Abstract
Bacterial resistance presents a difficult issue for fluoroquinolone treatment of bacterial infections. In previous work, we reported that 8-methoxy-quinazoline-2,4-diones are active against quinolone-resistant mutants of Escherichia coli. Here, we demonstrate the activity of a representative 8-methoxy-quinazoline-2,4-dione against quinolone-resistant gyrases. Furthermore, 8-methoxy-quinazoline-2,4-dione and other diones are shown to inhibit Staphylococcus aureus gyrase and topoisomerase IV with similar degrees of efficacy, suggesting that the diones might act as dual-targeting agents against S. aureus.
Antibiotic resistance is among the most difficult problems we currently face during the treatment of bacterial infections (10, 32). The fluoroquinolones are among the antibacterials affected by resistance, which can severely limit their clinical use (1, 6, 29). Thus, there is an urgent need to develop antimicrobial agents that are effective against drug-resistant pathogens. Two properties of quinolone-like compounds are likely to be useful for finding effective derivatives: (i) activity against quinolone-resistant mutants already present (12) and (ii) equal effectiveness against the two quinolone targets, DNA gyrase and topoisomerase IV (Topo IV) (dual-targeting agents; dual-targeting agents are expected to slow the emergence of drug-resistant mutants [4, 19, 23, 26, 31]). The presence of an 8-methoxy group (5, 22, 33) and changing the quinolone core structure to either quinazoline-2,4-dione (2, 8, 18) or pyrido[1,2-c]pyrimidine-1,3-dione (UI7) improve the activity of fluoroquinolone-like compounds against fluoroquinolone-resistant bacteria. We recently identified 8-methoxy-quinazoline-2,4-diones that show little increase in MIC due to gyrA or gyrB quinolone resistance mutations of Escherichia coli (12). To establish that this activity against mutant bacteria is due to improved activity against gyrase, we examined the activity of a representative 8-methoxy-quinazoline-2,4-dione (UING5-207; 8-methoxy 2,4-dione) with purified gyrase. We also assessed its effect on the activity of purified Topo IV to determine whether the in vivo results were likely due to a target switch. Furthermore, to better understand how dione structure influences target selection, we compared the effectiveness of diones for inhibition of catalytic activities of Staphylococcus aureus and E. coli topoisomerases.
Based on MIC values determined in previous work (12), we selected three mutant gyrases, GyrA S83W gyrase, GyrA G81C gyrase, and GyrA A67S gyrase, as examples exhibiting high, moderate, and low levels of quinolone resistance in vivo. Mutations were introduced into the E. coli gyrA gene using the overlap extension PCR technique (17), and subunits of E. coli and S. aureus gyrases and Topo IVs were expressed and purified. The active enzymes were reconstituted as described previously (13-16, 28). In vitro activities of the 8-methoxy 2,4-dione were compared with those of a cognate 8-methyl-quinazoline-2,4-dione (UIJR1-048; 8-methyl 2,4-dione), 5-methoxypyrido[1,2-c]pyrimidine-1,3-dione (UIJR1-100; 5-methoxy 1,3-dione), 8-methoxy fluoroquinolone (UING5-249), and ciprofloxacin, a clinically important fluoroquinolone. Synthesis of the diones and 8-methoxy fluoroquinolone was achieved using methods described previously (7, 12, 30); their structures are shown in Fig. 1. A DNA supercoiling assay (for example, see Fig. 2) was employed to examine the effects of these compounds on the catalytic activities of wild-type and mutant gyrases; a decatenation assay was used with Topo IV (Table 1). The abilities of these compounds to poison the E. coli topoisomerases were assessed using a DNA cleavage assay (Table 2). These assays were conducted as described previously (24). The activity against mutant enzymes was expressed as the ratio of either the 50% inhibitory concentration (IC50) or the CC3 value (the concentration required to triple the level of DNA cleavage from the background level in the absence of drug) for a mutant gyrase relative to that of wild-type gyrase for catalytic inhibition and poisoning assays, respectively. The CC3 value was used to minimize bias due to multiple cleavage events in a single DNA molecule.
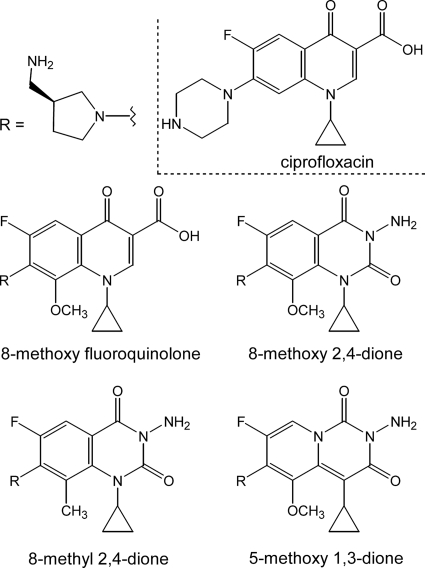
FIG. 1.
Structures of the diones and fluoroquinolones used in this study. The structures of 8-methoxy 2,4-dione (UING5-207), 8-methyl 2,4-dione (UIJR1-048), 5-methoxy 1,3-dione (UIJR1-100), 8-methoxy fluoroquinolone (UING5-249), and ciprofloxacin are shown.
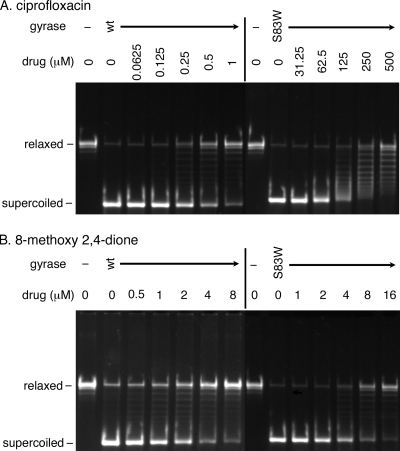
FIG. 2.
Representative results of supercoiling assays. The supercoiling assay was conducted using wild-type gyrase and GyrA S83W gyrase to examine the effect of either ciprofloxacin (A) or 8-methoxy 2,4-dione (B). wt, wild-type gyrase; S83W, GyrA S83W gyrase.
TABLE 1.
Inhibition of the catalytic activities of E. coli gyrase and Topo IV
| Compound | Median IC50 (μM) ± ADb |
||||
|---|---|---|---|---|---|
| Wild-type gyrase | GyrA S83W gyrase | GyrA G81C gyrase | GyrA A67S gyrase | Topo IV | |
| Ciprofloxacin | 0.45 ± 0.004 | 101 ± 1.9 (224)a | 28 ± 7.0 (62) | 1.0 ± 0.15 (2.2) | 15.9 ± 1.9 |
| 8-Methoxy fluoroquinolone | 0.16 ± 0.01 | 1.4 ± 0.1 (8.8) | 1.2 ± 0.1 (7.5) | 0.12 ± 0.02 (0.75) | 1.7 ± 0.1 |
| 8-Methoxy 2,4-dione | 2.8 ± 0.1 | 5.9 ± 0.9 (2.1) | 4.3 ± 0.4 (1.5) | 2.4 ± 0.1 (0.86) | 16 ± 0.4 |
| 8-Methyl 2,4-dione | 0.95 ± 0.15 | 3.8 ± 0.6 (4.0) | 1.7 ± 0.2 (1.8) | 1.2 ± 0.3 (1.3) | 6.0 ± 0.2 |
| 5-Methoxy 1,3-dione | 11 ± 1.2 | 67 ± 4.5 (6.1) | 31 ± 2.9 (2.8) | 19 ± 5.0 (1.7) | 163 ± 7.0 |
The ratio of the IC50 for a mutant gyrase to that for the wild-type gyrase is shown in parentheses.
AD, absolute deviation.
TABLE 2.
Poisoning of E. coli gyrase and Topo IV
| Compound | Median CC3 (μM) ± AD |
||||
|---|---|---|---|---|---|
| Wild-type gyrase | GyrA S83W gyrase | GyrA G81C gyrase | GyrA A67S gyrase | Topo IV | |
| Ciprofloxacin | 0.097 ± 0.003 | 39 ± 5.0 (402)a | 3.2 ± 0.7 (33) | 0.28 ± 0.05 (2.9) | 14.2 ± 3.3 |
| 8-Methoxy fluoroquinolone | 0.023 ± 0.001 | 0.15 ± 0.01 (6.5) | 0.30 ± 0.02 (13) | 0.035 ± 0.004 (1.5) | 1.4 ± 0.2 |
| 8-Methoxy 2,4-dione | 0.52 ± 0.04 | 1.1 ± 0.22 (2.1) | 1.2 ± 0.04 (2.3) | 0.88 ± 0.1 (1.7) | 11.1 ± 1.9 |
| 8-Methyl 2,4-dione | 0.13 ± 0.02 | 0.73 ± 0.02 (5.6) | 0.41 ± 0.02 (3.1) | 0.33 ± 0.05 (2.5) | 3.0 ± 0.2 |
| 5-Methoxy 1,3-dione | 3.8 ± 0.2 | 13 ± 2.2 (3.4) | 8.9 ± 1.6 (2.3) | 5.8 ± 1.0 (1.5) | 108 ± 8.0 |
The ratio of the CC3 value for a mutant gyrase to that for the wild-type gyrase is shown in parentheses.
Both assays showed that each methoxy-substituted compound, as well as the 8-methyl 2,4-dione, exhibited greater activity against mutant gyrase relative to wild-type gyrase than did ciprofloxacin (Tables 1 and 2). This increased relative activity correlated well with that observed in vivo (Fig. 3) (12). Methoxy-substituted diones and the 8-methyl 2,4-dione were more effective against mutant gyrases relative to wild-type gyrase than the 8-methoxy fluoroquinolone, although the absolute IC50s for the 8-methoxy fluoroquinolone and the 8-methyl 2,4-dione were lower than those for the methoxy-substituted diones. The 8-methoxy 2,4-dione exhibited the highest activity against mutant gyrases relative to wild-type gyrase. The absolute IC50 of the 5-methoxy 1,3-dione was the highest among the compounds tested (Table 1). However, it was more effective than ciprofloxacin against GyrA S83W gyrase. The IC50 of ciprofloxacin against either GyrA S83W gyrase or GyrA G81C gyrase was higher than that against Topo IV (Table 1); consequently, the three methoxy-substituted compounds and the 8-methyl 2,4-dione were more effective against mutant gyrases, including GyrA S83W gyrase, than Topo IV, the secondary target of these compounds (Tables 1 and 2). These results suggest that the elevated activity of the 8-methoxy 2,4-dione against gyrase mutants relative to wild-type E. coli (12) was due to its effectiveness against mutant gyrase and not to a target switch from gyrase to Topo IV. In addition, these studies are the first to report 1,3-dione activity against mutant gyrases. Further studies are necessary to identify the mutations that confer resistance to these diones on wild-type and quinolone-resistant bacteria. It will be interesting to investigate how the presence of a quinolone resistance-producing substitution, such as S83W in the GyrA protein, would affect the frequency and types of dione resistance mutations.
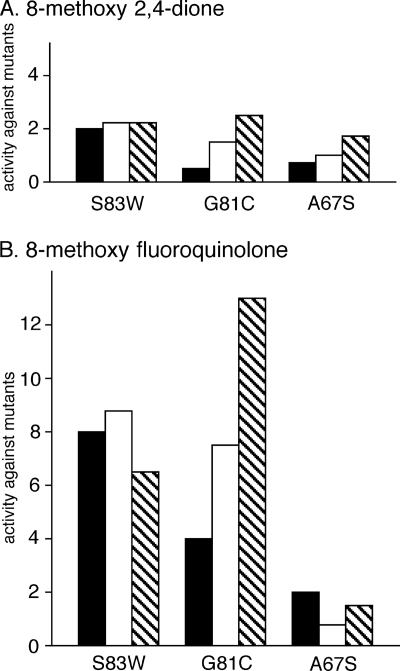
FIG. 3.
Comparison of in vitro and in vivo activities against mutant gyrase. Activities of the 8-methoxy 2,4-dione (A) and its cognate fluoroquinolone (B) against mutants measured in vivo (MIC values are from reference 12), in the supercoiling assay (Table 1), and in the DNA cleavage assay (Table 2) are expressed as the ratio of the MIC value, the IC50, or the CC3 value for each mutant gyrase to that of wild-type gyrase and are shown as solid, open, and striped bars, respectively. E. coli strains or purified gyrases containing the indicated mutation were used in the assays. As reported in reference 12, the absolute MIC values for the wild-type E. coli strain were 2.5 and 0.004 μg of the 8-methoxy 2,4-dione and the 8-methoxy fluoroquinolone/ml, respectively.
Each quinolone class drug typically has either gyrase or Topo IV, but not both, as the primary target in vivo. DNA gyrase is often the primary target of quinolones in Gram-negative bacteria (20, 21), whereas Topo IV is frequently the primary target in Gram-positive organisms (9). However, the preferred target can be switched by changes in quinolone structure (11, 25). It is unclear what determines the primary target of a quinolone. Some newer fluoroquinolones, such as moxifloxacin and other 8-methoxy fluoroquinolones, target gyrase and Topo IV of some organisms with nearly equipotent activity, and these dual-targeting fluoroquinolones seem to reduce the emergence of drug-resistant mutants (4, 19, 23, 26, 31). The quinolone sensitivity of gyrase and Topo IV, estimated by in vitro catalytic assays, is likely to be among the key factors that determine the primary target of a quinolone in vivo. For example, the inhibitory effects of ciprofloxacin on the supercoiling activity of gyrase and the decatenating activity of Topo IV showed that purified E. coli gyrase, the primary target of ciprofloxacin in E. coli, was more sensitive to ciprofloxacin than is purified E. coli Topo IV, whereas purified S. aureus Topo IV, the primary target of ciprofloxacin in S. aureus, was more sensitive to ciprofloxacin than purified S. aureus gyrase (Table 3; the high-salt conditions likely to be relevant with S. aureus topoisomerases [3, 16] precluded the use of CC3 as a comparator in Table 3).
TABLE 3.
Inhibition of the catalytic activities of E. coli and S. aureus topoisomerases
| Compound | Median IC50 (μM) ± AD for E. coli |
Selectivitya | Median IC50 (μM) ± AD for S. aureus |
Selectivitya | ||
|---|---|---|---|---|---|---|
| Gyrase | Topo IV | Gyrase | Topo IV | |||
| Ciprofloxacin | 0.45 ± 0.004 | 15.9 ± 1.9 | 35.3 | 31.5 ± 2.5 | 8.8 ± 1.4 | 3.6 |
| 8-Methoxy fluoroquinolone | 0.16 ± 0.01 | 1.7 ± 0.1 | 10.6 | 1.1 ± 0.1 | 0.5 ± 0.03 | 2.2 |
| 8-Methoxy 2,4-dione | 2.8 ± 0.1 | 16 ± 0.4 | 5.7 | 2.3 ± 0.1 | 5.5 ± 0.2 | 2.4 |
| 8-Methyl 2,4-dione | 0.95 ± 0.15 | 6.0 ± 0.2 | 6.3 | 1.6 ± 0.3 | 0.88 ± 0.07 | 1.8 |
| 5-Methoxy 1,3-dione | 11 ± 1.2 | 163 ± 7 | 14.8 | 36.5 ± 0.5 | 48 ± 8 | 1.3 |
Selectivity is defined as the ratio of the IC50 for the secondary target to that for the primary target. A selectivity value of 1 indicates perfect dual targeting.
Target selection by diones has not been extensively studied. Only one report describes the differential interaction of an 8-methyl 2,4-dione with Streptococcus pneumoniae gyrase and Topo IV (27). Thus, characterization and comparison of the effectiveness of assorted dione structures against the catalytic activities of S. aureus and E. coli topoisomerases in vitro should provide useful insight into target selection by a dione in vivo. These studies will also further our understanding of how the structure of a dione (e.g., 8-methyl versus 8-methoxy or 1,3- versus 2,4-) might influence target selection. Although absolute potency varied, the 8-methoxy fluoroquinolone and three diones inhibited the activities of S. aureus gyrase and S. aureus Topo IV with similar degrees of efficacy (Table 3). The 8-methoxy fluoroquinolone and the 8-methyl 2,4-dione were slightly more effective against S. aureus Topo IV, while the methoxy-substituted diones were slightly more effective against S. aureus gyrase. Thus, the diones, as well as the 8-methoxy fluoroquinolone, might act as dual-targeting drugs against S. aureus while preferentially targeting gyrase over Topo IV with E. coli. Additional structure-function studies are required to determine whether dual targeting of S. aureus is inherently general to diones or if specific substituents in the core structures are required (e.g., the 8-methoxy group on fluoroquinolones).
In conclusion, an 8-methoxy 2,4-dione, an 8-methyl 2,4-dione, and a 5-methoxy 1,3-dione exhibited greater in vitro activities against quinolone-resistant mutant gyrases relative to wild-type gyrase than did ciprofloxacin or an 8-methoxy fluoroquinolone. E. coli Topo IV was less sensitive to these diones than any of the quinolone-resistant mutant gyrases; thus, the activities of these quinolone class antimicrobial agents against gyrase mutants relative to wild-type E. coli were due to their effectiveness against mutant gyrases and not to a target switch from gyrase to Topo IV. In addition, the diones used in this study inhibited the catalytic activities of S. aureus gyrase and S. aureus Topo IV at similar effectiveness levels, indicating that some diones might function as dual-targeting agents against S. aureus.
Acknowledgments
We thank Xilin Zhao and Muhammad Malik for critical comments on the manuscript.
This work was supported by NIH grant AI073491 and a fund from the University of Minnesota Medical School.
Footnotes
Published ahead of print on 19 April 2010.
REFERENCES
- 1.Acar, J. F. 1997. Consequences of bacterial resistance to antibiotics in medical practice. Clin. Infect. Dis. 24(Suppl. 1):S17-S18. [DOI] [PubMed] [Google Scholar]
- 2.Bird, P., E. Ellsworth, D. Nguyen, J. Sanchez, H. Showalter, R. Singh, M. Stier, T. Tran, B. Watson, and J. Yip. August 2006. 3-Aminoquinazolin-2,4-dione antibacterial agents. U.S. patent 7,094,780 B1.
- 3.Blanche, F., B. Cameron, F. X. Bernard, L. Maton, B. Manse, L. Ferrero, N. Ratet, C. Lecoq, A. Goniot, D. Bisch, and J. Crouzet. 1996. Differential behaviors of Staphylococcus aureus and Escherichia coli type II DNA topoisomerases. Antimicrob. Agents Chemother. 40:2714-2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caeiro, J. P., and P. B. Iannini. 2003. Moxifloxacin (Avelox): a novel fluoroquinolone with a broad spectrum of activity. Expert Rev. Anti Infect. Ther. 1:363-370. [DOI] [PubMed] [Google Scholar]
- 5.Dong, Y., C. Xu, X. Zhao, J. Domagala, and K. Drlica. 1998. Fluoroquinolone action against mycobacteria: effects of C8 substituents on bacterial growth, survival, and resistance. Antimicrob. Agents Chemother. 42:2978-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drlica, K., H. Hiasa, R. J. Kerns, M. Malik, A. Mustaev, and X. Zhao. 2009. Quinolones: action and resistance updated. Curr. Top. Med. Chem. 9:981-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellsworth, E. L., and H. D. H. Showalter. June 2003. Antibacterial agents. U.S. patent 0114458 A1.
- 8.Ellsworth, E. L., T. Tran, H. H. Showalter, J. Sanchez, B. Watson, M. Stier, J. Domagala, S. Gracheck, E. Joannides, M. Shapiro, S. Dunham, D. Hanna, M. Huband, J. Gage, J. Bronstein, J. Liu, D. Nguyen, and R. Singh. 2006. 3-Aminoquinazolinediones as a new class of antibacterial agents demonstrating excellent antibacterial activity against wild-type and multidrug resistant organisms. J. Med. Chem. 49:6435-6438. [DOI] [PubMed] [Google Scholar]
- 9.Ferrero, L., B. Cameron, B. Manse, D. Lagneaux, J. Crouzet, A. Famechon, and F. Blanche. 1994. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: a primary target of fluoroquinolones. Mol. Microbiol. 13:641-653. [DOI] [PubMed] [Google Scholar]
- 10.Fischbach, M. A., and C. T. Walsh. 2009. Antibiotics for emerging pathogens. Science 325:1089-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fournier, B., X. Zhao, T. Lu, K. Drlica, and D. C. Hooper. 2000. Selective Targeting of Topoisomerase IV and DNA gyrase in Staphylococcus aureus: different patterns of quinolone-induced inhibition of DNA synthesis. Antimicrob. Agents Chemother. 44:2160-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.German, N., M. Malik, J. Rosen, K. Drlica, and R. J. Kerns. 2008. Use of gyrase resistance mutants to guide selection of 8-methoxyquinazoline-2,4-diones. Antimicrob. Agents Chemother. 52:3915-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiasa, H. 2002. The Glu-84 of the ParC subunit plays critical roles in both topoisomerase IV-quinolone and topoisomerase IV-DNA interactions. Biochemistry 41:11779-11785. [DOI] [PubMed] [Google Scholar]
- 14.Hiasa, H., R. J. DiGate, and K. J. Marians. 1994. Decatenating activity of Escherichia coli DNA gyrase and topoisomerases I and III during oriC and pBR322 DNA replication in vitro. J. Biol. Chem. 269:2093-2099. [PubMed] [Google Scholar]
- 15.Hiasa, H., and M. E. Shea. 2000. DNA gyrase-mediated wrapping of the DNA strand is required for the replication fork arrest by the DNA gyrase-quinolone-DNA ternary complex. J. Biol. Chem. 275:34780-34786. [DOI] [PubMed] [Google Scholar]
- 16.Hiasa, H., M. E. Shea, C. M. Richardson, and M. N. Gwynn. 2003. Staphylococcus aureus gyrase-quinolone-DNA ternary complexes fail to arrest replication fork progression in vitro: effects of salt on the DNA binding mode and the catalytic activity of Staphylococcus aureus gyrase. J. Biol. Chem. 278:8861-8868. [DOI] [PubMed] [Google Scholar]
- 17.Horton, R. M., S. N. Ho, J. K. Pullen, H. D. Hunt, Z. Cai, and L. R. Pease. 1993. Gene splicing by overlap extension. Methods Enzymol. 217:270-279. [DOI] [PubMed] [Google Scholar]
- 18.Huband, M. D., M. A. Cohen, M. Zurack, D. L. Hanna, L. A. Skerlos, M. C. Sulavik, G. W. Gibson, J. W. Gage, E. Ellsworth, M. A. Stier, and S. J. Gracheck. 2007. In vitro and in vivo activities of PD0305970 and PD 0326448, new bacterial gyrase/topoisomerase inhibitors with potent antibacterial activities versus multidrug-resistant gram-positive and fastidious organism groups. Antimicrob. Agents Chemother. 51:1191-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ince, D., X. Zhang, L. C. Silver, and D. C. Hooper. 2002. Dual targeting of DNA gyrase and topoisomerase IV: target interactions of garenoxacin (BMS-284756, T-3811ME), a new desfluoroquinolone. Antimicrob. Agents Chemother. 46:3370-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khodursky, A. B., E. L. Zechiedrich, and N. R. Cozzarelli. 1995. Topoisomerase IV is a target of quinolones in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 92:11801-11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreuzer, K. N., and N. R. Cozzarelli. 1979. Escherichia coli mutants thermosensitive for deoxyribonucleic acid gyrase subunit A: effects on deoxyribonucleic acid replication, transcription, and bacteriophage growth. J. Bacteriol. 140:424-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu, T., X. Zhao, and K. Drlica. 1999. Gatifloxacin activity against quinolone-resistant gyrase: allele-specific enhancement of bacteriostatic and bactericidal activity by the C-8-methoxy group. Antimicrob. Agents Chemother. 43:2969-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okumura, R., T. Hirata, Y. Onodera, K. Hoshino, T. Otani, and T. Yamamoto. 2008. Dual-targeting properties of the 3-aminopyrrolidyl quinolones, DC-159a and sitafloxacin, against DNA gyrase and topoisomerase IV: contribution to reducing in vitro emergence of quinolone-resistant Streptococcus pneumoniae. J. Antimicrob. Chemother. 62:98-104. [DOI] [PubMed] [Google Scholar]
- 24.Oppegard, L., B. Hamann, K. R. Streck, K. C. Ellis, H. Fieldler, A. B. Khodursky, and H. Hiasa. 2009. In vivo and in vitro patterns of the activity of simocyclinone D8, an angucyclineone antibiotic from Streptomyces antibioticus. Antimicrob. Agents Chemother. 53:2110-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan, X.-S., and L. M. Fisher. 1997. Targeting of DNA gyrase in Streptococcus pneumoniae by sparfloxacin: selective targeting of gyrase or topoisomerase IV by quinolones. Antimicrob. Agents Chemother. 41:471-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan, X. S., and L. M. Fisher. 1998. DNA gyrase and topoisomerase IV are dual targets of clinafloxacin action in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2810-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan, X.-S., K. A. Gould, and L. M. Fisher. 2009. Probing the differential interactions of quinazolinedione PD 0305970 and quinolones with gyrase and topoisomerase IV. Antimicrob. Agents Chemother. 53:3822-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng, H., and K. J. Marians. 1993. Escherichia coli topoisomerase IV. Purification, characterization, subunit structure, and subunit interactions. J. Biol. Chem. 268:24481-24490. [PubMed] [Google Scholar]
- 29.Peterson, L. R. 2005. Quinolone resistance in clinical practice: occurrence and importance, p. 119-137. In D. C. Hooper and J. S. Wolfson (ed.), Quinolone antimicrobial agents, 2nd ed. ASM Press, Washington, DC.
- 30.Rosen, J. D., N. German, and R. J. Kerns. 2009. Efficient synthesis of the 2-amino-6-chloro-4-cyclopropyl-7-fluoro-5-methoxy-pyrido[1,2-c]pyrimidine-1,3-dione core ring system. Tetrahedron Lett. 50:785-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strahilevitz, J., and D. C. Hooper. 2005. Dual targeting of topoisomerase IV and gyrase to reduce mutant selection: direct testing of the paradigm by using WCK-1734, a new fluoroquinolone, and ciprofloxacin. Antimicrob. Agents Chemother. 49:1949-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taubes, G. 2008. The bacteria fight back. Science 321:356-361. [DOI] [PubMed] [Google Scholar]
- 33.Zhao, X., C. Xu, J. Domagala, and K. Drlica. 1997. DNA topoisomerase targets of the fluoroquinolones: a strategy for avoiding bacterial resistance. Proc. Natl. Acad. Sci. U. S. A. 94:13991-13996. [DOI] [PMC free article] [PubMed] [Google Scholar]