Abstract
Amplification of pfmdr1 in Plasmodium falciparum is linked to resistance to aryl-amino-alcohols and in reduced susceptibility to artemisinins. We demonstrate here that duplicated pfmdr1 genotypes circulate in West Africa. The monitoring of this prevalence in Africa appears essential for determining the antimalarial policy and to maintain the efficiency of artemisinin-based combination therapy (ACT) for as long as possible.
Plasmodium falciparum malaria remains a major cause of morbidity and mortality in tropical and subtropical areas. One of the main characteristics of P. falciparum is its ability to become resistant to all the treatments used. To limit the evolution and spread of drug resistance, the World Health Organization (WHO) recommendations are now based on artemisinin (ART)-based combination therapies (ACTs) to treat uncomplicated falciparum malaria. By May 2008, 39 of the 42 countries in Africa where P. falciparum is endemic had adopted ACTs (artemether-lumefantrine [AL] or artesunate-amodiaquine [AS-AQ]) as the first-line treatments (Global AMDP Database [http://www.who.int/malaria/am_drug_policies_by_region_afro/en/index.html]). Even if accessibility to these compounds is still insufficient in Africa, the drug pressure by ART derivatives has increased dramatically in recent years. Furthermore, although mefloquine (MQ) is still not used in Africa, it is likely that it will be introduced within a few years. The current level of resistance to sulfadoxine-pyrimethamine (SP), the only malaria prophylaxis for pregnant women, is high, and therefore, SP is likely to be replaced by MQ (3), as has happened in Southeast Asia. Based on the expectation of new drug policies, the monitoring of the efficacy of ACTs and MQ against P. falciparum is necessary.
Amplification of pfmdr1 is a common molecular marker of ACT and MQ susceptibility. An increase in the copy number of pfmdr1 is associated with clinical failures and with in vitro resistance to aryl-amino-alcohols, particularly MQ, but also to lumefantrine (11, 13). pfmdr1 amplification has also been demonstrated to decrease the susceptibility to ART derivatives in the field as well as in vitro (2, 4, 8, 13), although the role of pfatp6 polymorphism in the phenomenon is unclear.
There is little data on pfmdr1 amplification in Africa. One study carried out in 1995 in Lambarené (Gabon) found 5% of isolates with more than 1 copy number of pfmdr1, but this was not confirmed in 2002 (15). In Kenya, Holmgren et al. (7) identified only one isolate with 2 pfmdr1 copies from 72 isolates tested. Except for one isolate from the Ivory Coast in 1993 (1), no pfmdr1 amplification has been identified in West Africa (6, 16).
Using falciparum malaria patients returning from Africa as a sentinel, we have investigated whether pfmdr1 amplification has occurred in isolates from these patients.
DNA was obtained from patients who returned from West and central Africa between 2005 and 2009 with falciparum malaria diagnosed in the Parasitology-Mycology Department of the Toulouse University Hospital. The copy number of pfmdr1 was determined by real-time PCR with a LightCycler 480 (Roche Diagnostics). The primers were obtained from Price et al. (11). β-Tubulin was used as the one-copy reference gene. The reaction was carried out in a final volume of 10 μl in a 96-well plate (5 μl of LightCycler 480 SYBR green I master mix [Roche Diagnostics], 0.25 mM each primer, 2-μl DNA sample). The amplification program was as follows: (i) 10 min at 95°C; and (ii) 45 cycles, with 1 cycle consisting of 15 s at 95°C, 15 s at 63°C, and 10 s at 70°C. In each experiment, DNAs from the laboratory strains FcM29-Cameroon (1 copy of pfmdr1) and Dd2 (2 or 3 copies of pfmdr1) (16) were used as controls. The efficiency of each PCR (pfmdr1 and β-tubulin) was determined using a scale dilution of the FcM29 DNA. Determination of the copy number was done by comparison of the ratio of pfmdr1/β-tubulin on the LightCycler 480 V1.5.0 software (Roche Diagnostic) taking into account the efficiency of each PCR. The PCR results of all the samples considered in this study could be localized to the linear portion of the efficiency curve in terms of the Cp (crossing point) (Cp value between 20 to 35). The cutoff value of a multicopy was considered to be >1.5. Each sample was analyzed three times (three replicate assays), and each one found with a copy number if more than 1.5 pfmdr1 copies was checked again.
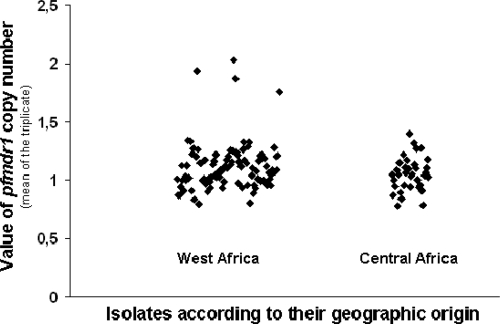
We identified 4 out of 131 isolates (3%) with a pfmdr1 copy number greater than 1. The 4 patients were cured by quinine treatment. These 4 isolates were all from patients in West Africa and were identified in 2008 and 2009. No isolates with pfmdr1 amplification were detected in Central Africa. However, the small number of samples from this area was not sufficient to enable us to reach a conclusion on the prevalence (Table 1 and Fig. 1). It would therefore be interesting to determine the exact prevalence of clones with pfmdr1 amplification and to monitor its evolution in a more extended study of Africa and at multiple test sites. This monitoring could be achieved through the WorldWide Antimalarial Resistance Network (WWARN), now operational (12).
TABLE 1.
Value and country of origin of the four isolates with a pfmdr1 copy number greater than 1
| pfmdr1 copy no. (mean ± SD)a | Country |
|---|---|
| 1.87 ± 0.1 | Ivory Coast |
| 2.03 ± 0.09 | Ivory Coast |
| 1.94 ± 0.2 | Burkina Faso |
| 1.76 ± 0.08 | Togo |
Values (means ± standard deviations) from the three replicate assays.
FIG. 1.
Value of the pfmdr1 copy number in isolates from West Africa and Central Africa. A total of 131 isolates were collected in 2005 to 2009 (2005 [n = 5], 2006 [n = 35], 2007 [n = 33], 2008 [n = 34], and 2009 [n = 24]). From 131 isolates, only 4 had pfmdr1 copy number amplification. The other 127 isolates had a mean pfmdr1 copy number value of 1.08 ± 0.14. Of these 131 isolates, 99 isolates were from West Africa (38 from Ivory Coast, 18 from Burkina Faso, 10 from Senegal, 9 from Guinea Conakry, 6 from Mali, 6 from Benin, 4 from Togo, 3 from Mauritania, 2 from Nigeria, 2 from Ghana, and 1 from Liberia) and 32 isolates were from Central Africa (17 from Cameroon, 8 from the Central Africa Republic, 4 from Gabon, 2 from Congo, and 1 from Chad). Each symbol shows the pfmdr1 copy number for one isolate (mean of three replicate assays).
The presence of these pfmdr1 multicopy clones in West Africa could be linked to rare but actual clinical MQ failures (5) and to the reduced susceptibility of a few isolates to MQ (10, 14) observed in vitro in this area.
In Southeast Asia, specifically in some parts of Thailand and Cambodia, the high level of isolates with a pfmdr1 copy number greater than 1 (30 to 40%) was due to MQ monotherapy for many years as the first-line treatment for uncomplicated falciparum malaria (11). As this treatment is not used in Africa, the prevalence of clones with pfmdr1 amplification remains low at the moment. Nevertheless, because of the widespread prescription of lumefantrine (aryl-amino-alcohol), the partner drug of artemether in ACT (AL) in Africa, monitoring pfmdr1 now appears necessary to determine the possible role of AL in the selection of pfmdr1 copy number amplification. Moreover, in addition to fake formulations, many drugs are used without the authorization of the Ministries of Public Health. In Africa, against WHO recommendations, artesunate and MQ are used in monotherapy and MQ-SP are already being used (9). A misuse of these antimalarial drugs could lead to a rapid increase in the prevalence of strains with amplified pfmdr1, since the transmission level of malaria in Africa is the highest of all areas where malaria is endemic. This situation could accelerate the emergence of resistance to ACTs. Identification of these 4 isolates with pfmdr1 amplification in 2008 and 2009 thus raises the question of the emergence of this genotype in West Africa.
Based on the role of pfmdr1 amplification in the susceptibility of P. falciparum to ART derivatives and aryl-amino-alcohol, avoiding the selection of strains with duplicated pfmdr1 in Africa is essential. This will be a difficult challenge but vital to ensure a longer period of efficacy for the ACTs.
Acknowledgments
We thank John Woodley for revising the English of the manuscript.
Footnotes
Published ahead of print on 19 April 2010.
REFERENCES
- 1.Basco, L. K., J. Le Bras, Z. Rhoades, and C. M. Wilson. 1995. Analysis of pfmdr1 and drug susceptibility in fresh isolates of Plasmodium falciparum from subsaharan Africa. Mol. Biochem. Parasitol. 74:157-166. [DOI] [PubMed] [Google Scholar]
- 2.Begum, K., H. S. Kim, Y. Okuda, Y. Wataya, M. Kimura, and T. Huruta. 2002. Genomic analysis of mefloquine-resistant Plasmodium falciparum. Nucleic Acids Res. 2002(Suppl. 2):223-224. [DOI] [PubMed] [Google Scholar]
- 3.Briand, V., J. Bottero, H. Noel, V. Masse, H. Cordel, J. Guerra, H. Kossou, B. Fayomi, P. Ayemonna, N. Fievet, A. Massougbodji, and M. Cot. 2009. Intermittent treatment for the prevention of malaria during pregnancy in Benin: a randomized, open-label equivalence trial comparing sulfadoxine-pyrimethamine with mefloquine. J. Infect. Dis. 200:991-1001. [DOI] [PubMed] [Google Scholar]
- 4.Carrara, V. I., J. Zwang, E. A. Ashley, R. N. Price, K. Stepniewska, M. Barends, A. Brockman, T. Anderson, R. McGready, L. Phaiphun, S. Proux, M. van Vugt, R. Hutagalung, K. M. Lwin, A. P. Phyo, P. Preechapornkul, M. Imwong, S. Pukrittayakamee, P. Singhasivanon, N. J. White, and F. Nosten. 2009. Changes in the treatment responses to artesunate-mefloquine on the northwestern border of Thailand during 13 years of continuous deployment. PLoS One 4:e4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gay, F., M. H. Binet, M. D. Bustos, B. Rouveix, M. Danis, C. Roy, and M. Gentilini. 1990. Mefloquine failure in child contracting falciparum malaria in West Africa. Lancet 335:120-121. [DOI] [PubMed] [Google Scholar]
- 6.Happi, C. T., G. O. Gbotosho, O. A. Folarin, A. Sowunmi, T. Hudson, M. O'Neil, W. Milhous, D. F. Wirth, and A. M. Oduola. 2009. Selection of Plasmodium falciparum multidrug resistance gene 1 alleles in asexual stages and gametocytes by artemether-lumefantrine in Nigerian children with uncomplicated falciparum malaria. Antimicrob. Agents Chemother. 53:888-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmgren, G., A. Bjorkman, and J. P. Gil. 2006. Amodiaquine resistance is not related to rare findings of pfmdr1 gene amplifications in Kenya. Trop. Med. Int. Health 11:1808-1812. [DOI] [PubMed] [Google Scholar]
- 8.Lim, P., A. P. Alker, N. Khim, N. K. Shah, S. Incardona, S. Doung, P. Yi, D. M. Bouth, C. Bouchier, O. M. Puijalon, S. R. Meshnick, C. Wongsrichanalai, T. Fandeur, J. Le Bras, P. Ringwald, and F. Ariey. 2009. Pfmdr1 copy number and arteminisin derivatives combination therapy failure in falciparum malaria in Cambodia. Malar. J. 8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meremikwu, M., U. Okomo, C. Nwachukwu, A. Oyo-Ita, J. Eke-Njoku, J. Okebe, E. Oyo-Ita, and P. Garner. 2007. Antimalarial drug prescribing practice in private and public health facilities in South-east Nigeria: a descriptive study. Malar. J. 6:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oduola, A. M., W. K. Milhous, L. A. Salako, O. Walker, and R. E. Desjardins. 1987. Reduced in-vitro susceptibility to mefloquine in West African isolates of Plasmodium falciparum. Lancet ii:1304-1305. [DOI] [PubMed] [Google Scholar]
- 11.Price, R. N., A. C. Uhlemann, A. Brockman, R. McGready, E. Ashley, L. Phaipun, R. Patel, K. Laing, S. Looareesuwan, N. J. White, F. Nosten, and S. Krishna. 2004. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet 364:438-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sibley, C. H., P. J. Guerin, and P. Ringwald. 20 March 2010. Monitoring antimalarial resistance: launching a cooperative effort. Trends Parasitol. [Epub ahead of print]. doi: 10.1016/j.pt.2010.02.008. [DOI] [PubMed]
- 13.Sidhu, A. B., A. C. Uhlemann, S. G. Valderramos, J. C. Valderramos, S. Krishna, and D. A. Fidock. 2006. Decreasing pfmdr1 copy number in plasmodium falciparum malaria heightens susceptibility to mefloquine, lumefantrine, halofantrine, quinine, and artemisinin. J. Infect. Dis. 194:528-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sowunmi, A., and L. A. Salako. 1992. Evaluation of the relative efficacy of various antimalarial drugs in Nigerian children under five years of age suffering from acute uncomplicated falciparum malaria. Ann. Trop. Med. Parasitol. 86:1-8. [DOI] [PubMed] [Google Scholar]
- 15.Uhlemann, A. C., M. Ramharter, B. Lell, P. G. Kremsner, and S. Krishna. 2005. Amplification of Plasmodium falciparum multidrug resistance gene 1 in isolates from Gabon. J. Infect. Dis. 192:1830-1835. [DOI] [PubMed] [Google Scholar]
- 16.Ursing, J., P. E. Kofoed, L. Rombo, and J. P. Gil. 2006. No pfmdr1 amplifications in samples from Guinea-Bissau and Liberia collected between 1981 and 2004. J. Infect. Dis. 194:716-719. [DOI] [PubMed] [Google Scholar]