Abstract
The simultaneous nutrient germination of hundreds of individual wild-type spores of three Bacillus species and a number of Bacillus subtilis strains has been measured by two new methods, and rates of release of the great majority of the large pool of dipicolinic acid (DPA) from individual spores of B. subtilis strains has been measured by Raman spectroscopy with laser tweezers. The results from these analyses and published data have allowed a number of significant conclusions about the germination of spores of Bacillus species as follows. (i) The time needed for release of the great majority of a Bacillus spore's DPA once rapid DPA release had begun (ΔTrelease) during nutrient germination was independent of the concentration of nutrient germinant used, the level of the germinant receptors (GRs) that recognize nutrient germinants used and heat activation prior to germination. Values for ΔTrelease were generally 0.5 to 3 min at 25 to 37°C for individual wild-type spores. (ii) Despite the conclusion above, germination of individual spores in populations was very heterogeneous, with some spores in wild-type populations completing germination ≥15-fold slower than others. (iii) The major factor in the heterogeneity in germination of individual spores in populations was the highly variable lag time, Tlag, between mixing spores with nutrient germinants and the beginning of ΔTrelease. (iv) A number of factors decrease spores' Tlag values including heat activation, increased levels of GRs/spore, and higher levels of nutrient germinants. These latter factors appear to affect the level of activated GRs/spore during nutrient germination. (v) The conclusions above lead to the simple prediction that a major factor causing heterogeneity in Bacillus spore germination is the number of functional GRs in individual spores, a number that presumably varies significantly between spores in populations.
Spores of various Bacillus species are metabolically dormant and can survive for years in this state (30). However, spores constantly sense their environment, and if appropriate small molecules termed germinants are present, spores can rapidly return to life in the process of germination followed by outgrowth (25, 29, 30). The germinants that most likely trigger spore germination in the environment are low-molecular-weight nutrient molecules, the identities of which are strain and species specific, including amino acids, sugars, and purine nucleosides. Metabolism of these nutrient germinants is not needed for the triggering of spore germination. Rather, these germinants are recognized by germinant receptors (GRs) located in the spore's inner membrane that recognize their cognate germinants in a stereospecific manner (17, 24, 25, 29). Spores have a number of such GRs, with three functional GRs in Bacillus subtilis spores and even more in Bacillus anthracis, Bacillus cereus, and Bacillus megaterium spores (6, 29, 30). Binding of nutrient germinants to some single GRs is sufficient to trigger spore germination, for example the triggering of B. subtilis spore germination by binding of l-alanine or l-valine to the GerA GR. However, many GRs cooperate such that binding of germinants by ≥2 different GRs is needed to trigger germination (2, 29): for example, the triggering of B. subtilis spore germination by the binding of components of a mixture of l-asparagine, d-glucose, d-fructose, and K+ ions (AGFK) to the GerB and GerK GRs. The binding of nutrient germinants to GRs triggers subsequent events in germination, although how this is accomplished is not known.
The first readily measured biochemical event after addition of nutrient germinants to Bacillus spores is the rapid release of the spore's large depot (∼10% of spore dry weight) of pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) plus its chelated divalent cations, predominantly Ca2+ (Ca-DPA), from the spore core (25, 29). Ca-DPA release then results in the activation of two redundant cortex-lytic enzymes (CLEs), CwlJ and SleB, which hydrolyze the spore's peptidoglycan cortex layer (16, 22, 27, 29). CwlJ is activated by Ca-DPA as it is released from the spore while SleB is activated only after most DPA is released (17, 20, 22, 26, 27). Cortex hydrolysis ultimately allows the spore core to expand and take up more water, raising the core water content from the 35 to 45% of wet weight in the dormant spore to the 80% of wet weight characteristic of growing cells. Full hydration of the spore core then allows enzyme action, metabolism, and macromolecular synthesis to resume in the now fully germinated spore.
Germination of spores in populations is very heterogeneous, with some spores germinating rapidly and some extremely slowly (4, 5, 9, 11, 13-15, 19, 26, 31, 32). Where it has been studied, the reason for this heterogeneity has been suggested to be due to a variable lag period (Tlag) between the time of mixing spores with a germinant and the time at which rapid DPA release begins, since once rapid DPA release begins, the time required for release of almost all DPA as well as for subsequent cortex hydrolysis is generally rather short compared to Tlag values in individual spores (5, 11, 13-15, 19, 26, 31, 32). The times required for DPA release and cortex hydrolysis are also similar in wild-type spores with both very short and long Tlag values (5, 15, 19, 27). The reasons for the variability in Tlag times between individual spores in populations are not known, although there are reports that both activation of spores for germination by a sublethal heat treatment (heat activation) as well as increasing concentrations of nutrient germinants can shorten Tlag values (12, 14, 15, 18, 32). However, there has been no detailed study of the causes of the variability in Tlag values between very large numbers of individual spores in populations.
In order to study the heterogeneity in spore germination thoroughly, methods are needed to follow the germination of hundreds of individual spores over several hours. Initial studies of the germination of individual spores examined a single spore in a phase-contrast microscope and followed the germination of this spore by changes in the core's refractive index due to DPA release and core swelling (14, 15, 32, 34). However, this method is labor-intensive for gathering data with hundreds of individual spores. More recently, confocal microscopy and then surface adsorption and optical tweezers have been used to capture single spores, and germination events have been followed by methods such as Raman spectroscopy to directly measure DPA release, as well as phase-contrast microscopy and elastic light scattering (3, 5, 9, 10, 19, 26). While the latter recent advances have allowed accumulation of much information about germination, collection of this type of data for large numbers of individual spores is still labor-intensive, although use of dual optical traps (35) and perhaps multiple traps in the future may alleviate this problem. However, phase-contrast microscopy plus appropriate computer software has recently allowed the monitoring of many hundreds of individual spores for several hours, with automated assessment of various changes in the cells during the period of observation (19). In the present work, we have used both phase-contrast and differential interference contrast (DIC) microscopy to monitor the germination of many hundreds of individual spores of three Bacillus species adhered on either an agarose pad or a glass coverslip for 1 to 2 h. This work, as well as examination of times needed for release of most DPA once rapid DPA release has begun during germination of individual spores under a variety of conditions, has allowed detailed examination of the effects of heat activation, nutrient germinant concentration, GR numbers per spore, and individual CLEs on spore germination heterogeneity and on values of Tlag for individual spores.
MATERIALS AND METHODS
Bacillus strains used and spore preparation.
The Bacillus strains used in this work are listed in Table 1. B. subtilis strains are isogenic derivatives of strain PS832, a laboratory 168 strain. Spores of B. subtilis strains were prepared on 2× SG medium agar plates at 37°C without antibiotics, B. cereus spores were prepared at 30°C in defined liquid medium (8), and B. megaterium spores were prepared at 30°C in supplemented nutrient broth (21); spores were harvested and purified as described previously (11, 13, 21). Purified spores were stored at 4°C in water protected from light and were free (>98%) of growing and sporulating cells, germinated spores and cell debris as observed by phase-contrast microscopy.
TABLE 1.
Bacillus species and strains used
| Bacillus straina | Genotype or phenotypeb | Source or reference |
|---|---|---|
| B. cereus T | Wild type | H. O. Halvorson |
| B. megaterium QM B1551 | Wild type | H. S. Levinson |
| B. subtilis | ||
| PS533 | Wild type; Kmr | 28 |
| PS3415 | PsspB::gerB* MLSr | 4 |
| PS3476 | PsspD::gerA MLSr | 4 |
| PS3502 | PsspD::gerB* MLSr | 4 |
| FB10 | gerB* | 23 |
| FB111 | cwlJ::tet Tcr | 22 |
| FB112 | sleB::spc Spr | 22 |
| FB113 | cwlJ::tet sleB::spc Tcr Spr | 22 |
All B. subtilis strains are isogenic.
Kmr, resistant to kanamycin (10 μg/ml); MLSr, resistant to the macrolides lincomycin (25 μg/ml) and erythromycin (1 μg/ml); Spr, resistant to spectinomycin (100 μg/ml); and Tcr, resistant to tetracycline (10 μg/ml).
Spore germination.
B. subtilis spores were germinated in (i) 25 mM HEPES or Tris-HCl buffer (pH 7.4) (l-valine or l-alanine) at 30, 34, or 37°C; (ii) 12.5 mM KPO4 buffer (pH 7.4) (AGFK) at 34°C; or (iii) 25 mM HEPES buffer (pH 7.4) (l-asparagine) at 30°C. B. cereus spores were germinated at 25 or 34°C in 25 mM Tris-HCl buffer (pH 7.4 or 8.8) with (i) 40 mM NH4Cl (l-alanine at pH 8.8) or (ii) various concentrations of inosine or l-alanine at pH 7.4. Finally, B. megaterium spores were germinated at 30°C in 25 mM KPO4 buffer (pH 7.4) (all germinants). Unless otherwise noted, spores were heat activated prior to germination by incubation of spores at an optical density at 600 nm (OD600) of 10 to 25 in water for 30 min at 70 or 75°C (B. subtilis), 15 min at 60°C (B. megaterium) or 25 to 30 min at 65 or 70°C (B. cereus), cooled on ice for at least 15 min, and used for germination experiments within 1 to 5 h.
Monitoring spore germination: method A.
Analysis of the germination of multiple individual spores by phase-contrast microscopy of spores adhered to a coverslip was carried out as described previously (18), and the same method was also effective using DIC microscopy (see below). Control experiments (data not shown) showed that method A scored spores as germinated when DPA had been released, since this results in an ∼3-fold decrease in spore refractility, although there is a further smaller decrease in spore refractility upon completion of cortex hydrolysis and core expansion (26, 27). A drop of heat-activated spores (20 μl, ∼3 × 108 spores/ml in water) was placed on the surface of a microscope coverslip. After 1 h at 4°C to allow spores to adhere, the spore suspension was removed with a pipette, leaving a thin film of spores. After this thin film was dried in the refrigerator, the slide was washed gently several times to remove unattached spores. Coverslips were then mounted on and sealed to a microscope sample holder at a constant appropriate temperature (37°C) after addition of ∼300 μl of germinant solution on the top of the coverslip. The DIC microscope was set such that the polarizer and analyzer were crossed, and thus the DIC bias phase was zero (1). In this setting, the dormant spores appear bright due to the spatial gradient of the refractive index and the background appears dark. As the spores release DPA, the DIC images become weak (19). After addition of the germinant solution to the spores on coverslips, a digital charge-coupled device (CCD) camera (16 bit, 1,600 by× 1,200 pixels) was used to record the DIC images at a rate of 1 frame per 15 s for up to 90 min. These images were analyzed with a computation program in Matlab to locate each spore and to calculate the averaged pixel intensity of an area of 40 × 40 pixels that covered the whole individual spore on each DIC image. The DIC image intensity of each individual spore was plotted as the function of the incubation time (with a resolution of 15 s), and times for the rapid fall of ∼75% in image intensity that is concomitant with the release of ∼85% of spore DPA (ΔTrelease values) (18; and see below) for individual spores were determined. Examples of the statistics from representative experiments using method A are given in Table 2.
TABLE 2.
Distribution of germination times for spores of Bacillus species under different conditionsa
| Bacillus species and germinant concn | No. of spores examined (% germinated) | Avg completion times (min) ± SDb |
|---|---|---|
| B. megaterium | ||
| d-Glucose | ||
| 10 mM | 226 (89) | 4 ± 1 |
| 100 μM | 397 (74) | 4.4 ± 6.7 |
| 50 μM | 449 (52) | 6.2 ± 10.1 |
| 15 μM | 1,018 (19) | 7.6 ± 10.2 |
| l-Proline | ||
| 5 mM | 253 (82) | 4.5 ± 4.8 |
| 1 mM | 517 (66) | 4.9 ± 4.6 |
| 500 μM | 347 (58) | 5.2 ± 5.2 |
| 250 μM | 442 (45) | 5.9 ± 9.6 |
| 150 μM | 588 (29) | 5.3 ± 9.8 |
| KBr | ||
| 50 mM | 477 (71) | 7.6 ± 11.8 |
| 5 mM | 492 (64) | 5.8 ± 8.1 |
| 1 mM | 374 (54) | 5.1 ± 3.6 |
| 500 μM | 412 (48) | 4.9 ± 8.2 |
| 100 μM | 900 (25) | 5.0 ± 5.5 |
| B. cereus | ||
| l-Alanine | ||
| 10 mM | 420 (99) | 5.6 ± 2.8 |
| 100 μM | 879 (75) | 21.6 ± 18.9 |
| B. subtilis | ||
| l-Alanine | ||
| 10 mM | 488 (98) | 16.3 ± 9.9 |
| 50 μM | 1,131 (45) | 26.4 ± 12.1 |
Heat-activated (60°C for B. megaterium, 65°C for B. cereus, and 70°C for B. subtilis) spores adhered on coverslips were germinated in 25 mM Tris-HCl buffer (pH 7.4) for B. cereus and B. subtilis and 25 mM KPO4 buffer (pH 7.4) for B. megaterium. Germination of individual spores at 25°C (B. cereus), 30°C (B. megaterium), or 37°C (B. subtilis) was monitored by method A using either phase-contrast microscopy (B. cereus) or DIC microscopy (B. megaterium and B. subtilis). The total observation times were 60 min for B. cereus spores and 90 min for B. megaterium and B. subtilis spores.
The times for completion are from t0 to completion of rapid DPA release.
Monitoring spore germination: method B.
For analysis of the germination of individual spores on an agarose pad using DIC microscopy, the microscope stage was first heated to the desired temperature. Sixteen milligrams of low-melting-temperature agarose was mixed with 330 μl of the desired germinant plus buffer, and the mixture was melted by incubation at 95°C for 15 min. The melted-agarose-germinant mixture (33 μl) was sandwiched between two cover glasses with a 1-mm spacer to create a small gel pad, upon which 0.5 μl of a well-mixed spore suspension (∼3 × 109 spores/ml) was added. The gel pad was then placed upside down into a microscopy observation chamber (FCS2; Bioptechs, Butler, PA) and imaged with an Olympus IX81 microscope. DIC images of spores were taken every minute. For each time point, multiple (4, 5) imaging fields were scanned with a motorized microscopy stage, which routinely allowed up to 1,000 spores to be continuously monitored in each experiment. The starting time, t0, of experiments is defined as the time of addition of spores to the gel pad. Imaging generally started 5 to 10 min after t0, and the temperature equilibration started 2 min after t0. ImageJ was used to manually count total spores in each imaging field and to score spores that had germinated every 10 min by the germinated spores' less-bright appearance and slightly larger size than dormant spores (see Results). Control experiments indicated that using method B, it was very difficult to score spores as germinated that had not hydrolyzed their cortex but had only released their DPA (e.g., cwlJ sleB B. subtilis spores) (22; data not shown). This was in contrast to method A, which could easily score individual spores as germinated that had released their DPA but had not hydrolyzed their cortex (data not shown). The reason for this difference between methods A and B is not clear, but it may be due to the agarose beneath the spores in method B and the differences in the microscopy setup in the two methods, as the polarizer and analyzer were set to produce pseudo-three-dimensional images with method B but with less contrast compared to images obtained with method A. All experiments with method B were carried out at least twice, and in all cases, the conclusions from results with different experiments were identical. Examples of the statistics for data from representative experiments using method A are given in Fig. 1 and Table 2.
FIG. 1.
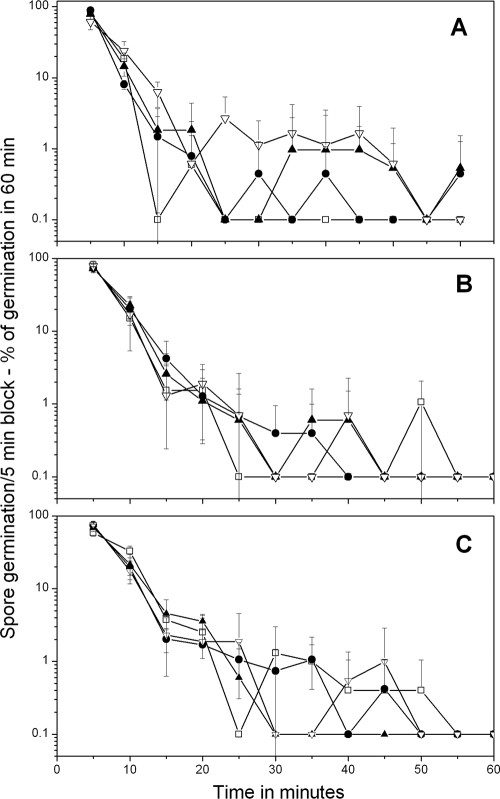
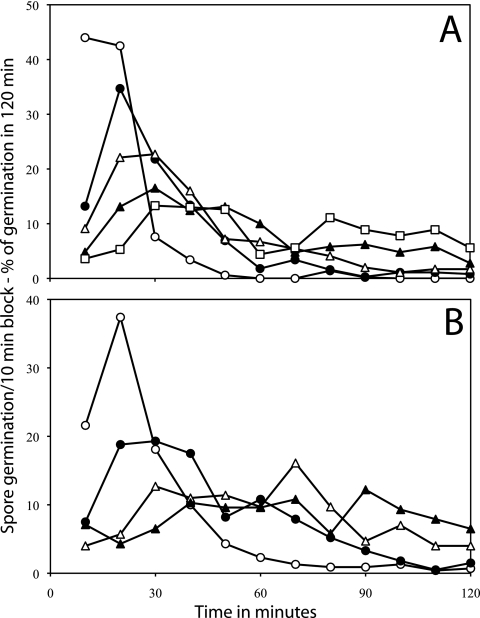
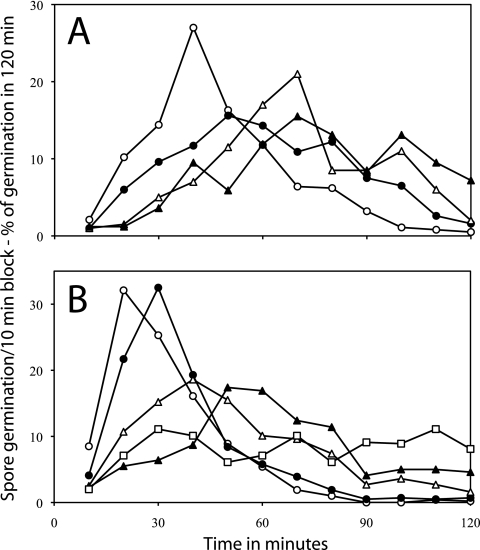
Germination of B. megaterium spores with various concentrations of d-glucose (A), l-proline (B), and KBr (C) analyzed by method A. Heat-activated B. megaterium spores were germinated, and the percentage of spores germinating in various time periods and the standard deviations of these values were determined by method A as described in Materials and Methods. The symbols used (and the percentage of spores that germinated in 90 min and total number of spores analyzed) are as follows: panel A, □, 10 mM d-glucose (89% of 226 spores), •, 100 μM d-glucose (74% of 397 spores), ▴, 50 μM d-glucose (52% of 449 spores), and ▿, 15 μM d-glucose (19% of 1,018 spores); panel B, □, 5 mM l-proline (82% of 253 spores), •, 1 mM l-proline (66% of 517 spores), ▴, 500 μM l-proline (58% of 347 spores), and ▿, 150 μM l-proline (29% of 588 spores); panel C, □, 50 mM KBr (71% of 477 spores), •, 5 mM KBr (64% of 492 spores), ▴, 1 mM KBr (54% of 374 spores), and ▿, 100 μM KBr (25% of 900 spores). In the absence of d-glucose, l-proline, or KBr there was ≤2% germination of these spores in 90 min. Note that all values plotted as 0.1% germination are actually 0%, but were plotted as 0.1% to allow use of a log scale to display the low levels of spores that germinated slowly, in particular at intermediate times. Note also that (i) the values given on the vertical axis are the percentage of spores that germinated in a particular 5-min period in the experiment and (ii) the latter values have been normalized to the total number of spores that germinated in the complete experimental period to allow better comparison of experiments in which high and low percentages of spores germinated.
Measuring kinetics of DPA release during germination of individual spores.
Raman spectra of individual spores trapped by laser tweezers in an optical trap were measured during the germination of these spores under various conditions as described previously (5, 35). A number of prominent bands in a dormant spore's Raman spectrum are due almost exclusively to DPA, in particular a very prominent band at 1,017 cm−1 (5, 19, 26, 35). During spore germination, the DPA-specific Raman bands disappear, thus making it possible to determine the kinetics of DPA release during germination of individual spores (5, 19, 26).
Kinetic parameters of nutrient germination of individual Bacillus spores.
As noted above, methods A and B have been developed to analyze the kinetics of nutrient germination of large numbers of Bacillus spores simultaneously. An obvious question concerns the kinetic parameters of nutrient germination that are determined by these two methods. Previous work examining nutrient germination of individual spores of Bacillus species using phase-contrast microscopy and/or Raman spectroscopy (5, 19, 26) have identified a number of kinetic parameters of the germination process. Beginning after mixing of spores with nutrients, there is a highly variable period, Tlag, in which there is only a small and slow decrease in spore refractility and a slow release of ≤15% of spore DPA (5, 19, 26). Tlag is followed by a rapid loss of ∼75% of remaining spore refractility as well as all remaining DPA. This rapid step is termed ΔTrelease: ΔTrelease = Trelease (the time for completion of DPA release [19]) − Tlag. ΔTrelease takes 0.5 to 3 min for individual wild-type spores, and the duration of ΔTrelease for an individual spore appears largely independent of that spore's Tlag value (5, 19, 26, 27). Following completion of ΔTrelease, there is a further small decline in spore refractility due to both the hydrolysis of the spore cortex and spore core swelling that also takes only 2 to 3 min for individual wild-type spores (26, 27).
Analysis of the nutrient germination of individual spores by DIC microscopy using method A showed that the DIC image intensities of individual spores exhibit the same kinetic features as seen using phase-contrast microscopy (see Results). In contrast, method B scored a spore as germinated only if cortex hydrolysis had been largely completed. Consequently (i) method A scores a spore as germinated only if has completed Tlag and most if not all of ΔTrelease; (ii) method B requires that Tlag and ΔTrelease be completed as well as most if not all cortex hydrolysis before a spore is scored as germinated; and (iii) since the times for ΔTrelease and cortex hydrolysis are relatively both short and constant between individual wild-type spores, even between individual spores with very different Tlag values (5, 19, 26), variability in times to germinate between individual spores as determined by method A or B is almost completely due to variability in Tlag.
RESULTS
Analysis of germination of multiple individual spores by methods A and B.
It is well known that spores' refractive index changes dramatically during germination due to both the release of the spore core's large DPA pool and some water uptake, as well as subsequent cortex hydrolysis that leads to core swelling and further water uptake (14, 15, 25, 29, 32). These changes in refractive index can also be seen by DIC microscopy, and this is particularly easy for the relatively large B. megaterium spores (see Fig. S1 [method A] in the supplemental material). Method A thus allowed facile discrimination between dormant and germinated spores in large spore populations (see Fig. S1 [arrows in 1- and 3-min images] in the supplemental material). Method A was also applicable to B. cereus and B. subtilis spores and gave identical results when phase-contrast microscopy was used instead of DIC microscopy, as expected (18; data not shown).
Method B in which spores were applied to an agarose pad could also be used for assessment of the germination of individual B. cereus (data not shown) and B. subtilis spores (se Fig. S2A to D in the supplemental material), although the degree of contrast between dormant and germinated spores was much less than with spores adhered on a glass slide (compare Fig. S1 and S2 in the supplemental material). However, method B could differentiate individual dormant and germinated spores (see Fig. S2A to D [arrows] in the supplemental material), even though many of the B. cereus and B. subtilis spores on the agarose pad were in clumps (see Fig. S2 in the supplemental material) (data not shown). Fortunately, this latter behavior did not significantly influence the germination of the spores in clumps (see Fig. S2B to D in the supplemental material) (data not shown).
Effect of germinant concentrations on the nutrient germination of individual B. megaterium spores.
With demonstration that germinated spores could readily be distinguished from dormant spores by method A, we then used this method to assess the effect of concentrations of various germinants on the distribution of germination times for B. megaterium spores (Fig. 1A to C). Most B. megaterium spores germinated in the first 10 min of the observation period. However, with the germinants d-glucose and l-proline, lower germinant concentrations resulted in (i) less overall germination in the observation period and (ii) more spores germinating much later than 10 min after mixing of spores with germinants (Fig. 1A and B; note log scale on the vertical axis). This latter effect was reflected in longer average times for B. megaterium spores to germinate as d-glucose concentrations decreased, as well as significant increases in the standard deviations of the average times for these spores to germinate as concentrations of d-glucose or l-proline decreased (Table 2). In contrast, with potassium bromide (KBr) as the germinant, there was no noticeable increase in either the average times to germinate or the standard deviations of these average values as KBr concentrations decreased; indeed, the average time to germinate and the standard deviation of this value were largest at the highest KBr concentration used (Table 2).
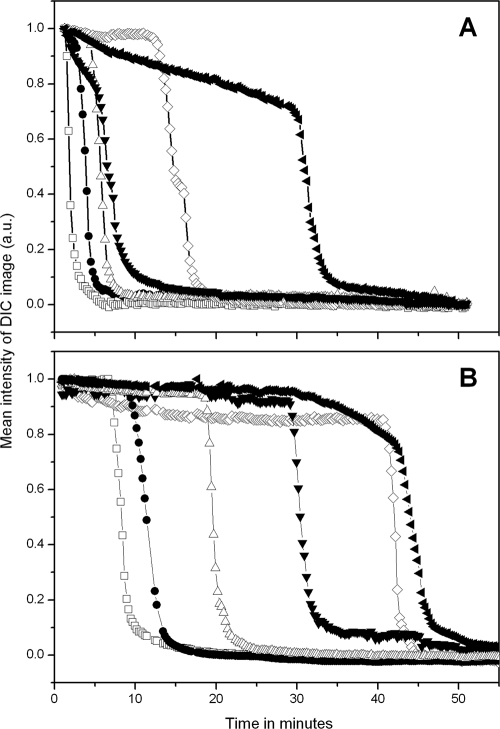
As noted above, the distribution of times for individual B. megaterium spores to complete germination became more heterogeneous as d-glucose or l-proline concentrations decreased. However, it was not clear if this effect was due to slower rates of change in DIC image intensities or to longer Tlag times prior to rapid rates of change in the DIC image intensities. To decide between these alternatives, the DIC image intensities of randomly chosen individual B. megaterium spores germinating with high or low germinant concentrations were measured (Fig. 2 A and B). This analysis showed that DIC image intensities of individual spores initially either remained constant or decreased slowly at both high and low d-glucose concentrations. However, this was followed by an extremely rapid fall in DIC image intensity (ΔTrelease; see Materials and Methods) that took 1 to 3 min. Notably, values for ΔTrelease were relatively similar for spores germinating at low or high glucose concentrations and for spores germinating shortly after addition of germinants or much later. Individual B. megaterium spores incubated with high and low KBr or l-proline concentrations exhibited similar patterns of change in DIC intensities during germination to those seen with glucose as the germinant. Values of ΔTrelease were 2.7 ± 0.8 min for B. megaterium spores germinated with both 10 mM and 15 μM glucose, and values for spores germinating with either high or low concentrations of KBr and l-proline were also very similar (Fig. 2A and B) (data not shown).
FIG. 2.
Mean intensities of DIC images from six randomly chosen B. megaterium spores during germination with 10 mM (A) and 15 μM (B) d-glucose. Heat-activated B. megaterium spores were germinated with d-glucose, and the DIC image intensities were determined on randomly chosen spores by method A as described in Materials and Methods. All DIC image intensities were averaged over a 40- by 40-pixel area that covered the whole individual spore. Curves with different symbols represent the germination of one individual spore. The final DIC image intensity values after 90 min of germination were subtracted from the intensities of DIC images at various times and expressed in arbitrary units (a.u.), and values were then normalized to the value at the time the germinant was added.
Effect of germinant concentrations and heat activation on germination of individual B. cereus spores.
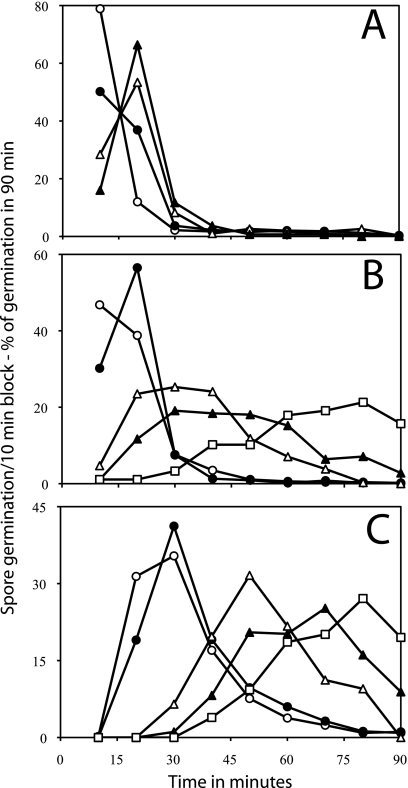
While the times to complete germination of individual B. megaterium spores showed distinctive changes depending on d-glucose or l-proline concentrations, it seemed important to ensure that this behavior was displayed by spores of other Bacillus species, especially since this behavior was not seen with B. megaterium spores germinating with KBr. Consequently, method B was used to monitor the germination of hundreds of individual B. cereus spores with various l-alanine concentrations (Fig. 3 A). This analysis indicated that features seen when B. megaterium spore germination was monitored by method A were also seen when B. cereus spore germination was monitored by method B in that (i) lower germinant concentrations gave lower total germination and (ii) germination of B. cereus spores was shifted to slightly longer times at lower l-alanine concentrations. Analysis of the germination of individual B. cereus spores with inosine as the germinant gave more dramatic results than with l-alanine (Fig. 3B). At the highest inosine concentration, these spores germinated almost exclusively in the first 30 min (Fig. 3B and 4 A). However, as the inosine concentrations decreased, not only did the percentage of total germination in a 90-min decrease, but the completion of spore germination occurred also at later times (Fig. 3B). This resulted in a large decrease in the ratio of spores germinating early to those germinating late as the inosine concentration was decreased (Fig. 4A). Limited analysis of the germination of individual B. cereus spores by method A using phase-contrast microscopy also gave qualitatively similar results to those obtained with method B (compare Table 2 and Fig. 3A).
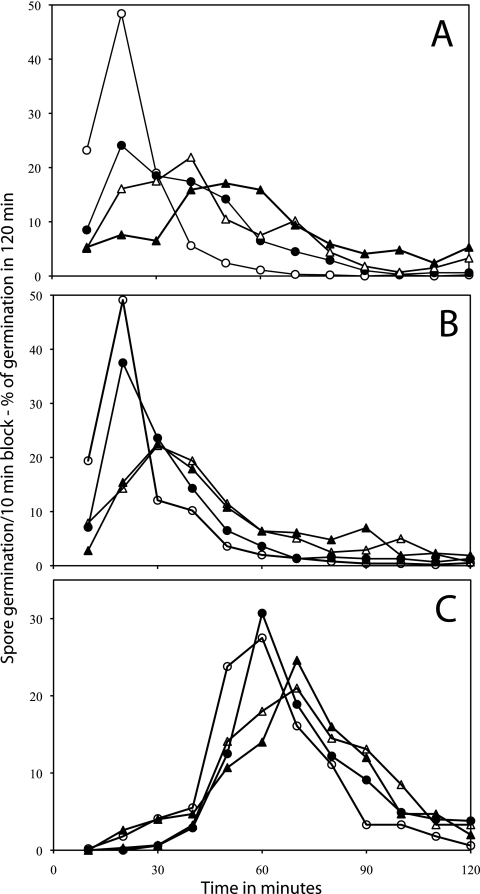
FIG. 3.
Distribution of germination times of spores of B. cereus during germination of heat-activated (A and B) and unactivated (C) spores with various l-alanine (A) or inosine (B and C) concentrations. B. cereus spores were germinated with or without heat activation (70°C) at pH 8.8, and the percentages of individual spores germinating at 25°C (A) or 34°C (B and C) in various 10-min periods were determined as described in Materials and Methods using method B. The symbols used (and the percentage of spores that had germinated in 90 min and total number of spores analyzed) are as follows: panel A, ○, 5 mM l-alanine (99% of 887 spores), •, 2 mM l-alanine (89% of 1,002 spores), ▵, 1 mM l-alanine (60% of 606 spores), and ▴, 0.3 mM l-alanine (28% of 489 spores); panel B, ○, 5 mM inosine (100% of 536 spores), •, 0.3 mM inosine (99% of 464 spores), ▵, 30 μM inosine (87% of 394 spores), ▴, 20 μM inosine (71% of 398 spores), and □, 0.085 μM inosine (25% of 363 spores); and panel C, ○, 1 mM inosine (99% of 450 spores), •, 0.3 mM inosine (98% of 441 spores), ▵, 0.1 mM inosine (85% of 347 spores), ▴, 50 μM inosine (60% of 468 spores), and □, 30 μM inosine (21% of 614 spores). In the absence of l-alanine or inosine, there was ≤1% germination of these spores in 90 min. Note that (i) the values given on the vertical axis are the percentages of spores that germinated in a particular 10-min period in the experiment and (ii) the latter values have been normalized to the total number of spores that germinated in the complete experimental period of the experiment.
FIG. 4.
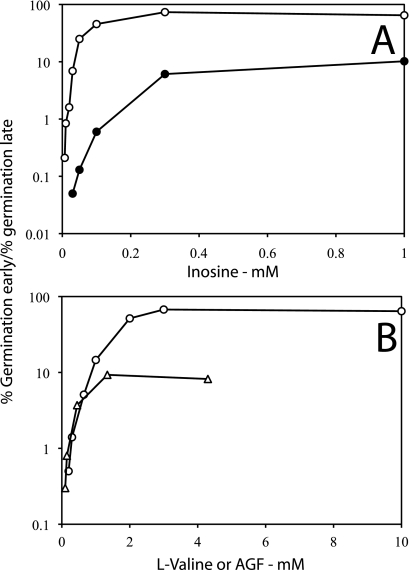
Ratios of individual B. cereus (A) and B. subtilis (B) spores germinating early to those germinating late as a function of germinant concentrations and with and without heat activation. (A) Data are from the experiments in Fig. 3B and C during germination with inosine of B. cereus spores with (○) or without (•) heat activation. The ratio is the total percentage of spores that germinated between 0 and 30 min over that between 50 and 90 min. (B) Data are from experiments with heat-activated B. subtilis spores in Fig. 5A and B during germination with either l-valine (○) or AGFK (▵) and include results using germinant concentrations not shown in Fig. 5. In the AGFK germination, only the concentration of each of the AGF components was varied, while the KPO4 buffer (pH 7.4) was constant at 12.5 mM. The ratio is the total percentage of spores that germinated between 0 and 30 min over that between 80 and 120 min.
Previous work has shown that heat activation significantly increases the rate of germination of spore populations (17). Thus, it was of obvious interest to examine the effect of heat activation on the inosine germination of individual B. cereus spores (Fig. 3C). The inosine germination of these spores without prior heat activation exhibited some similarity to that of heat-activated spores in that germination of unactivated spores required longer times at lower inosine concentrations (compare Fig. 3B and C). However, the unactivated spores required significantly higher levels of inosine to achieve the same degree of germination as the activated spores. In addition, at all inosine concentrations tested the unactivated spores had higher levels of individual spores germinating at later times, and this translated into much lower ratios of spores germinating early to spores germinating late for unactivated compared to heat-activated spores at all inosine concentrations (Fig. 3B and C and 4A).
Germination of individual B. subtilis spores with various germinant concentrations.
In addition to spores of B. cereus and B. megaterium, we also examined the germination of large numbers of individual B. subtilis spores, in particular because of the many isogenic mutant B. subtilis strains with alterations in levels of proteins, including GRs, that play crucial roles in spore germination (2, 4, 22, 23, 29). When measured by method B, the germination of individual B. subtilis spores as a function of nutrient germinant concentrations exhibited similar features to the germination of individual B. cereus and B. megaterium spores, as more B. subtilis spores germinated later when germinant concentrations were decreased (Fig. 5 A and B). This was true both with l-valine, which triggers germination via the GerA receptor, and with the AGFK mixture, which triggers germination by cooperative action of the GerB and GerK receptors (Fig. 5A and B). Again, the ratio of spores germinating early to those germinating late fell dramatically as germinant concentrations decreased, although less so for AGFK germination than for germination with l-valine (Fig. 4B). Measurement of the germination of B. subtilis spores with high and low l-alanine concentrations using method A again gave qualitatively similar results to those noted above with l-valine using method B (compare Table 2 and Fig. 5A).
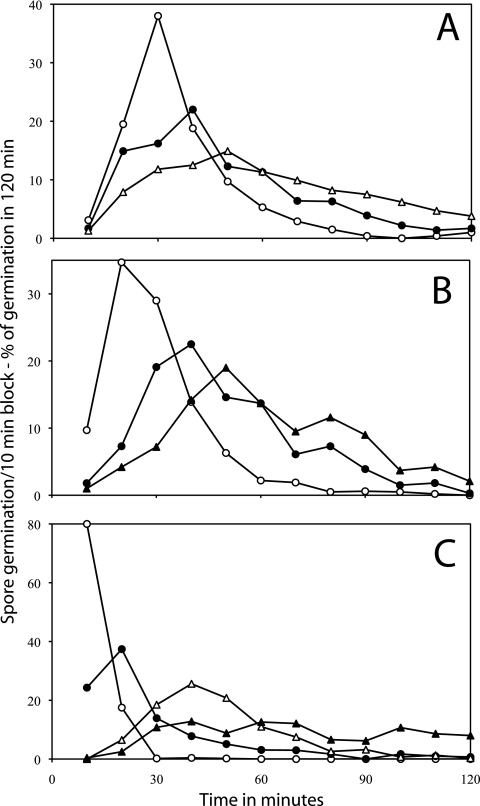
FIG. 5.
Germination of individual B. subtilis spores as a function of the concentrations of l-valine (A) or AGFK (B). Heat-activated (75°C) B. subtilis spores were germinated at 34°C in HEPES buffer for l-valine germination, and the percentages of spores that germinated in various 10-min periods were determined as described in Materials and Methods using method B. For AGFK germination, the concentration of only A, G, and F were varied, and the concentration shown is that of each of these individual components, while the KPO4 buffer concentration was constant at 12.5 mM. The symbols used (and the percentage of spores that had germinated in 90 min and the total number of spores analyzed) are as follows: panel A, ○, 10 mM l-valine (99% of 413 spores), •, 1 mM l-valine (95% of 395 spores), ▵, 0.65 mM l-valine (88% of 519 spores), ▴, 0.3 mM l-valine (60% of 485 spores), and □, 0.2 mM l-valine (16% of 561 spores); panel B, ○, 1.4 mM AGFK (98% of 480 spores), •, 0.45 mM AGFK (80% of 490 spores), ▵, 0.15 mM AGFK (53% of 564 spores), and ▴, 0.1 mM AGFK (28% of 492 spores). In the absence of AGFK or l-valine, there was ≤1% germination of these spores in 120 min. Note that (i) the values given on the vertical axis are the percentage of spores that germinated in a particular 10-min period in the experiment and (ii) the latter values have been normalized to the total number of spores that germinated in the complete experimental period.
Effect of loss of cortex-lytic enzymes on germination of individual B. subtilis spores.
The results given above and previously (5, 14, 15, 19, 26, 32) suggested that the most important variable contributing to heterogeneity in spore germination is the length of Tlag, although factors that modulate the length of Tlag have not been determined. One effector of Tlag might be a CLE, as loss of at least CwlJ has been reported to markedly decrease the rate of DPA release during spore germination (26, 27). However, loss of the CLE SleB had no significant effect on the germination of individual B. subtilis spores with l-alanine, and at most a minimal effect on the distribution of times to complete spore germination as a function of l-alanine concentration (Fig. 6 A compared with B). In contrast, loss of CwlJ had a large effect, as even at the highest l-alanine concentration, most cwlJ spores completed germination ∼40 min later than wild-type or sleB spores (Fig. 6, compare A with C). However, there was only a slight increase in the germination times at lower l-alanine concentrations with cwlJ spores (Fig. 6C). While these results might suggest that CwlJ is a major factor determining Tlag values for individual spores, the increased Tlag values in cwlJ spores are likely only apparent increases for two reasons: (i) during germination of individual cwlJ B. subtilis spores, ΔTrelease values are ≥10-fold greater than in wild-type spores (26); and (ii) method B did not score a spore as germinated until the spore core likely was fully hydrated (see Materials and Methods), and with cwlJ spores this is ≥10-fold longer after Trelease begins than with wild-type spores (26). Indeed, when cwlJ sleB spores that cannot degrade their cortex (22) were incubated for 2 h with 1 mM l-alanine, method B never scored these spores as germinated even though all DPA was released in 1 h (data not shown).
FIG. 6.
Germination of individual wild-type (A), sleB (B), and cwlJ (C) B. subtilis spores as a function of l-alanine concentration. Heat-activated (75°C) spores of B. subtilis strains were germinated in HEPES buffer at 34°C with l-alanine, and the percentages of germination of individual spores during a 10-min periods were determined as described in Materials and Methods using method B. The symbols used (and the percentage of spores that had germinated in 90 min and total number of spores analyzed) are as follows: panel A, ○, 1 mM l-alanine (97% of 569 spores), •, 70 μM l-alanine (95% of 381 spores), ▵, 30 μM l-alanine (61% of 457 spores), and ▴, 20 μM l-alanine (44% of 385 spores); panel B, ○, 1 mM l-alanine (96% of 519 spores), •, 100 μM l-alanine (98% of 398 spores), ▵, 30 μM l-alanine (53% of 405 spores), and ▴, 20 μM l-alanine (29% of 472 spores); and panel C, ○, 1 mM l-alanine (97% of 488 spores), •, 150 μM l-alanine (79% of 433 spores), ▵, 65 μM l-alanine (61% of 496 spores), and ▴, 30 μM l-alanine (31% of 479 spores). In the absence of l-alanine, there was ≤1% germination of these spores in 120 min. Note that (i) the values given on the vertical axis are the percentage of spores that germinated in a particular 10-min period in the experiment and (ii) the latter values have been normalized to the total number of spores that germinated in the complete experimental period.
Effect of levels of GRs/spore on germination of individual B. subtilis spores.
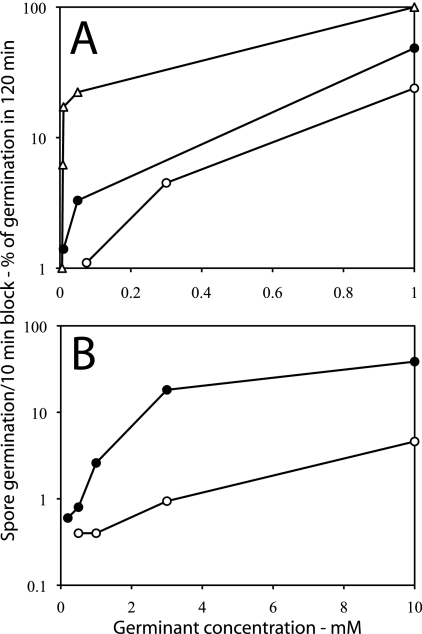
While it appears likely that CLEs do not directly affect values of Tlag but only those of ΔTrelease, it seemed likely that the number of GRs per spore might influence Tlag values, since populations of B. subtilis spores with increased average GR levels germinate faster than wild-type spores with nutrients that target overexpressed GRs (4). Consequently, we monitored the germination of B. subtilis spores with wild-type levels of the GerB* GR, a GerB GR variant that triggers spore germination with l-asparagine alone (2, 23), and spores of strains with 20-fold and ∼200-fold-higher average levels of the GerB* GR (Fig. 7 A to C). Spores with the GerB* receptor were chosen for this experiment because the wild-type GerB receptor does not trigger germination with l-asparagine alone, but only with AGFK, and then only if the GerK receptor is also present (29). Strikingly, the times to complete spore germination as scored by method B were decreased as the spores' average level of the GerB* GR increased. (Fig. 7) In addition, spores with higher GerB* GR levels exhibited both more germination and shorter germination times at lower l-asparagine concentrations (Fig. 7), as well as markedly higher ratios of spores germinating early over spores germinating late at the same l-asparagine concentration (Fig. 8 A).
FIG. 7.
Germination of heat-activated B. subtilis spores with various levels of the GerB* receptor. Heat-activated (75°C) spores of B. subtilis strains FB10 (A) (wild-type GerB* levels), PS3502 (B) (20-fold-elevated GerB* levels), and PS3415 (C) (200-fold-elevated GerB* levels) were germinated at 30°C with various l-asparagine concentrations, and the percentages of germination of individual spores in various time intervals were determined as described in Materials and Methods using method B. The symbols used (and the percentage of spores that had germinated in 90 min and total number of spores analyzed) are as follows: panel A, ○, 1 mM l-asparagine (93% of 558 spores), •, 0.3 mM l-asparagine (75% of 425 spores), and ▵, 75 μM l-asparagine (46% of 445 spores); panel B, ○, 1 mM l-asparagine (99% of 631 spores), •, 50 μM l-asparagine (75% of 437 spores), and ▴, 10 μM l-asparagine (28% of 678 spores); panel C, ○, 1 mM l-asparagine (99% of 525 spores), •, 50 μM l-asparagine (90% of 341 spores), ▵, 8.25 μM l-asparagine (73% of 405 spores), and ▴, 6 μM l-asparagine (24% of 435 spores). In panels B and C, use of 5 mM l-asparagine gave nearly identical results to those with 1 mM l-asparagine (data not shown). In the absence of l-asparagine, there was ≤1% germination of PS3415 and PS3502 spores in 120 min, although the preparation of FB10 spores used in this experiment exhibited ∼6% germination in 120 min in the absence of l-asparagine, and this was corrected for in the data used in panel A. Note that (i) the values given on the vertical axis are the percentage of spores that germinated in a particular 5- or 10-min period in the experiment and (ii) the latter values have been normalized to the total number of spores that germinated in the complete experimental period.
FIG. 8.
(A and B) Effects of levels of germinant receptor levels on ratios of individual B. subtilis spores germinating early to those germinating late. (A) Data are from the experiments in Fig. 7A to C during germination with l-asparagine of B. subtilis spores with wild-type levels of the GerB* receptor (○), with 20-fold higher levels of GerB* (•), and with 200-fold-higher levels of GerB* (▵), and include results with an l-asparagine concentration not shown in Fig. 7C. The ratio shown is the percentage of spores that germinated between 0 and 40 min over that between 70 to 120 min. (B) Data are from Fig. 9A and B during germination with l-valine using wild-type spores (○) or spores with elevated levels of the GerA receptor (•). The ratio is the percentage of spores that germinated between 0 and 40 min over that between 70 to 120 min.
Examination of the l-valine germination of spores with elevated average levels of the GerA GR gave similar results, since spore populations with higher average GerA GR levels germinated more completely and with shorter germination times than wild-type spores at similar germinant concentrations (Fig. 9 A and B). In addition, the spores with elevated average GerA GR levels exhibited markedly higher ratios of spores germinating early over spores germinating late at the same l-valine concentrations than wild-type spores (Fig. 8B).
FIG. 9.
Germination of heat-activated B. subtilis spores with either normal levels of the GerA receptor (A) or elevated GerA receptor levels (B). Heat-activated (75°C) spores of strains PS533 (normal GerA levels) and PS3476 (≥10-fold GerA receptor levels) were germinated at 30°C in HEPES buffer with various l-valine concentrations, and the percentages of germination of individual spores were determined as described in Materials and Methods using method B. The symbols used (and the percentage of spores that had germinated in 90 min and total number of spores analyzed) are as follows: panel A, ○, 10 mM l-valine (98% of 381 spores), •, 3 mM l-valine (84% of 454 spores), ▵, 1 mM l-valine (44% of 453 spores), and ▴, 0.5 mM l-valine (20% of 433 spores); and panel B, ○, 10 mM l-valine (100% of 483 spores), •, 3 mM l-valine (98% of 423 spores), ▵, 1 mM l-valine (84% of 532 spores), ▴, 0.5 mM l-valine (48% of 448 spores), and □, 0.2 mM l-valine (22% of 441 spores). There was ≤1% germination of the spores used in this figure in the absence of l-valine. Note that (i) the values given on the vertical axis are the percentage of spores that germinated in a particular 5- or 10-min time period in the experiment and (ii) the latter values have been normalized to the total number of spores that germinated in the complete experimental period.
Rates of DPA release from individual spores of various B. subtilis strains under different conditions.
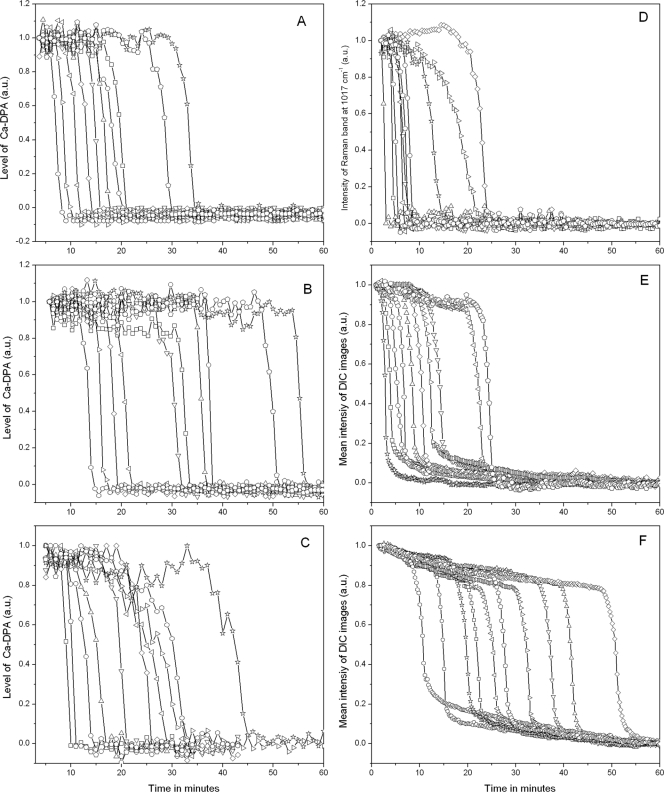
The results given above strongly indicated that the major factor determining when a spore completes germination is the lag time, Tlag, prior to the initiation of rapid DPA release following mixing of spores with germinants. However, it was possible that for some spores under some conditions, ΔTrelease itself might vary considerably, as a cwlJ mutation alone increases average values of ΔTrelease 6- to 15-fold for B. megaterium and B. subtilis spores, respectively (26, 27). Consequently, we measured rates of DPA release from individual spores of several B. subtilis strains under various conditions. Strikingly, when DPA release from individual spores was measured directly during germination of wild-type B. subtilis spores with high or low l-alanine concentrations or with AGFK, the times to release ≥85% of the spores' DPA (ΔTrelease; see Materials and Methods) were essentially identical (Fig. 10 A to C). Previous work has also strongly implied but by no means proved that ΔTrelease values are identical for individual spores of either B. cereus and B. megaterium germinating with high and low nutrient germinant concentrations and for B. cereus spores germinating with or without heat activation (9, 14, 15, 32). In addition, the ΔTrelease values were also identical for B. subtilis spores with or without elevated average levels of the GerB* or GerA GRs germinating with high concentrations of l-asparagine or l-valine, respectively (Fig. 10D to F).
FIG. 10.
Ca-DPA levels during nutrient germination of 10 randomly chosen individual heat-activated B. subtilis spores were determined by Raman spectroscopy (A to D) and DIC microscopy (E and F). (A to C) Heat-activated (70°C) spores of B. subtilis strain PS533 (wild type) were germinated at 37°C with 10 mM L-alanine in 25 mM Tris-HCl buffer (pH 7.4) (A), 50 μM l-alanine in 25 mM Tris-HCl buffer (pH 7.4) (B), and AGFK (2.5 mM l-asparagine, 5 mg/ml d-glucose, 5 mg/ml d-fructose, 5 mM KCl) (C), and Ca-DPA levels were determined by Raman spectroscopy as described in Materials and Methods. (D and E) Ca-DPA levels in spores of strain PS3415 (200-fold elevated GerB* levels) germinated at 37°C with 5 mM l-asparagine in 25 mM HEPES buffer (pH 7.4) were monitored by Raman spectroscopy (D) and DIC microscopy (E). (F) Ca-DPA levels in spores of strain FB10 (normal GerB* levels) germinated at 37°C with 5 mM L-asparagine in 25 mM HEPES buffer (pH 7.4) were monitored by DIC microscopy.
DISCUSSION
The work in this communication as well as previous results (14, 15, 32) leads to a number of conclusions about the germination of spores of B. cereus, B. megaterium, and B. subtilis and which likely represent all wild-type spores of Bacillus species. The first conclusion is that values of ΔTrelease during germination of wild-type spores with nutrient germinants are small: 0.5 to 3 min for individual spores in populations at 30 to 37°C. These ΔTrelease values were essentially the same in spores germinating soon after mixing spores with nutrient germinants or much later. A significant amount of data has been published previously supporting this conclusion (5, 9, 14, 15, 19, 26, 27, 32), and our present work is consistent with these reports. The present work also indicates that nutrient germinant concentrations and numbers of GRs/spore do not appreciably affect values of ΔTrelease. That nutrient germination concentration does not affect ΔTrelease was suggested many years ago, although this was not based on direct measurements of DPA release but was inferred from measurements of phase-contrast intensities of individual germinating spores (15, 32). This early work also suggested that heat activation did not affect values of ΔTrelease (14, 32), although we have not confirmed this in the present work. The one exception to this first conclusion is that ΔTrelease increases significantly in spores of B. megaterium and B. subtilis that lack the CLE CwlJ (26, 27). Presumably this is because the initial slow release of DPA during germination normally triggers rapid CwlJ action that in turn increases the rate of further DPA release, while this is not how SleB is activated during germination (20, 29).
A second major conclusion is that germination of individual spores in spore populations is very heterogeneous, as some spores germinate much slower than others. This has been noted previously in many other studies (3, 5, 9, 19, 26), but our work is the first to demonstrate this so clearly with large numbers of individual spores. This simple conclusion then leads to a third conclusion that the major factor causing the heterogeneity in the nutrient germination of individual Bacillus spores in spore populations is Tlag, the time between mixing of spores with nutrient germinants and the initiation of rapid DPA release. This value varies widely between individual spores in populations, while values of ΔTrelease are relatively constant, as is the rate of cortex hydrolysis, at least in wild-type spores (26).
The extremely slow germination of some individuals in spore populations has long been of major concern to the food industry for a variety of reasons, with such slow-germination spores often called “superdormant” spores (11-13). While the superdormant spores may make up only a small percentage of spores in populations, their potential importance is so great that it would be of significant interest to determine factors that affect values of Tlag. Consequently, the fourth conclusion from the current work is likely the most significant one and concerns factors that determine values of Tlag for individual spores in populations. Our present work identified three factors that decrease average values of Tlag: (i) heat activation, (ii) saturating levels of nutrient germinants, and (iii) increased levels of GRs recognizing the particular nutrient germinant being used. There are bits of data in the literature indicating that heat activation and high nutrient concentrations decrease values of Tlag for individual spores, but these data are generally with only small numbers of spores of only one species (14, 15, 32). However, there are no published data on effects of GR numbers/spore on Tlag values for individual spores. Interestingly, the three factors noted above that decrease Tlag values for individual spores in populations have also been identified as decreasing percentages of superdormant spores in spore populations (11-13), consistent with superdormant spores having extremely long Tlag periods. Indeed, it was suggested that the reason individual spores are superdormant is that they have extremely low numbers of a particular GR (11). This is an attractive idea since available data on average levels of GRs/spore indicate this value is low: ∼25 molecules/spore for at least the GerB and GerB* GRs (24, 29). In addition, the value for the average number of functional GRs/spore may be even lower if, as seems possible, GRs function as oligomers (24). Such low average GR numbers/spore could then result in significant percentages of spores with very few GRs for stochastic reasons, and these spores would tend to have long Tlag values even at saturating nutrient germinant concentrations.
There is, however, one set of results that appears to be at odds with the fourth conclusion above, and that is the somewhat increased average germination time for B. megaterium spores at the highest KBr concentration used. We do not have a firm explanation for this result, although KBr germination of B. megaterium spores is mediated by one or more GRs, albeit perhaps fortuitously (6, 7). However, given the chaotropic effects of KBr and the potential for nonspecific inhibition of germination at high KBr concentrations, perhaps very high KBr concentrations can both stimulate spore germination via GRs and nonspecifically inhibit germination, and the nonspecific effects decrease much more than the effects on GRs as KBr concentrations decrease.
The conclusions above, in particular the fourth one, now lead to a prediction: that the number of “activated” GRs per spore is what determines values of Tlag for individual spores, and this in turn is the factor responsible for heterogeneity in Tlag values. Note that the word “activated” is in quotation marks because we do not know what GRs do to trigger spore germination beyond binding nutrient germinants. However, available data (12, 18) indicate that heat activation somehow increases spores' levels of “activatable” GRs, either by making individual GRs more responsive to nutrient germinants or by making more GRs available to nutrient germinants. Indeed, heat activation appears to have no effect for spore germination processes that do not proceed via GRs (22, 33). Certainly higher nutrient germinant concentrations up to and including saturation of GRs would increase the level of “activated” GRs as would overexpression of GRs. It is of course possible that it is not the level of “activated” GRs that is crucial in determining Tlag values for individual spores, but rather some other spore component that acts between GRs and DPA release in the signal transduction pathway in spore germination. However, while the possible existence of such an intermediary in germination has been suggested (2), it has not been identified.
Barring the identification of such an intermediary in the signaling from “activated” GRs in the germination pathway, we favor the idea that it is “activated” GRs themselves that are the crucial factor, as has been suggested previously (2, 34). The question then becomes how to prove or disprove such a prediction. One way is to directly correlate the Tlag values of individual spores with their GR level, in particular at saturating nutrient germinant levels. A second way would be to demonstrate that spore populations display discrete Tlag values dependent on their GR levels, especially at low nutrient germinant concentrations. This latter idea is especially appealing given the low levels of at least one GR in spores. Indeed, the presence of discrete times for germination of spores in populations was suggested 30 years ago (34), although the data on which this suggestion was based were by no means definitive. Consequently, work to assess the validity of the prediction made above is currently ongoing.
Supplementary Material
Acknowledgments
This work was supported by a Multi-University Research Initiative award from the U.S. Department of Defense to J.Y., Y.Q.L., and P.S.
We are grateful to Barbara Setlow and Sonali Ghosh for preparation of some of the spores and to Lixin Peng and Jing Yu for measurements on some spores.
Footnotes
Published ahead of print on 14 May 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Arnison, M. R., K. G. Larkin, C. J. R. Sheppard, N. I. Smith, and C. J. Cogswell. 2004. Linear phase imaging using differential interference contrast microscopy. J. Microsc. 214:7-12. [DOI] [PubMed] [Google Scholar]
- 2.Atluri, S., K. Ragkousi, D. E. Cortezzo, and P. Setlow. 2006. Cooperativity between different nutrient receptors in germination of spores of Bacillus subtilis and reduction of this cooperativity by alterations in the GerB receptor. J. Bacteriol. 188:28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billon, C. M.-P., C. J. McKirgan, P. J. McClure, and C. Adair. 1997. The effect of temperature on the germination of single spores of Clostridium botulinum 62A. J. Appl. Microbiol. 82:48-56. [DOI] [PubMed] [Google Scholar]
- 4.Cabrera-Martinez, R.-M., F. Tovar-Rojo, V. R. Vepachedu, and P. Setlow. 2003. Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis. J. Bacteriol. 185:2457-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, D., S. S. Huang, and Y. Q. Li. 2006. Real-time detection of kinetic germination and heterogeneity of single Bacillus spores by laser tweezers Raman spectroscopy. Anal. Chem. 78:6936-6941. [DOI] [PubMed] [Google Scholar]
- 6.Christie, G., M. Lazarevska, and C. R. Lowe. 2008. Functional consequences of amino acid substitutions to GerVB, a component of the Bacillus megaterium spore germinant receptor. J. Bacteriol. 190:2014-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christie, G., and C. R. Lowe. 2008. Amino acid substitutions in transmembrane domains 9 and 10 of GerVB that affect the germination properties of Bacillus megaterium spores. J. Bacteriol. 190:8009-8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clements, M. O., and A. Moir. 1998. Role of the gerI operon of Bacillus cereus 569 in the response of spores to germinants. J. Bacteriol. 180:6729-6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coote, P. J., M.-P. Billon, S. Pennell, P. J. McClure, D. P. Ferdinando, and M. B. Cole. 1995. The use of confocal scanning laser microscopy (CSLM) to study the germination of individual spores of Bacillus cereus. J. Microbiol. Methods 21:193-208. [Google Scholar]
- 10.Evanoff, D. D., Jr., J. Heckel, T. P. Caldwell, K. A. Christensen, and G. Chumanov. 2006. Monitoring DPA release from a single germinating Bacillus subtilis endospore via surface-enhanced Raman scattering microscopy. J. Am. Chem. Soc. 128:12618-12619. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh, S., and P. Setlow. 2009. Isolation and characterization of superdormant spores of Bacillus species. J. Bacteriol. 191:1787-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh, S., P. Zhang, Y.-Q. Li, and P. Setlow. 2009. Superdormant spores of Bacillus species have elevated wet heat resistance and temperature requirements for heat activation. J. Bacteriol. 191:5584-5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh, S., and P. Setlow. 2010. The preparation, germination properties and stability of superdormant spores of Bacillus cereus. J. Appl. Microbiol. 108:582-590. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto, T., W. R. Frieben, and S. F. Conti. 1969. Germination of single bacterial spores. J. Bacteriol. 98:1011-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto, T., W. R. Frieben, and S. F. Conti. 1969. Microgermination of Bacillus cereus spores. J. Bacteriol. 100:1385-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heffron, J. D., E. A. Lambert, N. Sherry, and D. L. Popham. 2010. Contribution of four cortex lytic enzymes to germination of Bacillus anthracis spores. J. Bacteriol. 192:763-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudson, K. D., B. M. Corfe, E. H. Kemp, P. J. Coote, and A. Moir. 2001. Localization of GerAA and GerAC germination proteins in the Bacillus subtilis spore. J. Bacteriol. 183:4317-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keynan, A., and Z. Evenchick. 1969. Activation, p. 359-396. In G. W. Gould and A. Hurst (ed.), The bacterial spore. Academic Press, New York, NY.
- 19.Kong, L., P. Zhang, P. Setlow, and Y.-Q. Li. 2010. Characterization of bacterial spore germination using integrated phase microscopy, Raman spectroscopy, and optical tweezers. Anal. Chem. 82:3840-3847. [DOI] [PubMed] [Google Scholar]
- 20.Magge, A., A. C. Granger, P. G. Wahome, B. Setlow, V. R. Vepachedu, C. A. Loshon, L. Peng, D. Chen, Y.-Q. Li, and P. Setlow. 2008. Role of dipicolinic acid in the germination, stability, and viability of spores of Bacillus subtilis. J. Bacteriol. 190:4798-4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom.
- 22.Paidhungat, M., K. Ragkousi, and P. Setlow. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J. Bacteriol. 183:4886-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paidhungat, M., and P. Setlow. 1999. Isolation and characterization of mutations in Bacillus subtilis that allow spore germination in the novel germinant d-alanine. J. Bacteriol. 181:3341-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paidhungat, M., and P. Setlow. 2001. Localization of a germinant receptor protein (GerBA) to the inner membrane of Bacillus subtilis spores. J. Bacteriol. 183:3982-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paidhungat, M., and P. Setlow. 2002. Spore germination and outgrowth, p. 537-548. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its relatives: from genes to cells. American Society for Microbiology, Washington, DC.
- 26.Peng, L., D. Chen, P. Setlow, and Y.-Q. Li. 2009. Elastic and inelastic light scattering from single bacterial spores in an optical trap allows monitoring of spore germination dynamics. Anal. Chem. 81:4035-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Setlow, B., L. Peng, C. A. Loshon, Y. Q. Li, G. Christie, and P. Setlow. 2009. Characterization of the germination of Bacillus megaterium spores lacking enzymes that degrade the spore cortex. J. Appl. Microbiol. 107:318-328. [DOI] [PubMed] [Google Scholar]
- 28.Setlow, B., and P. Setlow. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 178:3486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550-556. [DOI] [PubMed] [Google Scholar]
- 30.Setlow, P., and E. A. Johnson. 2007. Spores and their significance, p. 35-67. In M. P. Doyle and L. R. Beuchat (ed.), Food microbiology: fundamentals and frontiers, 3rd ed. ASM Press, Washington, DC.
- 31.Stringer, S. C., M. D. Webb, S. M. George, C. Pin, and M. W. Peck. 2005. Heterogeneity of times required for germination and outgrowth from single spores of nonproteolytic Clostridium botulinum. Appl. Environ. Microbiol. 71:4998-5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vary, J. C., and H. O. Halvorson. 1965. Kinetics of germination of bacterial spores. J. Bacteriol. 89:1340-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vepachedu, V. R., and P. Setlow. 2007. Role of SpoVA proteins in the release of dipicolinic acid during germination of Bacillus subtilis spores triggered by dodecylamine or lysozyme. J. Bacteriol. 189:1565-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woese, C. R., J. C. Vary, and H. O. Halvorson. 1968. A kinetic model for bacterial spore germination. Proc. Natl. Acad. Sci. U. S. A. 59:869-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang, P., L. Kong, P. Setlow, and Y. Q. Li. 2010. Characterization of wet heat inactivation of single spores of Bacillus species by dual-trap Raman spectroscopy and elastic light scattering. Appl. Environ. Microbiol. 76:1796-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.