Abstract
The development of effective microbicides for the prevention of HIV-1 sexual transmission represents a primary goal for the control of AIDS epidemics worldwide. A promising strategy is the use of bacteria belonging to the vaginal microbiota as live microbicides for the topical production of HIV-1 inhibitors. We have engineered a human vaginal isolate of Lactobacillus jensenii to secrete the anti-HIV-1 chemokine RANTES, as well as C1C5 RANTES, a mutated analogue that acts as a CCR5 antagonist and therefore is devoid of proinflammatory activity. Full-length wild-type RANTES and C1C5 RANTES secreted by L. jensenii were purified to homogeneity and shown to adopt a correctly folded conformation. Both RANTES variants were shown to inhibit HIV-1 infection in CD4+ T cells and macrophages, displaying strong activity against HIV-1 isolates of different genetic subtypes. This work provides proof of principle for the use of L. jensenii-produced C1C5 RANTES to block HIV-1 infection of CD4+ T cells and macrophages, setting the basis for the development of a live anti-HIV-1 microbicide targeting CCR5 in an antagonistic manner.
Human immunodeficiency virus type 1 (HIV-1) is the cause of AIDS, a pandemic that has killed more than 25 million people in 3 decades. More than 33 million people are presently living with HIV-1 worldwide. Thus, the development of efficacious preventive measures represents a high priority. Although a protective HIV-1 vaccine would be the most effective strategy, topical anti-HIV-1 microbicides represent a more realistic alternative and a complementary option (7). However, the recent failure of clinical trials with broad-spectrum but nonspecific compounds has highlighted the need for the development of novel, target-specific microbicides (20). Among the potential molecular targets of a microbicide, the HIV-1 coreceptor CCR5 is of major interest, considering that it is expressed on a large number of CD4+ T lymphocytes, usually activated, present in the vaginal, rectal, and foreskin epithelia. Besides its physiological role as a chemokine, RANTES, a natural ligand of CCR5, is a potent HIV-1 inhibitor (6), and its three-dimensional structure has been extensively investigated (reviewed in reference 36). Therefore, RANTES has been recognized as a lead anti-HIV-1 molecule, and worldwide efforts are being pursued to engineer RANTES derivatives with high anti-HIV-1 potency (36). PSC-RANTES, a chemically modified version of RANTES that acts as a potent anti-HIV-1 blocker and CCR5 agonist, has been successfully applied in a monkey model to block vaginal HIV-1 transmission, providing a proof of principle for the relevance of CCR5 as a microbicide target and RANTES as a microbicide (14). Yet RANTES derivatives not only need to be potent blockers of HIV-1 entry but should also fail to activate CCR5. CCR5 antagonism is crucial to avoid proinflammatory side effects (or even to provide anti-inflammatory activities) to prevent mucosal inflammation, which in the long term could lead to enhancement of HIV-1 transmission.
Within the field of topical anti-HIV-1 microbicides, a promising approach is the development of live microbicides (1, 13). The live-microbicide concept is based on the engineering of commensal bacteria belonging to the human microbiota in order to achieve the in vivo and in situ production of anti-HIV-1 agents. A natural candidate for the development of vaginal live microbicides is Lactobacillus spp., the predominant commensal bacterial species in the female genital tract, which are currently under investigation both in their native and engineered forms. Lactobacilli are also particularly promising in view of the fact that an association has been reported between the depletion of vaginal lactobacilli and the establishment of opportunistic infections, including an increased risk of acquiring HIV-1 and herpes simplex virus type 2 (HSV-2) (5, 34).
HIV-1 entry into target cells is an important arena for inhibitors aimed at preventing virus infection, and several proteins interfering with this process have been produced by recombinant commensal bacteria (3, 4, 9, 15-17, 26-28). Given the dependence of most wild HIV-1 isolates on CCR5 for entry (19), RANTES has attracted particular interest in the field of live microbicides. In this work, we successfully engineered a human vaginal Lactobacillus jensenii isolate to secrete wild-type (wt) RANTES and, separately, its CCR5 antagonist analogue, C1C5 RANTES (23). Both proteins were expressed as full-length molecules that exerted strong anti-HIV-1 activity in CD4+ T cells and macrophages, which represent the two major target cells for HIV-1. These results provide the proof of principle for the possibility of engineering a CCR5-targeting live microbicide, and they set the basis for future development of this approach toward clinical applicability.
MATERIALS AND METHODS
Construction of expression vectors.
RANTES expression plasmids were constructed based on p1063, modified from Escherichia coli/Lactobacillus shuttle vector pOsel175 (16). The expression cassette contains the native ptsH promoter, the signal sequence of the Lactobacillus crispatus S-layer protein CbsA, necessary for secretion of the recombinant protein into the culture medium (4), and the RANTES variant cDNAs or their codon-optimized versions encoding amino acids (aa) 1 to 68. The construction and design of p1063-RANTES and p1063-C1C5 RANTES is described elsewhere (30). Lactobacilli are codon-biased organisms containing only about 36% G+C in their genomes (24). In order to increase the protein expression level, the wt RANTES nucleotide sequence was recoded by assembly PCR (33) to conform more closely to the optimal lactobacillus codon usage. The codon-optimized (CO) nucleotide RANTES sequence (RANTES-CO) was first TA cloned into the pCRII-TOPO vector (Invitrogen, Carlsbad, CA) and then subcloned (NheI-NheI) to create p1063-RANTES-CO. Subsequently, p1063-C1C5 RANTES-CO was obtained by the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) from p1063-RANTES-CO using specific primers (C1C5-CO-forward [5′-GTTTCTACTGTTTCAGCTTGTCCTTATAGCTGTGATACTACTCCATG-3′] and C1C5-CO-reverse [5′-CATGGAGTAGTATCACAGCTATAAGGACAAGCTGAAACAGTAGAAAC- 3′]; bases in bold correspond to mutated codons). The final constructs were verified by DNA sequencing.
Bacterial strains and culture.
The human vaginal isolate L. jensenii 1153 was routinely cultivated at 37°C (5% CO2) in MRS or Rogosa SL broth (Becton, Dickinson and Company, Sparks, MD). E. coli XL1-Blue (Stratagene) was electroporated for plasmid transformation, and recombinant colonies were selected and maintained in LB broth (Becton, Dickinson and Company) at 37°C, supplemented with erythromycin (300 μg/ml). L. jensenii was transformed with purified plasmids by electroporation essentially as described previously (4). Transformed lactobacilli were routinely propagated in liquid medium containing 20 μg/ml erythromycin.
Throughout all experiments, RANTES variants were analyzed by Western blotting and quantified using a commercial enzyme-linked immunosorbent assay (ELISA) kit (RANTES/CCL5 DuoSet; R&D Systems, Minneapolis, MN), using purified E. coli-produced RANTES (kindly donated by Amanda Proudfoot, Merck Serono, Geneva, Switzerland) as a standard.
Protein purification.
Recombinant wt RANTES and C1C5 RANTES, derived from codon-optimized constructs, were purified to homogeneity from culture supernatants of transformed L. jensenii by a four-step ion-exchange chromatography protocol, as described in detail elsewhere (30), essentially taking advantage of the high isoelectric point of the two proteins. Semipurified C1C5 RANTES fractions, containing both major forms as purified material, were applied and fractionated on a Superdex 75 column (GE Healthcare Amersham, Chalfont St. Giles, United Kingdom) in phosphate-buffered saline (PBS), allowing separation of the two forms.
Fractions containing semipurified wt and C1C5 RANTES were also applied on a 5-ml heparin column (HiTrap; GE Healthcare Amersham), equilibrated in 10 mM phosphate buffer at pH 7.5, and eluted using a stepped gradient ranging from 300 mM to 2 M NaCl in the same buffer. Purified full-length RANTES variants eluted in the 1 M NaCl fraction.
Western blotting.
Western blot analysis was performed according to standard procedures. Comparable amounts of protein samples were separated by 13% SDS-PAGE, blotted onto Protran-83 nitrocellulose membranes (Schleicher & Schuell, Keene, NH), and incubated overnight with polyclonal rabbit anti-human RANTES antibodies (1:1,000) (PeproTech, Rocky Hill, NJ), followed by 1 h of incubation with horseradish peroxidase-conjugated polyclonal goat anti-rabbit antibodies (1:5,000) (Sigma, St. Louis, MO). Chemiluminescent signals were developed using the ECL reagent (GE Healthcare Amersham).
HIV-1 infection.
The R5 HIV-1 isolates used in this study were as follows: two laboratory-adapted clade B strains, BaL and SF162; two primary clade B isolates obtained from infected children, 5513 and 10005 (kindly donated by Gabriella Scarlatti, San Raffaele Scientific Institute, Milan, Italy); and two primary clade C isolates, 92BR025 (ARP179.11) and 98IN007 (ARP1027.3). The acute HIV-1 infection assay was performed by incubating PM1 cells (2 × 104/well) with viral stocks (50 50% tissue culture infective doses [TCID50]/well) in complete RPMI 1640 medium (Lonza BioWhittaker, Valais, Switzerland) in the presence or absence of wt or C1C5 RANTES. PM1 is a unique CD4+ CCR5+ T-cell clone susceptible to a wide variety of primary HIV-1 isolates, including those exclusively using CCR5 as a coreceptor (18). Experiments were performed in triplicate using 96-well round-bottom microtiter plates. After incubation at 37°C for 16 h, the wells were washed twice and complete medium, with or without inhibitors, was added. Virus replication was assayed at day 4 postinfection using a p24 antigen ELISA. Supernatants were diluted in 1% Empigen BB detergent (Calbiochem, Gibbstown, NJ) to disrupt virions and added to a 96-well ELISA plate coated with anti-HIV-1 p24 polyclonal antibodies (Aalto Bio Reagents Ltd., Dublin, Ireland) and incubated for 2 h at room temperature. The plate was then washed three times in TBS buffer (1.5 M NaCl, 250 mM Tris [pH 7.5]), and an alkaline phosphatase-conjugated anti-HIV-1 p24 monoclonal antibody (Aalto Bio Reagents Ltd.) was added for 1 h at room temperature. After three washes with Tropix buffer (10 mM MgCl2, 200 mM Tris [pH 9.8]), p24 was detected with the luminescence substrate CSPD Tropix (Applied Biosystems, Foster City, CA) and the signal was analyzed using a Mithras LB 940 luminometer (Berthold Technologies, Bad Wildbad, Germany). Levels of p24 were calculated by extrapolation from a standard curve generated with serial dilutions of p24 antigen standard.
Human monocyte cultures were established from peripheral blood mononuclear cells (PBMC) isolated from Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density gradient centrifugation of buffy coat preparations obtained from healthy HIV-1-seronegative blood donors. PBMC (8 × 106/ml) were cultured in Dulbecco's modified Eagle medium (DMEM) (Lonza BioWhittaker) supplemented with 5% AB serum (Lonza BioWhittaker), 10% FCS (Lonza BioWhittaker), 2 mM glutamine, 50 μg/ml streptomycin, and 100 U/ml penicillin (Lonza BioWhittaker), and monocytes were allowed to adhere in T75 flasks for 2 h at 37°C. Nonadherent cells were then removed by washing with medium. After 24 h, adherent cells were recovered, seeded (1 × 105/well) in 96-well flat-bottom plates in DMEM supplemented with 10% fetal calf serum (FCS) and 5% AB serum, and allowed to differentiate into monocyte-derived macrophages (MDM) 7 to 10 days before infection. MDM were infected in quadruplicate with HIV-1BaL (50 TCID50/well) in a total volume of 0.2 ml in the presence or absence of inhibitors. After overnight incubation, unbound virus was removed by extensive washing, fresh medium was added, and cultures were further incubated at 37°C. Supernatants were harvested at day 4 for p24 antigen determination as described above.
Dose-response curves were fit using the GraphPad Prism software program (GraphPad Software, San Diego, CA) in order to calculate 50% inhibitory concentrations (IC50s).
Mass spectrometry and N-terminal sequencing.
Purified wt and C1C5 RANTES fractions deriving from ion-exchange chromatography were desalted and concentrated on a ZipTip C4 pipette tip (Millipore, Bedford, MA), following the standard protocol. Briefly, 20-μl volumes of samples were acidified to 5% formic acid (Sigma) and eluted in 1 μl of 20% formic acid in 50% CH3CN directly into a nanospray needle (Proxeon Biosystems, Odense, Denmark). Mass spectra were acquired on an ESI-QqTOF mass spectrometer (Api Q-STAR pulsar PE Sciex, Toronto, Canada). Capillary voltage was set to 850 V, and the mass range was set to m/z 600 to 1,500. Spectra were acquired and processed using the Analyst QS 1.1 software program (Applied Biosystems).
The two major forms of C1C5 RANTES (full-length and lower-molecular-mass peptide), purified and separated by gel filtration, were run on an SDS-PAGE gel, blotted onto a polyvinylidene difluoride (PVDF) membrane, and stained with Coomassie brilliant blue R-250 (Applichem, Darmstadt, Germany). The PVDF membrane was then destained with 50% methanol and rinsed with deionized water. N-terminal sequence analysis was performed on an Applied Biosystems sequencer.
RESULTS
Engineering of L. jensenii to produce RANTES variants.
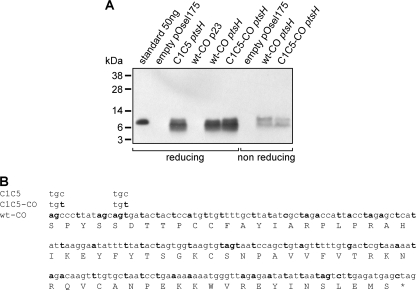
The human vaginal isolate L. jensenii 1153 was selected for engineering of wt and C1C5 RANTES. This Lactobacillus strain has proven to be ideal for its efficient growth and transformation characteristics, and its genome has been fully sequenced to facilitate comprehension and exploitation of regulatory elements, such as promoters and suitable sites for chromosomal integration (16). A modified version of plasmid pOsel175 was used for the construction of wt and C1C5 RANTES expression vectors employing a strong native ptsH promoter (16). Sequences encoding RANTES variants were fused with the signal sequence of the L. crispatus S-layer gene (encoding CbsA), a strategy successfully used for the secretion of the two N-terminal Ig domains of human CD4 (4) and cyanovirin N (CV-N) (16). In an attempt to further improve the production level, wt and C1C5 RANTES cDNAs were codon optimized for lactobacillus expression. RANTES secretion was also tested in an identical codon-optimized construct under the weaker p23 promoter from Lactococcus lactis (4), but as expected, the level of protein produced was dramatically lower (compared to that of the construct under the ptsH promoter), with only a faint band detectable in a Western blot over a long film exposure time (data not shown). Expression levels of the various constructs were compared for the amount of secreted proteins (Fig. 1 A). Although codon optimization (Fig. 1B) did not yield any substantial improvement in the plasmid-based RANTES secretion level, it cannot be excluded that it might provide an enhancement of expression in the planned chromosomal integration of C1C5 RANTES cDNA, a fundamental future step in the development of a safe live microbicide for studies in human volunteers. Based on these considerations, codon-optimized wt RANTES and C1C5 RANTES under the ptsH promoter, secreted at 0.5 to 0.6 mg/liter and 0.3 to 0.4 mg/liter, respectively, were selected for further characterization.
FIG. 1.
Expression and codon optimization of wt and C1C5 RANTES as secreted molecules in L. jensenii. (A) wt and C1C5 RANTES nonoptimized or codon-optimized (CO) versions were visualized in Western blots using polyclonal rabbit anti-human RANTES antibodies. Proteins were expressed under the p23 or ptsH promoter. Supernatant from L. jensenii transformed with the empty pOsel175 vector and E. coli-purified wt RANTES (standard) were used as controls. (B) Codon-optimized nucleotide sequence for wt (wt-CO) or C1C5 (C1C5-CO) RANTES. Modified nucleotides from native cDNA are in bold. Only codons for residues C1 and C5 (optimized and nonoptimized) are indicated for C1C5 RANTES, since the remaining sequence is identical to that of wt-CO or wt nonoptimized RANTES, respectively.
wt and C1C5 RANTES produced by L. jensenii block R5 HIV-1 infection.
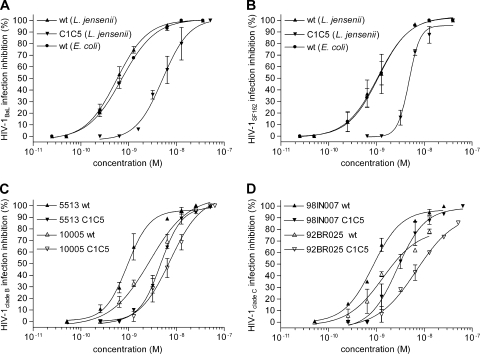
To validate the delivery of RANTES by recombinant lactobacilli as an efficient anti-HIV-1 preventive approach, the first requirement to be fulfilled is the secretion of the chemokine in its active form, i.e., binding to CCR5 and inhibition of R5 HIV-1 infection. An acute infection assay, based on CCR5-expressing target cells (the human CD4+ T cell clone PM1) infected with two laboratory-adapted clade B R5 HIV-1 strains, BaL and SF162, was used to evaluate activities of both purified lactobacillus-expressed wt and C1C5 RANTES variants. The protocol established for the purification of lactobacillus-secreted RANTES variants is based on four-step ion-exchange chromatography and has been reported in full detail elsewhere (30). Purified wt RANTES produced in E. coli and refolded from inclusion bodies was used as a reference control. As shown in Fig. 2 A and B, wt RANTES produced in lactobacilli potently inhibited acute HIV-1 infection, with IC50s of 0.54 nM against HIV-1BaL and 1.14 nM against HIV-1SF162, while the E. coli-produced protein showed IC50s of 0.69 nM (HIV-1BaL) and 1.17 nM (HIV-1SF162). Surprisingly, however, L. jensenii-secreted C1C5 RANTES showed a lower antiviral activity than the wt protein, with IC50s of 5.00 nM (HIV-1BaL) and 4.8 nM (HIV-1SF162). These findings are in contrast with previously reported data for C1C5 RANTES (23). This issue was therefore further investigated by mass spectrometry analysis of the purified proteins (see below).
FIG. 2.
Anti-HIV-1 activities of L. jensenii-purified wt and C1C5 RANTES. R5 HIV-1 inhibition was tested by an acute infection assay on the human CD4+ T cell clone PM1. (A and B) Inhibition of laboratory-adapted clade B HIV-1BaL (A) or HIV-1SF162 (B) was tested using L. jensenii-purified wt and C1C5 RANTES and E. coli-purified wt RANTES as a control. (C) Inhibition of primary clade B isolates HIV-15513 and HIV-110005 by L. jensenii-purified wt and C1C5 RANTES. (D) Inhibition of primary clade C isolates HIV-198IN007 and HIV-192BR025 by L. jensenii-purified wt and C1C5 RANTES. HIV-1 inhibition was measured by a p24-based assay after 4 days of infection; values indicate the means ± SD for two independent experiments performed in triplicate.
In view of development as live microbicides targeting cellular CCR5, L. jensenii-purified RANTES variants were also tested against primary R5 HIV-1 isolates, using the same acute infection assay. Two clade B strains (5513 and 10005), isolated from infected children, were used that confirmed the suitability of this approach (Fig. 2C), with IC50s of 0.94 nM and 2.75 nM (5513 and 10005, respectively) for wt RANTES and 4.70 nM and 7.37 nM (5513 and 10005, respectively) for C1C5 RANTES. Even more important given the prospective application of anti-HIV-1 microbicides for those countries where HIV-1 infection is most prominent and which are in need of low-cost intervention, two primary clade C (the most common clade affecting these countries) isolates were tested, 98IN007 and 92BR025. Indeed, wt and C1C5 RANTES presented consistent cross-clade anti-HIV-1 potency, providing a definitive in vitro proof of concept for the CCR5-targeting live microbicide strategy. As shown in Fig. 2D, IC50s for wt RANTES were 0.74 nM and 1.37 nM (98IN007 and 92BR025, respectively), with a consistent difference from C1C5 RANTES (IC50s of 2.74 nM and 6.81 nM for 98IN007 and 92BR025, respectively).
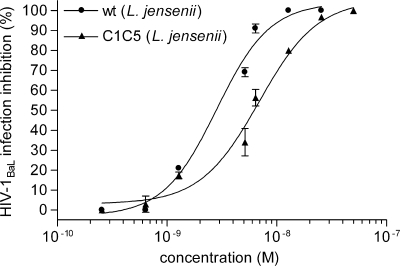
Since the cells of the mononuclear phagocytic system play a major role in HIV-1 infection and resident macrophages in the vaginal epithelium might contribute to sexual HIV-1 transmission, the inhibitory activities of RANTES variants were also investigated in human peripheral blood MDM. Figure 3 shows that both purified wt and C1C5 RANTES produced by L. jensenii inhibited HIV-1BaL infection in MDM cultures. Interestingly, in MDM cultures the two RANTES variants showed a lower difference in antiviral potency than in PM1 cells infected with the same virus strain, with an IC50 of 6.89 nM for C1C5 RANTES, compared to 2.83 nM for the wt protein. However, the HIV-1 blocking activity of wt RANTES was less efficient in MDM than in PM1 cells.
FIG. 3.
Inhibition of HIV-1BaL infection in human MDM. HIV-1 inhibition was tested using L. jensenii-purified wt and C1C5 RANTES. Anti-HIV-1 activity was measured by a p24-based assay after 4 days of infection; values indicate the means ± SD for two independent experiments performed in quadruplicate.
Biochemical characterization of L. jensenii-secreted wt and C1C5 RANTES.
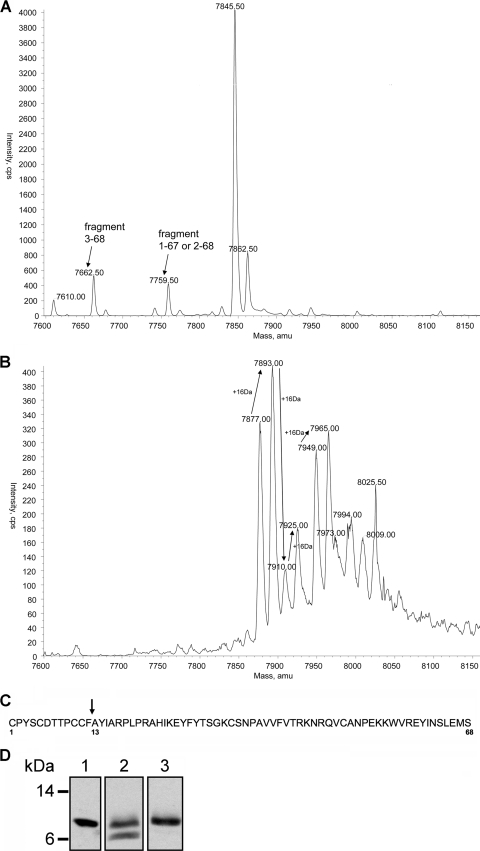
As mentioned above, L. jensenii-secreted wt and C1C5 RANTES were purified to homogeneity by a procedure involving four-step ion-exchange chromatography (30). In the secreted material, both RANTES variants presented two major forms (Fig. 1A, lanes 8 and 9), likely corresponding to the full-length protein and a degradation product. Analysis of the intracellular lactobacillus content for both RANTES variants revealed that degradation is likely to occur after secretion, since the form of lower molecular mass appears to be absent in the intracellular RANTES pool (30). Some fractions of the purified lactobacillus-secreted material contained exclusively the form putatively corresponding to full-length RANTES (either wt or C1C5). These fractions were used to test the anti-HIV-1 activity on PM1 cells (Fig. 2) and MDM (Fig. 3). Given the consistent difference in anti-HIV-1 potency between wt and C1C5 RANTES, we decided to characterize further the putative full-length forms. Therefore, the two preparations were analyzed by mass spectrometry, which confirmed the presence of a predominant wt RANTES form with a mass corresponding to that of the full-length protein (Fig. 4A). In addition, two truncated forms were present as minor components, a fragment comprised of aa 3 through 68 (3-68) and a fragment lacking a serine residue, hence possibly a 1-67 or a 2-68 form, since wt RANTES presents a serine at both termini. In contrast, purified C1C5 RANTES presented several forms in addition to the full-length form, including one retaining the last amino acid from the CbsA leader sequence (Fig. 4B). Several putative oxidation forms exist for both the expected full-length form (7,877 Da) and the form retaining the last alanine residue from the CbsA leader (7,949 Da), with a further complexity involving other nonidentified forms and their oxidation states. These data suggested that the lower anti-HIV-1 activity of L. jensenii-produced C1C5 RANTES might be due to a lower concentration of biologically active protein in the secreted purified preparation. Nevertheless, the major requirements for this system, i.e., production of C1C5 RANTES as a full-length product with potent anti-HIV-1 activity, were clearly confirmed in our experiments.
FIG. 4.
Molecular characterization of purified wt and C1C5 RANTES secreted by L. jensenii. (A) Mass spectrometry analysis of purified full-length wt RANTES. The deconvoluted mass spectrum is shown, which identifies the major component as the full-length protein and minor components corresponding to a 3-68 fragment and a 1-67 or 2-68 fragment (a serine residue is lacking, and RANTES presents a serine at both termini, positions 1 and 68). (B) The deconvoluted mass spectrum of purified full-length C1C5 RANTES is shown. Arrows connect peaks differing by 16 Da, likely corresponding to different oxidation states of the same protein form (i.e., the 7,877-Da full-length form and the 7,949-Da extra N-terminal alanine form). (C) The proteolytic site of the low-molecular-mass C1C5 RANTES peptide was identified at residue 13 from the N terminus. (D) Western blot analysis of heparin-purified RANTES. Lane 1, RANTES standard; lane 2, RANTES semipurified by ion-exchange chromatography; lane 3, RANTES purified by heparin binding affinity.
In an attempt to identify the low-molecular-mass form and a possible proteolytic cleavage site, after ion-exchange chromatography, the two major forms of C1C5 RANTES were separated by a gel filtration step. Mass spectrometry analysis and N-terminal sequencing (data not shown) identified the lower-mass form as a truncated product beginning at residue 13 (Fig. 4C). Together, the N-terminal amino acid sequence and mass spectrometry data indicated that L. jensenii is able to express C1C5 RANTES as a full-length protein but also produces a 12-aa truncated form, possibly derived from proteolytic degradation. Although the same characterization has not been carried out on wt RANTES, it is conceivable that the form with reduced molecular mass detected by Western blotting could also be the result of proteolysis at the same position as for C1C5 RANTES.
The oligomeric state of L. jensenii-secreted wt and C1C5 RANTES was previously shown to correspond to the expected pattern of monomer-dimer-higher-order oligomers (30). The oligomerization state is correlated with the affinity of chemokine interaction with cell surface glycosaminoglycans, a typical physiological feature of RANTES (36). High-affinity binding of RANTES to heparin is very similar to glycosaminoglycan binding, and this interaction has been characterized in detail (25, 31). Both wt and C1C5 RANTES variants secreted by lactobacilli showed heparin binding activity, further confirming their expected features and providing an additional tool for protein purification. As shown in Fig. 4D, a semipurified L. jensenii-secreted wt RANTES was applied on a heparin column, and the eluted material was shown to contain exclusively the full-length form. L. jensenii-secreted C1C5 RANTES showed identical heparin binding properties (data not shown). Altogether, data from anti-HIV-1 activity, mass spectrometry, and heparin binding confirmed the correct folding of L. jensenii-secreted full-length chemokines.
DISCUSSION
The HIV-1 coreceptor CCR5 represents a primary target in the development of anti-HIV-1 microbicides aimed at preventing virus transmission during sexual intercourse. Indeed, CCR5 is the coreceptor almost exclusively used by HIV-1 isolates involved in the initial viral transmission. Considering the presence of CD4+ T lymphocytes and macrophages within the human vaginal epithelium (10, 32), blockade of HIV-1 by CCR5 targeting appears to be a realistic option. RANTES is among the most potent natural HIV-1 inhibitors (6), and its engineering to obtain highly potent anti-HIV-1 derivatives is considered a strategic track for providing efficient protein-based inhibitors (36). In addition to blocking HIV-1, the ideal RANTES derivatives should not activate CCR5, in order to avoid proinflammatory activity and prevent mucosal inflammation, a condition that could, in the long term, enhance HIV-1 transmission. These aspects are particularly relevant in the live microbicide scenario, provided that engineered lactobacilli should colonize the genital tract for prolonged periods, continuously delivering the anti-HIV-1 protein with a considerable risk of inducing chronic inflammation upon persistent activation of CCR5. Targeting of CCR5 at the vaginal mucosa also demands a lack of interference with the role played by this receptor in host physiology and pathology. In this context, recent reports have demonstrated that CCR5 expression and a functional RANTES-CCR5 axis are directly linked to the control of Chlamydia and HSV-2 infection (29, 35). Interaction with CCR5 in an antagonistic manner should maintain CCR5 expression on the cellular surface. Conversely, despite their potent anti-HIV-1 activity, RANTES derivatives with CCR5 agonist activity, such as PSC-RANTES, could induce unwanted effects both by eliciting inflammation and by persistently eliminating CCR5 from the cell surface, which is their major mechanism of antiviral action (22). CCR5 activation together with its cell surface disappearance would perturb the function of CCR5 in host physiology due to unwanted chronic receptor activation (a proinflammatory condition) and an absence of the receptor at the time of viral infection. In contrast, an antagonist analogue, such as C1C5 RANTES (23), should provide the necessary anti-HIV activity in the absence of receptor activation yet preserve CCR5 cell surface expression.
Engineering of commensal bacteria to serve as anti-HIV-1 live microbicides is a promising approach and a flourishing field of investigation. The first two domains of human CD4 have been produced both as a secretory protein and as a lactobacillus-anchored moiety to block or capture the virus, respectively (4, 17). Other investigators have used fusion inhibitory peptides derived from the gp41 transmembrane envelope glycoprotein, which exhibit virus-blocking properties similar to those of the T20 peptide (12, 27, 28). MIP-1β, another CCR5-ligand chemokine (15), and a single-chain variable fragment (scFv) derived from an anti-intercellular adhesion molecule 1 (ICAM-1) monoclonal antibody (MAb) (3) have also been produced in lactobacilli to block cell-associated HIV-1 transmission. Moreover, human vaginal commensal bacteria have been engineered to produce CV-N (9, 17, 26), a lectin protein displaying anti-HIV-1 activity owing to its high-affinity recognition of gp120 carbohydrate moieties (2, 21). The Lactobacillus-derived CV-N exerts potent anti-HIV-1 activity (17), and the original strain of that report, L. jensenii 1153, was also used in the present work. Importantly, the CV-N-engineered vaginal Lactobacillus strain was shown to persistently colonize the vaginal mucosa in Chinese rhesus macaques (37).
When applied as a microbicide in a monkey model, PSC-RANTES, a potent CCR5 agonist, blocked vaginal HIV-1 transmission, providing the proof of principle for CCR5 as a possible target for prevention of HIV-1 sexual transmission (14). Therefore, the production of an anti-HIV-1 CCR5 antagonist, such as C1C5 RANTES, by L. jensenii is of major interest, particularly in view of the possibility of implementing a dual delivery system, e.g., the provision of virus-targeting (CV-N) and cell-targeting (C1C5 RANTES) compounds, hindering the virus-cell interaction surfaces and acting in an additive manner. The first stage of a CCR5-targeting live microbicide has now been established, since we showed that both wt and C1C5 RANTES could be engineered in L. jensenii to be delivered as secretory proteins in their anti-HIV-1 active forms. wt and C1C5 RANTES, purified from L. jensenii-producing supernatants, blocked HIV-1 infection in CD4+ T cells and in primary human macrophages. The antiviral activities were very similar with six different R5 HIV-1 strains, providing an important in vitro proof for cross-clade protection.
Plasmid-based expression of RANTES variants by L. jensenii revealed that part of the secreted protein undergoes degradation, with the proteolytic cleavage site identified in a region crucial for RANTES integrity and activity. We are currently exploring strategies to reduce or eliminate this proteolytic cleavage and obtain a predominant secretion of full-length C1C5 RANTES in lactobacilli. A C1C5 RANTES anti-HIV-1 activity lower than that of wt RANTES was observed (Fig. 2 and 3). This observation could result from the conformation or oxidation state of the lactobacillus-derived C1C5 RANTES and merits further investigation. Nevertheless, the characterization of the anti-HIV-1 activity, dimerization/oligomerization state (30), and heparin binding attested to the native-like fold of both wt and C1C5 RANTES, although conformational differences are necessarily occurring at the N terminus due to the intramolecular disulfide bond established by C1 and C5. Indeed, in a recent report on P2-RANTES, N-terminal modifications were shown to lead to differences in conformation with respect to wt RANTES (11). A novel N-terminal RANTES mutant, 5P12-RANTES, has been reported to act as a CCR5 antagonist (8); hence, it would be interesting to compare 5P12-RANTES and C1C5 RANTES in an identical experimental assay. Furthermore, 5P12-RANTES expression in lactobacilli cannot be considered obvious, since different RANTES mutants recently produced in lactobacilli yielded very low expression levels (M. Secchi and L. Vangelista, unpublished data).
In conclusion, our results provide proof of principle for the efficient secretion of an anti-HIV-1 active CCR5 antagonist by an engineered vaginal commensal bacterium, which represents an important advancement toward realistic, safe, and low-cost prevention of sexual transmission of HIV-1.
Acknowledgments
This work was supported by NIH grant 1U19AI060615 and 5R21AI079799.
We thank Kyungmi Min and Jack Presley (University of California at Davis) for linear matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF) analysis and N-terminal amino acid sequence determination, respectively, Qing Xia and Thomas P. Parks (Osel, Inc.) for fermentation of L. jensenii and help with plasmid construction, respectively, and Francesca Sironi (San Raffaele Scientific Institute) for viral stock preparation. HIV-1 strains 92BR025 (ARP179.11) and 98IN007 (ARP1027.3) were obtained from the Programme EVA Centre for AIDS Reagents, NIBSC, United Kingdom, which was supported by the EU Europrise Network of Excellence, AVIP and NGIN consortia, and the Bill and Melinda Gates GHRC-CAVD Project; strains were donated by the WHO-UNAIDS Network for HIV Isolation and Characterization.
Footnotes
Published ahead of print on 17 May 2010.
REFERENCES
- 1.Bolton, M., A. van der Straten, and C. R. Cohen. 2008. Probiotics: potential to prevent HIV and sexually transmitted infections in women. Sex. Transm. Dis. 35:214-225. [DOI] [PubMed] [Google Scholar]
- 2.Boyd, M. R., K. R. Gustafson, J. B. McMahon, R. H. Shoemaker, B. R. O'Keefe, T. Mori, R. J. Gulakowski, L. Wu, M. I. Rivera, C. M. Laurencot, M. J. Currens, J. H. Cardellina II, R. W. Buckheit, Jr., P. L. Nara, L. K. Pannell, R. C. Sowder II, and L. E. Henderson. 1997. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob. Agents Chemother. 41:1521-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chancey, C. J., K. V. Khanna, J. F. Seegers, G. W. Zhang, J. Hildreth, A. Langan, and R. B. Markham. 2006. Lactobacilli-expressed single-chain variable fragment (scFv) specific for intercellular adhesion molecule 1 (ICAM-1) blocks cell-associated HIV-1 transmission across a cervical epithelial monolayer. J. Immunol. 176:5627-5636. [DOI] [PubMed] [Google Scholar]
- 4.Chang, T. L., C. Chang, D. A. Simpson, Q. Xu, P. K. Martin, L. A. Lagenaur, G. K. Schoolnik, D. D. Ho, S. L. Hillier, M. Holodniy, J. A. Lewicki, and P. P. Lee. 2003. Inhibition of HIV infectivity by a natural human isolate of Lactobacillus jensenii engineered to express functional two-domain CD4. Proc. Natl. Acad. Sci. U. S. A. 100:11672-11677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherpes, T. L., M. A. Melan, J. A. Kant, L. A. Cosentino, L. A. Meyn, and S. L. Hillier. 2005. Genital tract shedding of herpes simplex virus type 2 in women: effects of hormonal contraception, bacterial vaginosis, and vaginal group B streptococcus colonization. Clin. Infect. Dis. 40:1422-1428. [DOI] [PubMed] [Google Scholar]
- 6.Cocchi, F., A. L. DeVico, A. Garzino-Demo, S. K. Arya, R. C. Gallo, and P. Lusso. 1995. Identification of RANTES, MIP-1αα, and MIP-1ββ as the major HIV-suppressive factors produced by CD8++ T cells. Science 270:1811-1815. [DOI] [PubMed] [Google Scholar]
- 7.Cutler, B., and J. Justman. 2008. Vaginal microbicides and the prevention of HIV transmission. Lancet Infect. Dis. 8:685-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaertner, H., F. Cerini, J. M. Escola, G. Kuenzi, A. Melotti, R. Offord, I. Rossitto-Borlat, R. Nedellec, J. Salkowitz, G. Gorochov, D. Mosier, and O. Hartley. 2008. Highly potent, fully recombinant anti-HIV chemokines: reengineering a low-cost microbicide. Proc. Natl. Acad. Sci. U. S. A. 105:17706-17711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giomarelli, B., R. Provvedi, F. Meacci, T. Maggi, D. Medaglini, G. Pozzi, T. Mori, J. B. McMahon, R. Gardella, and M. R. Boyd. 2002. The microbicide cyanovirin-N expressed on the surface of commensal bacterium Streptococcus gordonii captures HIV-1. AIDS 16:1351-1356. [DOI] [PubMed] [Google Scholar]
- 10.Hladik, F., P. Sakchalathorn, L. Ballweber, G. Lentz, M. Fialkow, D. Eschenbach, and M. J. McElrath. 2007. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 26:257-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin, H., I. Kagiampakis, P. Li, and P. J. Liwang. 2010. Structural and functional studies of the potent anti-HIV chemokine variant P2-RANTES. Proteins 78:295-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilby, J. M., S. Hopkins, T. M. Venetta, B. DiMassimo, G. A. Cloud, J. Y. Lee, L. Alldredge, E. Hunter, D. Lambert, D. Bolognesi, T. Matthews, M. R. Johnson, M. A. Nowak, G. M. Shaw, and M. S. Saag. 1998. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat. Med. 4:1302-1307. [DOI] [PubMed] [Google Scholar]
- 13.Lagenaur, L. A., and E. A. Berger. 2005. An anti-HIV microbicide comes alive. Proc. Natl. Acad. Sci. U. S. A. 102:12294-12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lederman, M. M., R. S. Veazey, R. Offord, D. E. Mosier, J. Dufour, M. Mefford, M. Piatak, Jr., J. D. Lifson, J. R. Salkowitz, B. Rodriguez, A. Blauvelt, and O. Hartley. 2004. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science 306:485-487. [DOI] [PubMed] [Google Scholar]
- 15.Liu, J. J., G. Reid, Y. Jiang, M. S. Turner, and C. C. Tsai. 2007. Activity of HIV entry and fusion inhibitors expressed by the human vaginal colonizing probiotic Lactobacillus reuteri RC-14. Cell Microbiol. 9:120-130. [DOI] [PubMed] [Google Scholar]
- 16.Liu, X., L. A. Lagenaur, D. A. Simpson, K. P. Essenmacher, C. L. Frazier-Parker, Y. Liu, D. Tsai, S. S. Rao, D. H. Hamer, T. P. Parks, P. P. Lee, and Q. Xu. 2006. Engineered vaginal lactobacillus strain for mucosal delivery of the human immunodeficiency virus inhibitor cyanovirin-N. Antimicrob. Agents Chemother. 50:3250-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu, X., L. A. Lagenaur, P. P. Lee, and Q. Xu. 2008. Engineering of a human vaginal Lactobacillus strain for surface expression of two-domain CD4 molecules. Appl. Environ. Microbiol. 74:4626-4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lusso, P., F. Cocchi, C. Balotta, P. D. Markham, A. Louie, P. Farci, R. Pal, R. C. Gallo, and M. S. Reitz, Jr. 1995. Growth of macrophage-tropic and primary human immunodeficiency virus type 1 (HIV-1) isolates in a unique CD4++ T-cell clone (PM1): failure to downregulate CD4 and to interfere with cell-line-tropic HIV-1. J. Virol. 69:3712-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lusso, P. 2006. HIV and the chemokine system: 10 years later. EMBO J. 25:447-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moscicki, A. B. 2008. Vaginal microbicides: where are we and where are we going? J. Infect. Chemother. 14:337-341. [DOI] [PubMed] [Google Scholar]
- 21.O'Keefe, B. R., S. R. Shenoy, D. Xie, W. Zhang, J. M. Muschik, M. J. Currens, I. Chaiken, and M. R. Boyd. 2000. Analysis of the interaction between the HIV-inactivating protein cyanovirin-N and soluble forms of the envelope glycoproteins gp120 and gp41. Mol. Pharmacol. 58:982-992. [DOI] [PubMed] [Google Scholar]
- 22.Pastore, C., G. R. Picchio, F. Galimi, R. Fish, O. Hartley, R. E. Offord, and D. E. Mosier. 2003. Two mechanisms for human immunodeficiency virus type 1 inhibition by N-terminal modifications of RANTES. Antimicrob. Agents Chemother. 47:509-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polo, S., V. Nardese, C. De Santis, C. Arcelloni, R. Paroni, F. Sironi, A. Verani, M. Rizzi, M. Bolognesi, and P. Lusso. 2000. Enhancement of the HIV-1 inhibitory activity of RANTES by modification of the N-terminal region: dissociation from CCR5 activation. Eur. J. Immunol. 30:3190-3198. [DOI] [PubMed] [Google Scholar]
- 24.Pouwels, P. H., and J. A. Leunissen. 1994. Divergence in codon usage of Lactobacillus species. Nucleic Acids Res. 22:929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Proudfoot, A. E., S. Fritchley, F. Borlat, J. P. Shaw, F. Vilbois, C. Zwahlen, A. Trkola, D. Marchant, P. R. Clapham, and T. N. Wells. 2001. The BBXB motif of RANTES is the principal site for heparin binding and controls receptor selectivity. J. Biol. Chem. 276:10620-10626. [DOI] [PubMed] [Google Scholar]
- 26.Pusch, O., D. Boden, S. Hannify, F. Lee, L. D. Tucker, M. R. Boyd, J. M. Wells, and B. Ramratnam. 2005. Bioengineering lactic acid bacteria to secrete the HIV-1 virucide cyanovirin. J. Acquir. Immune Defic. Syndr. 40:512-520. [DOI] [PubMed] [Google Scholar]
- 27.Pusch, O., R. Kalyanaraman, L. D. Tucker, J. M. Wells, B. Ramratnam, and D. Boden. 2006. An anti-HIV microbicide engineered in commensal bacteria: secretion of HIV-1 fusion inhibitors by lactobacilli. AIDS 20:1917-1922. [DOI] [PubMed] [Google Scholar]
- 28.Rao, S., S. Hu, L. McHugh, K. Lueders, K. Henry, Q. Zhao, R. A. Fekete, S. Kar, S. Adhya, and D. H. Hamer. 2005. Toward a live microbial microbicide for HIV: commensal bacteria secreting an HIV fusion inhibitor peptide. Proc. Natl. Acad. Sci. U. S. A. 102:11993-11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakthivel, S. K., U. P. Singh, S. Singh, D. D. Taub, J. U. Igietseme, and J. W. Lillard, Jr. 2008. CCL5 regulation of mucosal chlamydial immunity and infection. BMC Microbiol. 8:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Secchi, M., Q. Xu, P. Lusso, and L. Vangelista. 2009. The superior folding of a RANTES analogue expressed in lactobacilli as compared to mammalian cells reveals a promising system to screen new RANTES mutants. Protein Expr. Purif. 68:34-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw, J. P., Z. Johnson, F. Borlat, C. Zwahlen, A. Kungl, K. Roulin, A. Harrenga, T. N. Wells, and A. E. Proudfoot. 2004. The X-ray structure of RANTES: heparin-derived disaccharides allows the rational design of chemokine inhibitors. Structure 12:2081-2093. [DOI] [PubMed] [Google Scholar]
- 32.Shen, R., H. E. Richter, R. H. Clements, L. Novak, K. Huff, D. Bimczok, S. Sankaran-Walters, S. Dandekar, P. R. Clapham, L. E. Smythies, and P. D. Smith. 2009. Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type-1 infection. J. Virol. 83:3258-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stemmer, W. P., A. Crameri, K. D. Ha, T. M. Brennan, and H. L. Heyneker. 1995. Single-step assembly of a gene and entire plasmid from large numbers of oligodeoxyribonucleotides. Gene 164:49-53. [DOI] [PubMed] [Google Scholar]
- 34.Taha, T. E., D. R. Hoover, G. A. Dallabetta, N. I. Kumwenda, L. A. Mtimavalye, L. P. Yang, G. N. Liomba, R. L. Broadhead, J. D. Chiphangwi, and P. G. Miotti. 1998. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS 12:1699-1706. [DOI] [PubMed] [Google Scholar]
- 35.Thapa, M., W. A. Kuziel, and D. J. Carr. 2007. Susceptibility of CCR5-deficient mice to genital herpes simplex virus type 2 is linked to NK cell mobilization. J. Virol. 81:3704-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vangelista, L., M. Secchi, and P. Lusso. 2008. Rational design of novel HIV-1 entry inhibitors by RANTES engineering. Vaccine 26:3008-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu, R. R., A. T. Cheng, L. A. Lagenaur, W. Huang, D. E. Weiss, J. Treece, B. E. Sanders-Beer, D. H. Hamer, P. P. Lee, Q. Xu, and Y. Liu. 2009. A Chinese rhesus macaque (Macaca mulatta) model for vaginal Lactobacillus colonization and live microbicide development. J. Med. Primatol. 38:125-136. [DOI] [PMC free article] [PubMed] [Google Scholar]