- 23.Top, J., N. M. Banga, R. Hayes, R. J. Willems, M. J. Bonten, and M. K. Hayden. 2008. Comparison of multiple-locus variable-number tandem repeat analysis and pulsed-field gel electrophoresis in a setting of polyclonal endemicity of vancomycin-resistant Enterococcus faecium. Clin. Microbiol. Infect. 14:363-369. [DOI] [PubMed] [Google Scholar]
- 24.Top, J., R. Willems, H. Blok, M. de Regt, K. Jalink, A. Troelstra, B. Goorhuis, and M. Bonten. 2007. Ecological replacement of Enterococcus faecalis by multiresistant clonal complex 17 Enterococcus faecium. Clin. Microbiol. Infect. 13:316-319. [DOI] [PubMed] [Google Scholar]