Abstract
Use of high ultrafiltrate flow rates with continuous venovenous hemofiltration (CVVHF) in critically ill patients is an emerging setting, for which there are few data to guide drug dosing. The objectives of this study were, firstly, to investigate the pharmacokinetics of meropenem in critically ill patients with severe sepsis who are receiving high-volume CVVHF with high-volume exchanges (≥4 liters/h); secondly, to determine whether standard dosing regimens (1,000 mg intravenously [i.v.] every 8 h) are sufficient for treatment of less susceptible organisms such as Burkholderia pseudomallei (MIC, 4 mg/liter); and, finally, to compare the clearances observed in this study with data from previous studies using lower-volume exchanges (1 to 2 liters/h). We recruited 10 eligible patients and collected serial pre- and postfilter blood samples and ultrafiltrate and urine samples. A noncompartmental method was used to determine meropenem pharmacokinetics. The cohort had a median age of 56.6 years, a median weight of 70 kg, and a median APACHE II (acute physiology and chronic health evaluation) score of 25. The median (interquartile range) values for meropenem were as follows: terminal elimination half-life, 4.3 h (2.9 to 6.0); terminal volume of distribution, 0.2 liters/kg (0.2 to 0.3); trough concentration, 7.7 mg/liter (6.2 to 12.9); total clearance, 6.0 liters/h (5.2 to 6.2); hemofiltration clearance, 3.5 liters/h (3.4 to 3.9). In comparing the meropenem clearance here with those in previous studies, ultrafiltration flow rate was found to be the parameter that accounted for the differences in clearance of meropenem (R2 = 0.89). In conclusion, high-volume CVVHF causes significant clearance of meropenem, necessitating steady-state doses of 1,000 mg every 8 h to maintain sufficient concentrations to treat less susceptible organisms such as B. pseudomallei.
Sepsis is a leading cause of morbidity and mortality among critically ill patients (8), and the mainstay of treatment is early initiation of broad-spectrum antibiotics and advanced supportive care. Acute kidney injury (AKI) occurs in up to 5% of all critically ill patients, and 70% of these patients will require renal replacement therapy (RRT) (16). An ongoing concern for these patients is the persisting high mortality rates that have been reported to be as high as 60% (24). Optimized antibiotic dosing may help reduce this burden. However, despite our knowledge of the significant problems associated with the presence of infection in critically ill patients, there is a paucity of data to guide antibiotic dosing with differing RRT settings and modalities.
Meropenem is an antibiotic that is often used for empirical treatment of infections in critically ill patients with AKI (10). It has clinically insignificant protein binding (2 to 3%) (10) and, as a carbapenem antibiotic, shows time-dependent bacterial killing, meaning that the unbound or free (f) antibiotic concentration in blood should be maintained above the MIC of the pathogen for at least 40% of the dosing interval (f 40% T>MIC) (7). Emerging retrospective human data suggest that clinical advantages may exist for maintaining meropenem concentrations for longer periods and at concentrations up to five times the MIC (f 100% T>5×MIC) (1, 17). While the elimination half-life of meropenem is increased in patients with renal dysfunction, the addition of RRT will cause some level of meropenem clearance (6, 9, 14, 23, 26). Therefore, dosing strategies specific for critically ill patients receiving RRT are essential to optimize antibiotic exposure and minimize the poor clinical outcomes observed in these patients (19).
The pharmacokinetics of meropenem have been described in patients being treated with continuous venovenous hemofiltration (CVVHF), continuous venovenous hemodialysis (CVVHD), and continuous venovenous hemodiafiltration (CVVHDF) (4, 9, 11, 13-15, 21-23, 25, 26). There is significant variability in the pharmacokinetics of meropenem in these studies. RRT clearance of meropenem is thought to be related to the volume of ultrafiltrate, the surface area of the filter membrane, the blood flow, and the duration of renal replacement therapy (RRT) (14). Previous studies have used filter membrane surface areas ranging from 0.45 to 0.9 m2, and fluid exchanges used between 1 and 2 liters/h. The recommended meropenem dosing regimens arising from these studies have ranged between 500 mg every 12 hours and 1,000 mg every 12 hours, which were reported to provide therapeutic concentrations to treat susceptible pathogens with a meropenem MIC90 of 0.1 to 0.2 mg/liter.
Despite these detailed studies, there are no data to describe the pharmacokinetics of meropenem during high-volume CVVHF. High-volume CVVHF uses high-volume exchanges and filters with larger membrane surface areas. This modality of CVVHF is reported to provide more rapid clearance of inflammatory mediators and stabilization of physiologic parameters. Furthermore, there is some evidence that high-volume filtration may be of clinical benefit in patients with severe sepsis (3).
However, treatment of more-resistant bacteria, such as Burkholderia pseudomallei (MIC90, 4 mg/liter) and some strains of Pseudomonas aeruginosa, is problematic. B. pseudomallei is a significant part of the sepsis burden in tropical Australia. It is an intracellular, Gram-negative bacterium causing melioidosis, a community-acquired infection with high mortality rates (5, 12). For this serious infection, meropenem is the optimal antibiotic choice (5).
The objectives of this study were, first, to investigate the pharmacokinetics of meropenem in critically ill patients with severe sepsis who are receiving high-volume CVVHF with high-volume exchanges (≥4 liters/h); second, to determine whether standard dosing regimens are sufficient for treatment of less susceptible organisms such as B. pseudomallei; and, finally, to compare the clearances observed in this study with data from previous studies using lower-volume exchanges (1 to 2 liters/h).
(This study was presented, in part, at the 2009 Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA.)
MATERIALS AND METHODS
This was a prospective, observational pharmacokinetic trial, performed in the intensive care unit of a 350-bed teaching hospital in Australia. The study was approved by the Human Research Ethics Committees of the Northern Territory Department of Health and Families and Menzies School of Health Research (ethics approval 08/37, 16 July 2008).
Patient selection and data collection.
Patients who met the American College of Chest Physicians/Society of Critical Care Medicine (ACCP/SCCM) criteria for sepsis (2) and were receiving high-volume CVVHF for oligo- or anuric-renal failure were eligible for enrollment. Meropenem was prescribed at the discretion of the treating intensive care physician. Informed consent was obtained from the patient or the patient's legally authorized representative.
Meropenem administration.
All patients were administered a standard dose of meropenem, 1,000 mg, via central venous catheter every 8 hours in line with local departmental guidelines. Meropenem was reconstituted with 20 ml of sterile water for injection and given as a bolus.
Renal replacement therapy.
Continuous venovenous hemofiltration (CVVHF) was performed in all the patients using the Nephral ST500 (AN69 hollow-fiber) filter with a surface area of 2.15 m2. All patients were initiated on the CVVHF at least 8 hours prior to the sampling period. Vascular access was obtained via the subclavian, internal jugular, or femoral vein, using a double-lumen 10G 14Fr catheter. The ultrafiltrate rate was set between 4 and 6 liters/h, with a target blood flow rate of 15 liters/h. The circuit was anticoagulated with heparin or citrate at the physician's discretion.
Sample collection.
Pharmacokinetic sampling occurred during one 8-hour dosage interval at steady state (defined as after the fourth meropenem dose). To minimize interruptions from filter failure from clotting, the filter was changed if it was more than 3 h old at the commencement of the sampling period. Two milliliters of blood was collected in a lithium heparin tube pre- and postfilter, predose, and at 15, 30, 45, 60, 120, 240, and 480 min. Ultrafiltrate samples were collected at the same time points. Total ultrafiltrate and urine for the dosing period were measured, and 10-ml aliquots were kept for analysis. All samples were immediately refrigerated at 4°C, and plasma was separated and frozen at −80°C within 24 h of sample collection. The blood samples were centrifuged at 3,000 rpm for 10 min.
Meropenem assay.
Meropenem concentrations in plasma, ultrafiltrate, and urine were determined by validated assay methods on a Shimadzu Prominence high-pressure liquid chromatography (HPLC) system. The stationary phase was a Phenomenex Gemini C18 column (5 μm, 150 × 3 mm), and the mobile phase was acetonitrile with 10 mM phosphate buffer at pH 6.5 (10%:90% organic agent/buffer). Meropenem and the internal standard (ertapenem) were detected by UV detection at 298 nm.
Meropenem standards and quality controls were prepared in matrices of plasma, ultrafiltrate, and urine. For all three matrices 100 μl of sample was diluted with buffer at pH 6.5 and internal standard, while plasma samples were subsequently deproteinated with acetonitrile and washed with dichloromethane before injection. Linearity was validated from 1 to 500 μg/ml (plasma), 1 to 200 μg/ml (ultrafiltrate), and 10 to 1,000 μg/ml (urine; dilution for samples over 1,000 μg/ml was validated at within 2%). Accuracy and precision were determined from n = 6 replicates of quality controls at high, medium, and low concentrations; results were within 6% for all matrices at all levels.
Pharmacokinetic analysis.
The pharmacokinetic values were calculated using noncompartmental methods. The area under the concentration-time curve from 0 to 8 h (AUC0-8) was calculated using the linear trapezoidal rule. Total body clearance (CLtot) was calculated as dose/AUC0-8. The area under the moment curve (AUMC0-8) was calculated using the linear trapezoidal rule. Mean residence time (MRT) was calculated as AUMC0-8/AUC0-8. The maximum concentration for the dosing period (Cmax) and the minimum concentration for the dosing period (Cmin) were the observed values; the apparent terminal elimination rate constant (λz) was determined from log-linear least squares regression analysis of concentrations from 2 to 8 h; the apparent volume of distribution during terminal phase (Vz) was CL/λz; the half-life (t1/2) was ln(2)/λz. The extraction ratio (ER) across the filter was calculated as the ratio of the meropenem venous blood sample concentration to the arterial blood sample concentration, and the sieving coefficient (SC) was calculated as the ratio of the concentration of meropenem in the ultrafiltrate to the concentration in the arterial blood sample. Renal clearance (CLrenal) was calculated using the equation CLrenal = Aurine0-8/AUC0-8 where Aurine0-8 is the total amount of meropenem recovered in the urine from 0 to 8 h. Clearance by CVVHF (CLCVVHF) was calculated using the equation CLCVVHF = ACVVHF/AUCCVVHF0-8 where ACVVHF is the total amount of meropenem recovered in the ultrafiltrate from 0 to 8 h and AUCCVVHF0-8 is the area under the concentration-time curve in CVVHF from 0 to 8 h. Clearance not mediated by CVVHF (CLnon-CVVHF) was calculated using the equation CLnon-CVVHF = CLtot − CLCVVHF.
Statistical analysis.
Statistical analyses were performed using Prism (GraphPad, version 4.03; San Diego, CA) and Microsoft Excel (Microsoft Office 2007; Redmond, WA). Correlations between factors were determined using linear regression.
RESULTS
Clinical data.
Ten patients were enrolled in the study. The clinical and demographic characteristics of the patients are described in Table 1. The plasma pharmacokinetic data are described in Table 2. Figure 1 shows the median (interquartile range) plasma meropenem concentrations over one dosing interval in the 10 patients. The hemofiltration settings and pharmacokinetic data are described in Table 3.
TABLE 1.
Clinical and demographic characteristics of patientsa
| Patient | Age (yr) | Gender | Wt (kg) | INR | Albumin | Diagnosis | APACHE II score (admission) | Clinical outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 71 | Male | 95 | 1.4 | 22 | Septic shock; source unknown | 25 | Death |
| 2 | 48 | Female | 50 | 1.1 | 25 | Pneumonia and septic shock | 18 | Home |
| 3 | 56 | Male | 70 | 1.6 | 26 | Pneumonia and septic shock | 32 | Death |
| 4 | 61 | Male | 105 | 0.8 | 41 | Pneumonia and septic shock | 22 | Death |
| 5 | 45 | Female | 70 | 1.1 | 31 | Biliary sepsis and septic shock | 27 | Home |
| 6 | 57 | Male | 110 | 1.3 | 28 | Septic arthritis and septic shock | 29 | Transferred |
| 7 | 51 | Female | 60 | 1.0 | 21 | Skin abscess and septic shock | 30 | Home |
| 8 | 69 | Male | 110 | 1.4 | 20 | Cellulitis and septic shock | 18 | Home |
| 9 | 59 | Male | 70 | 1.1 | 27 | Peritonitis and septic shock | 25 | Home |
| 10 | 29 | Female | 65 | 1.2 | 29 | Urinary sepsis and septic shock | 22 | Home |
| Median | 57 | 70 | 25 | |||||
| 25th percentile | 49 | 66 | 22 | |||||
| 75th percentile | 61 | 103 | 28 |
Abbreviations: INR, international normalized ratio; APACHE, acute physiology and chronic health evaluation.
TABLE 2.
Pharmacokinetic parameters from plasma dataa
| Patient | Cmax (mg/liter) | Cmin (mg/liter) | AUC0-8 (mg·h/liter) | AUMC0-8 (mg·h2/liter) | MRT (h) | CLtot (liters/h) | CLrenal (liters/h) | CLnon-CVVHF (liters/h) | Kel (h−1) | t1/2 (h) | Vz (liters/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 50.4 | 14.3 | 169.1 | 31,538.3 | 186.5 | 5.9 | 0.007 | 2.0 | 0.11 | 6.53 | 0.21 |
| 2 | 58.3 | 5.70 | 139.8 | 20,905.9 | 149.6 | 7.2 | 0.003 | 3.2 | 0.26 | 2.72 | 0.76 |
| 3 | 54.8 | 7.57 | 162.2 | 26,012.7 | 160.4 | 6.2 | 0.000 | 2.7 | 0.20 | 3.46 | 0.46 |
| 4 | 61.9 | 4.21 | 174.3 | 38,821.5 | 222.7 | 5.7 | 0.000 | 2.3 | 0.10 | 6.90 | 0.21 |
| 5 | 74.7 | 21.5 | 397.5 | 112,647.4 | 283.4 | 2.5 | 0.000 | 0.0 | 0.09 | 8.07 | 0.35 |
| 6 | 42.7 | 7.2 | 163.8 | 38,016.8 | 232.1 | 6.1 | 0.024 | 3.1 | 0.16 | 4.30 | 0.34 |
| 7 | 74.8 | 16.9 | 281.0 | 49,981.5 | 177.9 | 3.6 | 0.000 | 0.2 | 0.16 | 4.47 | 0.46 |
| 8 | 49.4 | 8.73 | 159.9 | 27,308.6 | 170.7 | 6.3 | 0.018 | 2.5 | 0.26 | 2.68 | 0.40 |
| 9 | 42.4 | 5.93 | 115.3 | 18,919.1 | 164.1 | 8.7 | 0.011 | 4.6 | 0.35 | 2.00 | 0.81 |
| 10 | 66.9 | 7.63 | 199.4 | 31,218.3 | 156.6 | 5.0 | 0.008 | 1.5 | 0.16 | 4.35 | 0.31 |
| Median | 56.6 | 7.6 | 166.5 | 31,378.3 | 174.3 | 6.0 | 0.005 | 2.4 | 0.16 | 4.32 | 0.37 |
| 25th percentile | 49.7 | 6.2 | 160.5 | 26,336.7 | 161.3 | 5.2 | 0.000 | 1.6 | 0.12 | 2.90 | 0.32 |
| 75th percentile | 65.7 | 12.9 | 193.1 | 38,620.3 | 213.7 | 6.2 | 0.010 | 3.0 | 0.24 | 6.02 | 0.46 |
Abbreviations: Cmax, observed maximum concentration during sampling period; Cmin, observed minimum concentration during sampling period; AUC0-8, area under the concentration-time curve during 8-hour dosing period; AUMC0-8, area under the moment curve during 8-hour dosing period; MRT, mean residence time; CLtot, total clearance; CLrenal, renal clearance; CLnon-CVVHF, clearance not mediated by CVVHF; Kel, elimination rate constant; t1/2, elimination half-life; Vz, apparent volume of distribution during terminal phase.
FIG. 1.
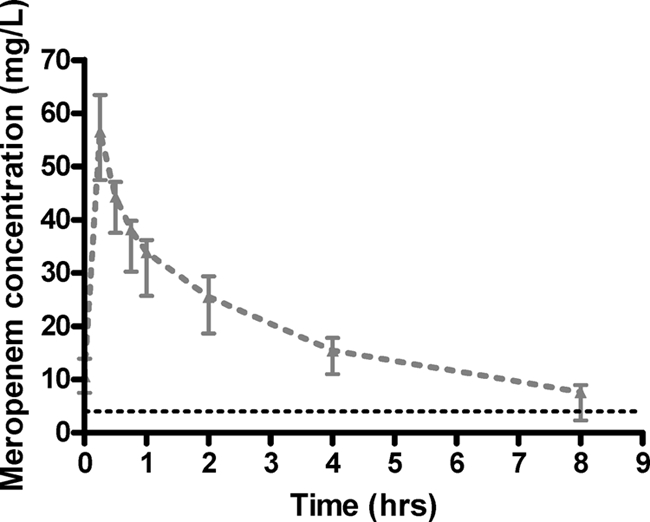
Median (and interquartile) concentration-versus-time data for the enrolled patients. The black broken line represents the MIC for B. pseudomallei (4 mg/liter).
TABLE 3.
Hemofiltration settings and pharmacokinetic dataa
| Patient | Filter age (min) | Mean blood flow (liters/h) | Mean UFR (liters/h) | Urine vol (0-8 h) (liters) | AUC0-8 (mg·h/liters) | AUMC0-8 (mg·h2/liters) | MRT (h) | Extraction ratio | CLCVVHF (liters/h) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 | 12.7 | 4.7 | 0.06 | 109.1 | 13,762.5 | 126.1 | 0.71 | 3.9 |
| 2 | 15 | 18.0 | 6.0 | 0.03 | 126.0 | 18,542.6 | 147.2 | 0.80 | 4.0 |
| 3 | 80 | 17.4 | 4.6 | 0.00 | 155.5 | 25,563.3 | 164.4 | 0.77 | 3.5 |
| 4 | 5 | 16.9 | 3.8 | 0.00 | 130.6 | 27,856.1 | 213.4 | 0.71 | 3.4 |
| 5 | 5 | 13.8 | 3.8 | 0.00 | 292.3 | 79,766.3 | 272.9 | 0.75 | 2.7 |
| 6 | 7 | 16.8 | 4.4 | 0.19 | 128.5 | 30,265.1 | 235.6 | 0.71 | 3.0 |
| 7 | 10 | 14.4 | 3.9 | 0.00 | 218.3 | 38,325.4 | 175.6 | 0.74 | 3.4 |
| 8 | 25 | 17.4 | 4.6 | 0.14 | 112.8 | 18,303.7 | 162.2 | 0.70 | 3.8 |
| 9 | 5 | 21.0 | 4.0 | 0.09 | 118.7 | 18,439.9 | 155.3 | 0.81 | 4.1 |
| 10 | 60 | 16.8 | 4.5 | 0.06 | 175.8 | 28,025.3 | 159.4 | 0.73 | 3.5 |
| Median | 24 | 16.5 | 4.4 | 0.08 | 129.5 | 26,709.7 | 163.3 | 0.74 | 3.5 |
| 25th percentile | 5 | 15.0 | 3.9 | 0.04 | 120.5 | 18,465.6 | 156.4 | 0.71 | 3.4 |
| 75th percentile | 29 | 17.4 | 4.6 | 0.114 | 170.7 | 29,705.2 | 203.9 | 0.77 | 3.9 |
Abbreviations: UFR, ultrafiltrate flow rate; AUC0-8, area under the concentration-time curve during 8-hour dosing period; AUMC0-8, area under the moment curve during 8-hour dosing period; MRT, mean residence time; CLCVVHF, hemofiltration clearance.
We omitted the 8-h sample from patients 4, 5, and 6 from analysis as we observed values more than 4 standard deviations outside the likely value. The likely reason for the unexplained values was inadvertent early administration of the subsequent dose. In the absence of the actual 8-hour sample for these patients, we used the trough concentration from the previous dose as a surrogate concentration in the pharmacokinetic analyses. To confirm the appropriateness of this assumption, we determined an elimination rate constant using linear regression from the 2-h and 4-h samples to confirm that the previous trough concentration was appropriate given the rate of clearance observed in the present dosing interval.
Previous studies have been published describing the pharmacokinetics of meropenem in various forms of CVVHF with low-volume exchanges. The settings and pharmacokinetics parameters observed in these studies are compared with those observed in this study in Table 4. When attempting to explain the different CVVHF clearances that have been reported by these previous studies, only ultrafiltrate flow rate (UFR) enabled normalization of data for drug clearance (R2 = 0.89). Neither membrane surface area (R2 = 0.30) nor blood flow rate (R2 = 0.18) could sufficiently describe meropenem hemofiltration clearance. No other demographic, clinical, or dialysis factors were found to describe meropenem CVVHF clearance adequately.
TABLE 4.
Comparative operational settings and pharmacokinetic data from this study and previous studies of meropenem clearance during CVVHFa
| Study (reference) | Membrane SA (m2) | BFR (liters/h) | UFR (liters/h) | SC | t1/2 (h) | V (liters/kg) | CLCVVHF (liters/h) | CLtot (liters/h) | CLCVVHF/SA | CLCVVHF/BFR | CLCVVHF/UFR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Thalhammer et al., 1998 (23) | 0.43 | 9 | 2.7 | 1.09 | 2.5 | 0.34 | 2.98 | 8.62 | 6.93 | 0.33 | 1.10 |
| Tegeder et al., 1999 (21) | 0.9 | 10 | 1.1 | 1.17 | 8.7 | 0.19 | 1.32 | 3.10 | 1.47 | 0.13 | 1.20 |
| Ververs et al., 2000 (26) | 0.9 | 12 | 1.6 | 0.63 | 6.3 | 0.37 | 0.96 | 4.57 | 1.07 | 0.08 | 0.60 |
| Giles et al., 2000 (9) | 0.9 | 9 | 1.7 | 0.95 | 5.8 | 0.35 | 1.50 | 3.63 | 1.67 | 0.17 | 0.88 |
| Valtonen et al., 2000 (25) | 0.7 | 6 | 0.4 | NS | 7.5 | NS | NS | 3.27 | NS | NS | NS |
| Krueger et al., 2003 (13) | 0.9 | 1 | 1.6 | 0.91 | 3.6 | 0.28 | 1.47 | 4.98 | 1.63 | 1.47 | 0.92 |
| Bilgrami et al., 2010 | 2.15 | 15 | 4.4 | 0.93 | 4.6 | 0.26 | 3.49 | 6.00 | 1.76 | 0.25 | 0.86 |
Abbreviations: SA, surface area; BFR, blood flow rate; UFR, ultrafiltrate flow rate; SC, sieving coefficient; t1/2, elimination half-life; V, stated apparent volume of distribution (V was at steady state in all studies except for references 23 and 25, which did not specify the value; in this study, the apparent V during terminal phase was used); CLCVVHF, hemofiltration clearance; CLtot, total clearance; NS, data not stated.
DISCUSSION
This is the first study to investigate the pharmacokinetics of meropenem during high-volume CVVHF. The results of this study show significant meropenem clearance in patients with acute kidney injury receiving CVVHF with high-volume exchanges.
Meropenem is a frequently used empirical antibiotic treatment in the critical care setting because of its broad spectrum of action. Pharmacodynamically, meropenem shows time-dependent bacterial killing, and optimal bactericidal activity suggests that maintaining a concentration above the MIC for at least 40% of the dosing time is required. However, emerging retrospective clinical studies involving meropenem support a longer f T>MIC in critically ill patients of up to 100% of the dosing interval (18, 19). However, for critically ill patients with renal dysfunction or those requiring renal replacement therapy such as CVVHF, there remains significant concern from clinicians that dosing recommendations facilitate optimal pharmacodynamic exposures of meropenem.
The pharmacokinetics of meropenem in CVVHF have been studied previously. However, the operational characteristics of the CVVHF used in these studies have varied greatly; the membrane surface areas have varied between 0.43 and 0.9 m2, the ultrafiltrate flow rate (UFR) between 1 and 2 liters/h, and the blood flow rates between 0.6 and 12 liters/h, and different types of membranes have been used. The local protocol used at our institution uses significantly higher blood flow rates (250 ml/min), higher UFRs (4.4 liters/h), and a membrane with a surface area of 2.15 m2. Hence, our study is able to provide a valuable contrast between each of the studies on the relative effect of UFR on meropenem clearance.
In this study, we observed a median clearance due to this form of hemofiltration of 3.49 liters/h. Compared to the previous studies using low ultrafiltrate flow rates, we have observed that meropenem clearance is largely explained by the differing UFRs. In evaluating the total clearance (CLtot) from each of the studies, the reasons for the differences are not apparent. The effects of residual renal function and the levels of sickness severity were reported inconsistently between these studies, making a systematic interpretation not possible.
Knowledge of drug clearance during a form of renal replacement therapy is important. However, dosing can rarely be based solely on clearance data. Once steady state has been achieved, dosing should be based on drug clearance. In renally impaired patients receiving renal replacement therapy, consideration of possible residual renal clearance or other nonrenal clearance is essential. Although data for upregulated nonrenal clearance exist for ciprofloxacin in renal dysfunction (20), we are not aware of any similar data for meropenem.
This paper has shown that meropenem clearance during CVVHF is heavily influenced by UFR. It follows that the clinical use of high-volume CVVHF requires a higher meropenem dose than previously considered necessary for CVVHF. Our data suggest that CVVHF settings similar to that used in our study require a steady-state meropenem dose of 1,000 mg every 8 h to maintain concentrations above the MIC of less susceptible pathogens such as B. pseudomallei (MIC90, 4.0 mg/liter, compared with the median [90th percentile] trough concentration observed in this study of 7.6 mg/liter [5.5 mg/liter]). For more susceptible organisms, a lower dose may be used, although a lower dose would be inappropriate for empirical therapy in most centers.
A possible limitation to the comparisons made between this study and others using CVVHF with lower-volume exchanges is the use of prefilter replacement fluid in this study. Of the other studies undertaken, each of them used postfilter dilution with replacement fluid. This is unlikely to affect the conclusions on the importance of UFR for meropenem clearance but may be important.
In conclusion, CVVHF with high-volume exchanges results in significant clearance of meropenem. Comparing the results of our study with previous studies that use lower-volume exchanges, we have been able to show that UFR is the main determinant of meropenem clearance during CVVHF. It follows that, when using CVVHF with high-volume exchanges, higher doses than those usually used in CVVHF are appropriate. Our data suggest that steady-state dosing of 1,000 mg every 8 h provides appropriate meropenem concentrations for less susceptible organisms such as B. pseudomallei.
Acknowledgments
We thank Kim Piera and Tonia Woodberry for the laboratory assistance, the nursing and medical staff of the ICU, and the patients and their relatives who agreed to be part of this study.
This study was funded by the National Health and Medical Research Council of Australia (project grant 519702, fellowship to J. A. Roberts and scholarship to J. Davis).
Footnotes
Published ahead of print on 17 May 2010.
REFERENCES
- 1.Ariano, R. E., A. Nyhlen, J. P. Donnelly, D. S. Sitar, G. K. Harding, and S. A. Zelenitsky. 2005. Pharmacokinetics and pharmacodynamics of meropenem in febrile neutropenic patients with bacteremia. Ann. Pharmacother. 39:32-38. [DOI] [PubMed] [Google Scholar]
- 2.Bone, R. C., R. A. Balk, F. B. Cerra, R. P. Dellinger, A. M. Fein, W. A. Knaus, R. M. Schein, and W. J. Sibbald. 1992. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101:1644-1655. [DOI] [PubMed] [Google Scholar]
- 3.Bouman, C. S., H. M. Oudemans-van Straaten, M. J. Schultz, and M. B. Vroom. 2007. Hemofiltration in sepsis and systemic inflammatory response syndrome: the role of dosing and timing. J. Crit. Care 22:1-12. [DOI] [PubMed] [Google Scholar]
- 4.Bugge, J. F. 2001. Pharmacokinetics and drug dosing adjustments during continuous venovenous hemofiltration or hemodiafiltration in critically ill patients. Acta Anaesthesiol. Scand. 45:929-934. [DOI] [PubMed] [Google Scholar]
- 5.Cheng, A. C., and B. J. Currie. 2005. Melioidosis: epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 18:383-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chimata, M., M. Nagase, Y. Suzuki, M. Shimomura, and S. Kakuta. 1993. Pharmacokinetics of meropenem in patients with various degrees of renal function, including patients with end-stage renal disease. Antimicrob. Agents Chemother. 37:229-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drusano, G. L. 2004. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug.’ Nat. Rev. Microbiol. 2:289-300. [DOI] [PubMed] [Google Scholar]
- 8.Finfer, S., R. Bellomo, J. Lipman, C. French, G. Dobb, and J. Myburgh. 2004. Adult-population incidence of severe sepsis in Australian and New Zealand intensive care units. Intensive Care Med. 30:589-596. [DOI] [PubMed] [Google Scholar]
- 9.Giles, L. J., A. C. Jennings, A. H. Thomson, G. Creed, R. J. Beale, and A. McLuckie. 2000. Pharmacokinetics of meropenem in intensive care unit patients receiving continuous veno-venous hemofiltration or hemodiafiltration. Crit. Care Med. 28:632-637. [DOI] [PubMed] [Google Scholar]
- 10.Hurst, M., and H. M. Lamb. 2000. Meropenem: a review of its use in patients in intensive care. Drugs 59:653-680. [DOI] [PubMed] [Google Scholar]
- 11.Isla, A., J. Maynar, J. A. Sanchez-Izquierdo, A. R. Gascon, A. Arzuaga, E. Corral, and J. L. Pedraz. 2005. Meropenem and continuous renal replacement therapy: in vitro permeability of 2 continuous renal replacement therapy membranes and influence of patient renal function on the pharmacokinetics in critically ill patients. J. Clin. Pharmacol. 45:1294-1304. [DOI] [PubMed] [Google Scholar]
- 12.Jenney, A. W., G. Lum, D. A. Fisher, and B. J. Currie. 2001. Antibiotic susceptibility of Burkholderia pseudomallei from tropical northern Australia and implications for therapy of melioidosis. Int. J. Antimicrob. Agents 17:109-113. [DOI] [PubMed] [Google Scholar]
- 13.Krueger, W. A., G. Neeser, H. Schuster, T. H. Schroeder, E. Hoffmann, A. Heininger, H. J. Dieterich, H. Forst, and K. E. Unertl. 2003. Correlation of meropenem plasma levels with pharmacodynamic requirements in critically ill patients receiving continuous veno-venous hemofiltration. Chemotherapy 49:280-286. [DOI] [PubMed] [Google Scholar]
- 14.Krueger, W. A., T. H. Schroeder, M. Hutchison, E. Hoffmann, H. J. Dieterich, A. Heininger, C. Erley, A. Wehrle, and K. Unertl. 1998. Pharmacokinetics of meropenem in critically ill patients with acute renal failure treated by continuous hemodiafiltration. Antimicrob. Agents Chemother. 42:2421-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuti, J. L., and D. P. Nicolau. 2005. Derivation of meropenem dosage in patients receiving continuous veno-venous hemofiltration based on pharmacodynamic target attainment. Chemotherapy 51:211-216. [DOI] [PubMed] [Google Scholar]
- 16.Levy, E. M., C. M. Viscoli, and R. I. Horwitz. 1996. The effect of acute renal failure on mortality. A cohort analysis. JAMA 275:1489-1494. [PubMed] [Google Scholar]
- 17.Li, C., X. Du, J. L. Kuti, and D. P. Nicolau. 2007. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob. Agents Chemother. 51:1725-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts, J. A., and J. Lipman. 2009. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 37:840-851. [DOI] [PubMed] [Google Scholar]
- 19.Roberts, J. A., S. Webb, D. Paterson, K. M. Ho, and J. Lipman. 2009. A systematic review on clinical benefits of continuous administration of beta-lactam antibiotics. Crit. Care Med. 37:2071-2078. [DOI] [PubMed] [Google Scholar]
- 20.Rohwedder, R., T. Bergan, S. B. Thorsteinsson, and H. Scholl. 1990. Transintestinal elimination of ciprofloxacin. Chemotherapy 36:77-84. [DOI] [PubMed] [Google Scholar]
- 21.Tegeder, I., F. Neumann, F. Bremer, K. Brune, J. Lotsch, and G. Geisslinger. 1999. Pharmacokinetics of meropenem in critically ill patients with acute renal failure undergoing continuous venovenous hemofiltration. Clin. Pharmacol. Ther. 65:50-57. [DOI] [PubMed] [Google Scholar]
- 22.Thalhammer, F., and W. H. Horl. 2000. Pharmacokinetics of meropenem in patients with renal failure and patients receiving renal replacement therapy. Clin. Pharmacokinet. 39:271-279. [DOI] [PubMed] [Google Scholar]
- 23.Thalhammer, F., P. Schenk, H. Burgmann, I. El Menyawi, U. M. Hollenstein, A. R. Rosenkranz, G. Sunder-Plassmann, S. Breyer, and K. Ratheiser. 1998. Single-dose pharmacokinetics of meropenem during continuous venovenous hemofiltration. Antimicrob. Agents Chemother. 42:2417-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchino, S., J. A. Kellum, R. Bellomo, G. S. Doig, H. Morimatsu, S. Morgera, M. Schetz, I. Tan, C. Bouman, E. Macedo, N. Gibney, A. Tolwani, and C. Ronco. 2005. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294:813-818. [DOI] [PubMed] [Google Scholar]
- 25.Valtonen, M., E. Tiula, J. T. Backman, and P. J. Neuvonen. 2000. Elimination of meropenem during continuous veno-venous haemofiltration and haemodiafiltration in patients with acute renal failure. J. Antimicrob. Chemother. 45:701-704. [DOI] [PubMed] [Google Scholar]
- 26.Ververs, T. F., A. van Dijk, S. A. Vinks, P. J. Blankestijn, J. F. Savelkoul, J. Meulenbelt, and F. T. Boereboom. 2000. Pharmacokinetics and dosing regimen of meropenem in critically ill patients receiving continuous venovenous hemofiltration. Crit. Care Med. 28:3412-3416. [DOI] [PubMed] [Google Scholar]


