Abstract
Lipoprotein T (LppT), a membrane-located 105-kDa lipoprotein of Mycoplasma conjunctivae, the etiological agent of infectious keratoconjunctivitis (IKC) of domestic sheep and wild Caprinae, was characterized. LppT was shown to promote cell attachment to LSM 192 primary lamb joint synovial cells. Adhesion of M. conjunctivae to LSM 192 cells is inhibited by antibodies directed against LppT. The RGD (Arg-Gly-Asp) motif of LppT was found to be a specific site for binding of M. conjunctivae to these eukaryotic host cells. Recombinant LppT fixed to polymethylmethacrylate slides binds LSM 192 cells, whereas LppT lacking the RGD site is deprived of binding capacity to LSM 192, and LppT containing RGE rather than RGD shows reduced binding. Synthetic nonapeptides derived from LppT containing RGD competitively inhibit binding of LSM 192 cells to LppT-coated slides, whereas nonapeptides containing RAD rather than RGD do not inhibit. RGD-containing, LppT-derived nonapeptides are able to directly inhibit binding of M. conjunctivae to LSM 192 cells by competitive inhibition, whereas the analogous nonapeptide containing RAD rather than RGD or the fibronectin-derived RGD hexapeptide has no inhibitory effect. These results reveal LppT as the first candidate of a RGD lectin in Mycoplasma species that is assumed to bind to β integrins.
Mycoplasma conjunctivae, the etiological agent of infectious keratoconjunctivitis (IKC), causes severe ocular infections that lead to blindness and perforation of the cornea, particularly in Alpine ibex (Capra ibex ibex) and chamois (Rupicapra rupicapra rupicapra) (4). In view of the harsh physiochemical conditions that protect the eye from being colonized and infected by pathogenic microorganisms, M. conjunctivae is expected to exhibit efficient adhesion functions in order to avoid being flooded off by lachrymal fluid. Adhesion is thought to play a central role in the pathogenicity of bacteria in general and of Mycoplasma species in particular, both directly as a basic condition of colonization (10, 23, 42, 43) and indirectly by adherence coupled to cytopathic functions. In the latter, adhering mycoplasmas may induce oxidative damage to the host cell by targeted release of peroxide and oxygen radical species (7, 27) or disrupt K+ channels of ciliated bronchial epithelial cells, which leads to ciliostasis (13). Extracellular matrix proteins and glycosaminoglycans play important roles as receptors for adhesion of bacterial pathogens, including those of Mycoplasma species. In Mycoplasma hyopneumoniae, protein P159 has recently been identified as a heparin binding protein that promotes adherence to eukaryotic cells (10). Furthermore, the R1 region near the carboxy terminus of protein P97 of M. hyopneumoniae has been shown to mediate adherence to swine cilia (23, 41). Mycoplasmal adhesion structures have extensively been studied in virulent Mycoplasma pneumoniae, where two surface proteins, P1 of 169 kDa and P30 of 30 kDa, are densely clustered to form the tip organelle that provides strong polarity to the cytoadherence process (12, 20). Moreover, a putative cytoskeleton-forming protein with a proline-rich, acidic domain was speculated to be involved in the formation of the adhesion tip (28). In contrast to the well-structured adherence organelle of M. pneumoniae, adhesins of most other Mycoplasma species appear to be distributed on the mycoplasmal surface, and no particular receptor-ligand mechanisms have to date been identified (29).
In M. conjunctivae, a serine-rich membrane-located lipoprotein, LppS, was found to be involved in the adhesion to LSM 192 lamb joint synovial cells. LppS was shown to have sequence similarity to the fibrinogen binding protein, clumping factor A (ClfA) of Staphylococcus aureus, which has a repeated serine-aspartate domain at the analogous polyserine location (6). In the lamb joint synovial cell model, adherence of M. conjunctivae was inhibited using Fab fragments from immunoglobulin G (IgG) directed against recombinant purified LppS (6). Lipoprotein T (LppT) of M. conjunctivae, which is encoded by the same bicistronic operon downstream of lppS, shows significant similarity to the heparin binding protein P159, protein P102, and Mhp494 of M. hyopneumoniae, which are involved in adhesion to swine cilia (10, 17, 19, 38). We report here the characterization of LppT and its role in adhesion. LppT contains an RGD cell attachment motif that consists of the amino acids Arg-Gly-Asp, which is shown to be directly involved in binding to primary lamb joint synovial cells. RGD adhesins belong to a large class of integrin binding proteins that bind the extracellular matrix and which are known to induce important biological events such as cell differentiation, malignant transformation, immune recognition, and blood coagulation (25, 31).
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Mycoplasma conjunctivae strain HRC/581T was grown in standard mycoplasma broth medium, solid mycoplasma agar medium containing 20% horse serum, 2.5% yeast extract, and 1% glucose (Axcell Biotechnologies, Lyon, France) at 37°C to end exponential growth phase at a density of 5 × 108 CFU/ml. The numbers of CFU of M. conjunctivae cultures or suspensions were measured by first passing the suspensions several times through a 27-gauge needle and then spotting aliquots of sequential 10-fold dilutions on mycoplasma agar medium and counting the colonies after 7 days of incubation at 37°C. The mycoplasmas were harvested by centrifugation at 13,000 × g for 20 min, washed three times in TES buffer (10 mM Tris-HCl, 1 mM EDTA, 0.8% NaCl at pH 7.5), and then resuspended in TES buffer to reach a concentration of 109 cells/ml. For gene cloning, Escherichia coli strain XL1-Blue MRF′ (Stratagene, La Jolla, CA) was used. Expression of recombinant, polyhistidine-tailed peptides from genes cloned on vector pETHIS-1 (33) in E. coli strain BL21(DE3) was obtained (Novagen, Madison, WI) (36). E. coli strains were grown in Luria-Bertani (LB) broth at 37°C in an orbital shaker-incubator. Ampicillin at 50 μg/ml was added for selection of cloning vectors. Genomic DNA of M. conjunctivae strains 2777, My-66/95, My-7/96, N50, and G9 was taken from a previous study (5).
Cloning and site-directed mutagenesis of lppT.
For the cloning and expression of recombinant LppT in the E. coli host strain, the lppT gene from genomic DNA of M. conjunctivae HRC/581T was first amplified with the primers LppT-NdeI-N and LppT-NotI-C (Table 1). The PCR product was cloned into the pGEM-T Easy vector (Promega Corp., Madison, WI) for subsequent site-directed mutagenesis. In order to replace the two Mycoplasma-specific TGATrp codons at amino acid (aa) positions 376 and 647 with the universal TGGTrp codon, we used the overlap extension PCR method (9) sequentially with the primer pair LppT-NdeI-N/LppT-NotI-C and the overlapping mutagenesis primer pairs LppT-mut1L/LppT-mut1R and LppT-mut2L/LppT-mut2R (Table 1). The DNA fragment containing both TGATrp codons mutated to TGGTrp was named lppT* and ligated in vector pETHIS-1 (33) using the NdeI and NcoI sites to obtain plasmid pJFFLppT-His1, which was confirmed by DNA sequencing and then introduced into E. coli BL21(DE3) for expression of the LppT-His fusion protein. Note that attempts to express recombinant LppT from a clone without the codons for the 34-aa signal sequence resulted in very low yields compared to those obtained with the full precursor protein of LppT.
TABLE 1.
Oligonucleotide primers used
| Primer | Oligonucleotide sequencea | Annealing temp (°C)b |
|---|---|---|
| LppT-NdeI-N | AACATATGAAAAAACCTAATTTTAAAACAT | 54 |
| LppT-NotI-C | TCGCGGCCGCTCTTAGCAATTGCTTGTCTTGC | 57 |
| LppT-mut1L | GATTTTTGGTTTGAGCAAAGAGA | 54 |
| LppT-mut1R | CTCAAACCAAAAATCAAATTTTTG | 51 |
| LppT-mut2L | TTCCAATGGGAAGCTGACCAA | 56 |
| LppT-mut2R | AGCTTCCCATTGGAATTTATTG | 53 |
| RGD-mutL | GCCCGTGGTGAAGTTTAC | 51 |
| RGD-mutR | GTAAACTTCACCACGGGC | 51 |
| RGD-reverse | TTTTGTAAACGGCATCAACTACGACATC | 60 |
| RGD-forward | GTTGATGCCGTTTACAAAACTGTAGTAG | 59 |
Underlined nucleotides represent restriction enzyme sites, and nucleotides presented in boldface indicate site-specific mutations.
Obtained with the Oligonucleotide Properties Calculator (http://www.basic.northwestern.edu/biotools/oligocalc.html), using the nearest neighbor method and the parameters 300 nM primer and 50 mM salt (Na+), not considering nucleotides added to create the restriction enzyme recognition sites.
DNA sequence analysis.
DNA sequence analysis was performed with a DNA Sequenator AB3100 genetic analyzer and a Taq DyeDeoxy Terminator cycle sequencing kit (Applied Biosystems, Norwalk, CT), with oligonucleotide primers derived from the DNA sequence of lppS of type strain HRC/581 and by primer walking. The presence of lppT in field strains of M. conjunctivae was confirmed by PCR using genomic DNA and primers LppT-NdeI-N and LppT-NotI-C (Table 1) and subsequent sequence analysis using primer LppT mut1L (Table 1) to cover the segment encoding the RGD site.
Construction of genes encoding poly-His-tailed LppT(ΔRGD) and LppT(RGE).
A gene expressing LppT without the RGD region was constructed in two steps. First, the 5′-terminal segment was PCR amplified from the clone lppT* on plasmid pJFFLppT-His1 as a template using the primers LppT-NdeI-N and RGD-forward (Table 1), with an annealing temperature of 55°C. The 3′-terminal part of lppT* was amplified by the primers RGD-reverse and LppT-NotI-C (Table 1), with an annealing temperature of 60°C. The two gene segments obtained that are complementary to regions within opposite strands were used to prime the geometric multiplication of the DNA segment extending between the primers. For this, the two segments together with the primers LppT-NotI-C and LppT-NdeI-N (Table 1) were used in a standard PCR, with an annealing temperature of 45°C.
The gene encoding LppT(RGE), where the RGD site at aa positions 462 to 464 was changed to RGE, was amplified by PCR with the overlapping extension method (9) using the primers RGD-mutL and RGD-mutR, the primer pair LppT-NotI-C and LppT-NdeI-N (Table 1), and the plasmid pJFFLppT-His1 as a template.
The amplified lppT*(ΔRGD) and lppT*(RGE) genes were ligated into the expression vector pETHIS-1 by means of the NdeI and NotI restriction sites. The plasmids were purified using the QIAprep Spin plasmid kit. They were sequenced using the primers complementary to the T7 promoter and to the 3′-terminal region flanking the multicloning site of pETHIS-1 to confirm the correct poly-His-tagged lppT*(ΔRGD) (plasmid pJFFLppTΔRGD-His1) and lppT*(RGE) (plasmid pJFFLppTRGE-His1) genes. The constructs were then transformed into Escherichia coli BL21(DE3) cells for expression.
Expression of recombinant, poly-His-tagged LppT and LppT derivates.
Recombinant proteins were produced in E. coli BL21(DE3) containing the various lppT* constructions on pETHIS-1 fusion expression vectors as described for other proteins (33), purified by Ni2+ chelate chromatography (16), dialyzed extensively against PN buffer (50 mM NaH2PO4 at pH 8.0, 300 mM NaCl), and analyzed on sodium dodecyl sulfate (SDS)-10% polyacrylamide gels.
Production of monospecific rabbit anti-LppT antibodies, IgG purification, and Fab preparation.
Monospecific, polyclonal antibodies directed against LppT were obtained by immunizing rabbit subcutaneously with 50 μg LppT-His protein in 500 μl PN buffer mixed with 50 μl of adjuvant 10 (Gerbu Biotechnik GmbH, Gaiberg, Germany), followed by two booster immunizations each with the same amount of protein and the same adjuvant 2 and 4 weeks later. Immunoglobulin G (IgG) was purified from the hyperimmune sera using the HiTrap protein G HP kit (Amersham Pharmacia Biotech, Uppsala, Sweden), dialyzed against Na-carbonate buffer (0.05 M NaHCO3 at pH 8.0, 0.125 M NaCl), and adjusted to 10 mg/ml. An aliquot of 1 ml was then dialyzed at 4°C overnight against 1,000 volumes of pepsin digestion buffer (20 mM CH3COONa, pH 4.5). Preparation of the Fab fragments was performed with the ImmunoPure Fab preparation kit (Pierce, Rockford, IL), according to the manufacturer's protocol. Fab and Fc fragments were generated from IgG by incubating 1.7 mg of purified IgG with immobilized pepsin at 37°C for 4 h. The crude digest was then applied to a column of immobilized protein A. Separation of 1 mg of Fab fragments from the Fc fragments bound to protein A was obtained by washing the column. The Fab fragments were then dialyzed overnight against the PN supplement with 2.7 mM KCl and analyzed on an SDS-polyacrylamide gel electrophoresis (PAGE) Coomassie blue-stained gel. The protein concentration was determined to be 620 μg/ml.
Triton X-114 phase partitioning.
M. conjunctivae cell components were separated into hydrophobic and hydrophilic fractions by the Triton X-114 (Fluka Chemicals, Buchs, Switzerland) partitioning method (8), using washed M. conjunctivae cells from a 5-ml end-exponential-phase culture.
Culture of lamb joint synovial cells.
Primary lamb cells from carpal joint synovial tissue (LSM 192) were cultivated in six-well tissue culture plates using tissue culture medium (minimal essential medium [MEM]; Biochrom, Berlin, Germany) supplemented with 10% fetal calf serum, 2.5 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin in a CO2 incubator. The medium was changed every 3 days. For assays on binding to protein-coated slides, the cells were labeled, after reaching a 75% confluence by [H3]thymidine (PerkinElmer, Boston, MA) overnight in the CO2 incubator. Medium was then exchanged with medium devoid of streptomycin. The cells were then trypsinized and suspended in fresh medium to be used for the adhesion and inhibition assays.
Adhesion assay of Mycoplasma conjunctivae and LSM 192 cells.
For adhesion assays, M. conjunctivae strain HRC/581T was grown in 200 ml of standard mycoplasma culture medium containing 20 μCi (740 kBq) of [U-14C]palmitic acid for 3 days at 37°C with 5% CO2, as described previously (37). The cultures were harvested by centrifugation at 13,000 × g for 20 min, and the mycoplasmas were washed three times in buffer A (0.05 M Tris-HCl at pH 7.2, 0.1 M NaCl, and 1 mM CaCl2), resuspended in this buffer, and adjusted to a concentration of 109 CFU/ml. Small aliquots were frozen at −80°C until use. Thawed, labeled M. conjunctivae cells were used immediately. To determine the optimal concentration of mycoplasmal cultures for adhesion assay, sequential dilutions (1:2 to 1:128) of the labeled M. conjunctivae cells were prepared in buffer A. Aliquots of 200 μl of each dilution were transferred onto each well containing LSM 192 cells and incubated for 2 h at 37°C. After excess liquid was removed, cells were washed twice with 500 μl of buffer A and subsequently solubilized with 100 μl of 1% (wt/vol) SDS solution plus 500 μl buffer A for 2 h at 37°C with shaking. The lysis suspension of each well was then transferred into a vial containing 3 ml of the scintillation cocktail Emulsifier Scintillator Plus (Packard Instrument Company, CT), and counts per minute were measured in a scintillation spectrometer (Wallac 1410 liquid scintillation counter; PerkinElmer, Regensdorf, Switzerland). Saturation of adhesion was obtained at 1,000 CFU of M. conjunctivae per LSM 192 cell, which was referred to as 100% adhesion throughout the investigation. In order to measure inhibition of adhesion of M. conjunctivae to LSM 192 cells by anti-LppT antibodies, the M. conjunctivae suspension was mixed with Fab anti-LppT IgG at a final concentration ranging from 0.1 μg/ml to 10 μg/ml and incubated for 2 h at room temperature with shaking. The Mycoplasma-Fab mix was transferred into wells containing the LSM 192 cells and incubated for 2 additional hours at 37°C. Cells were then treated as described above. As a negative control, Fab fragments from IgG obtained from the unvaccinated rabbit were used at a final concentration of 10 μg/ml, as described previously (6). The assays were performed in triplicate. Results are given as mean values from 3 assays, with the corresponding standard errors. Statistical significance of the differences of the different groups was measured by Student's unpaired t test.
In order to directly measure the impact of the RGD site of LppT on the binding of M. conjunctivae to LSM 192 cells, the LppT-derived RGD-containing nonapeptide VDARGDVYK or the derivative of it, VDARADVYK containing RAD instead of RGD, or fibronectin-derived hexapeptide GRGDSP, was used in a competitive binding assay. In this assay, LSM 192 cells were incubated with various concentrations of RGD or RAD nonapeptides at 20°C for 60 min prior to the addition of the labeled mycoplasmas. The assays were performed five times. Results are given as mean values from 5 assays, with the corresponding standard errors.
Assay for adherence of LSM 192 cells to slides coated with LppT.
Plastic (polymethylmethacrylate) slides were cleaned with 70% ethanol for 5 min and then dried in the oven at 60°C. Each slide was then dipped in 1 ml sterile PN buffer supplemented with 1 mg/ml gelatin for 1 h at 20°C before the liquid was removed by a Pasteur pipette and the slides were transferred to a new tube containing 1 ml PN. After 5 min, PN was removed, and the slides were incubated with 1 ml LppT or LppT(ΔRGD), or LppT(RGE) (20 μg/ml), in PN in a new tube at 37°C for 1 h. Subsequently, the liquid was removed, and the slides were washed for 5 min with 1 ml PN. The semidry slides were then transferred to a new tube containing 1 ml of labeled LSM 192 cells (8 × 105 cells/ml) incubated at 37°C with shaking for 40 min. The liquid was removed, and the slides were washed twice with 1 ml 140 mM NaCl for 5 min and then for 30 min. Subsequently, the semidry slides were transferred into a scintillation vial with 5 ml scintillation liquid and counted in the scintillation counter. Slides coated with bovine serum albumin (20 μg/ml) were used to determine nonspecific binding of LSM 192 cells, which was subtracted as background binding from the adhesion assay. Slides coated with fibronectin from bovine plasma (Sigma-Aldrich F4759) (20 μg/ml) were used as positive controls for binding. The background binding represented 31% of total binding to positive-control slides coated with fibronectin.
The RGD nonapeptide VDARGDVYK, the RAD nonapeptide VDARADVYK, and as a control, the fibronectin-derived standard RGDfibr hexapeptide GRGDSP were used in order to analyze the inhibitory effects of LppT-derived RGD-containing nonapeptides on the adherence of LSM 192 cells to LppT-coated slides. [H3[thymidine-labeled LSM 192 cells were incubated separately with different concentrations of the nona- or hexapeptides at 5 μg/ml, 10 μg/ml, 15 μg/ml, and 20 μg/ml for 15 min. Then, 1 ml of LSM 192 cell suspension (8 × 105 cells/ml) preincubated with the corresponding nona- or hexapeptide was added to the slides. Incubation was done at 37°C by shaking slowly for 30 min. Incubation was stopped by removing the liquid and following the adhesion protocol as described above. Three measurements were made on 2 independent days. Results are given as mean values from 6 measurements, with the corresponding standard errors.
RESULTS
Characterization of the lppST operon and the LppT lipoprotein.
The gene lppT is encoded downstream of lppS, separated by a short, 9-bp intergenic segment and a ribosome binding site (RBS) located 12 bp upstream of the ATG initiation codon. Based on DNA sequence data, LppT is a 105-kDa protein of 947 aa and with a calculated isoelectric point (pI) of 5.5 (EMBL/GenBank accession number AJ318939). It shows a typical signal peptidase cleavage site at residue 34. The leader sequence predicts a typical inside-to-outside helix structure and is followed by two short transmembrane structures, suggesting that LppT is a membrane-located lipoprotein. Triton X-114 extraction of total cultures of M. conjunctivae strain HRC/581T revealed LppT was in the detergent phase and not in the aqueous phase, as detected by immunoblot analysis using monospecific anti-LppT IgG (Fig. 1). This confirmed the membrane location of LppT. BLAST-p analysis revealed that the N-terminal and C-terminal moieties of LppT showed amino acid sequence similarity to the adhesins P102 and P159 of Mycoplasma hyopneumoniae (EMBL/GenBank accession numbers AAV27762 and AAV27918, respectively), with a 22% identity/41% similarity and a 21% identity/43% similarity, respectively. Furthermore, an analysis of the amino acid sequence of LppT revealed a characteristic RGD (Arg-Gly-Asp) cell attachment motif (in boldface) that is flanked by several valine residues, as follows: VVVDARGDVYKTVVA. DNA sequence analysis revealed that this segment is fully conserved on LppT of five field strains of M. conjunctivae isolated from animals with IKC, strains 2777 (chamois), My-66/95 (sheep), My-7/96 (goat), N50 (sheep), and G9 (ibex).
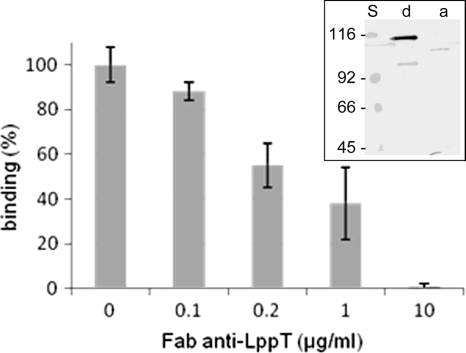
FIG. 1.
Anti-LppT IgG Fab fragments inhibit the binding of M. conjunctivae to LSM 192 cells. Binding studies were performed with 103 M. conjunctivae bacteria per LSM 192 cell. For inhibition of binding, Mycoplasma conjunctivae was incubated with purified anti-LppT IgG Fab fragments at concentrations as indicated. Binding of 100% refers to saturation of adhesion at 1,000 CFU Mycoplasma conjunctivae per LSM 192 cell, as described in Materials and Methods. The data shown are the mean values from three independent measurements. Standard errors calculated are indicated by error bars. Insert, immunoblot of Triton X-114 extracts of the M. conjunctivae detergent phase (d) and aqueous phase (a), reacted with hyperimmune rabbit antiserum directed against recombinant LppT. S, molecular mass standard. Note the minor bands on the blot represent nonspecific binding of the rabbit serum, since these bands are different and of lower molecular mass when the anti-LppT serum from a second rabbit was used.
LppT-promoted adhesion.
In order to study the role of LppT in the cell adhesion of M. conjunctivae, we performed assays for adhesion of C14-labeled M. conjunctivae strain HRC/581T to LSM 192 lamb synovial cells in the presence and absence of Fab fragments of monospecific rabbit anti-LppT IgG. Anti-LppT Fab fragments were able to inhibit the adhesion of M. conjunctivae to LSM 192 cells in a dose-dependent manner. At an anti-LppT Fab concentration of 10 μg/ml, binding was fully inhibited (Fig. 1), whereas Fab fragments prepared from IgG obtained from the serum of the rabbit collected prior to immunization had a minor effect on binding, reducing it by 13%. This demonstrates that LppT is involved in the adhesion of M. conjunctivae to sheep cells.
Role of RGD in adhesion.
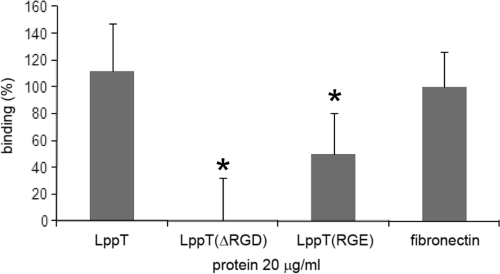
In order to assess whether the RGD sequence of LppT is involved in the adhesion mechanism, we determined the binding of [H3]thymidine-labeled LSM 192 cells to plastic slides coated with LppT, LppT(ΔRGD), or LppT(RGE). As a positive standard for RGD-promoted binding, we used slides coated with fibronectin, a known RGD-based adhesin of mammalian cells (Fig. 2). In this assay, LSM 192 cells bound to LppT with an efficacy similar to that of cells binding to fibronectin. In contrast, protein LppT(ΔRGD), where RGD was deleted, lost the binding capacity for LSM 192 cells (P = 0.003), and LppT(RGE), a recombinant LppT protein in which RGD was mutated to RGE, retained only 45% of the binding activity for LSM 192 cells (P = 0.044) (Fig. 2).
FIG. 2.
Binding of [H3]thymidine-labeled LSM 192 cells to slides coated with recombinant proteins LppT, LppT(ΔRGD), LppT(RGE), or fibronectin. The graphics show the percentage of binding relative to the binding to fibronectin-coated slides, which was used as an internal standard (100%). The data shown are the mean values from three independent measurements. Standard errors calculated are indicated by error bars. Groups with statistically relevant differences are marked by asterisks, and P values are given in the text.
To further verify the role of the RGD sequence of LppT as a potential binding site for the integrin receptor on the host cell, we developed a competitive inhibition assay. LppT-derived RGD-containing nonapeptides competed with recombinant LppT for the binding side on the receptors of LSM 192 cells. In this titration assay, the RGD nonapeptide VDARGDVYK reduced the attachment of [H3]thymidine-labeled LSM 192 cells to LppT to a level of 25%, whereas the RAD analogue VDARADVYK had only a minor effect, leaving the binding at 80% (Fig. 3). Interestingly, the fibronectin-derived RGD-containing hexapeptide GRGDSP did indeed inhibit binding of LSM 192 cells to LppT-coated slides (50% inhibition; P = 0.035) as well as to fibronectin-coated slides (80% inhibition; P < 0.01), whereas the LppT-derived nonapeptide VDARGDVYK failed to prevent LSM 192 cells from binding to fibronectin (10% inhibition; P = 0.05) but inhibited binding to LppT (70% inhibition; P = 0.035).
FIG. 3.
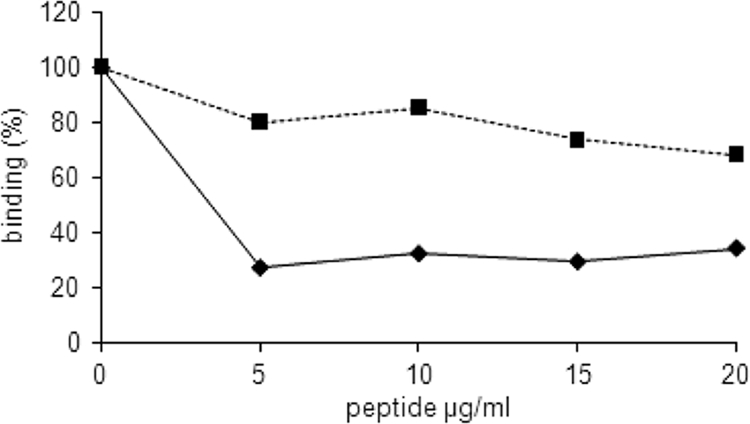
Competitive inhibition of RGD binding. Binding of LSM 192 cells to plastic slides coated with LppT was measured without or with competitive inhibition with the RGD nonapeptide (VDARGDVYK) (diamonds) or with the RAD nonapeptide (VDARADVYK) (squares). The LSM 192 cells were preincubated with RGD or RAD peptides at various concentrations 20 min prior to addition to the plastic slides. The graphics show the percentage of binding relative to the binding of LSM 192 cells in the absence of peptides (100%). The data shown are the mean values from 6 measurements. The standard errors were below 5% of the mean values. The error bars are too small to be visible.
Effects of LppT(RGD) binding capacity on mycoplasmal adherence.
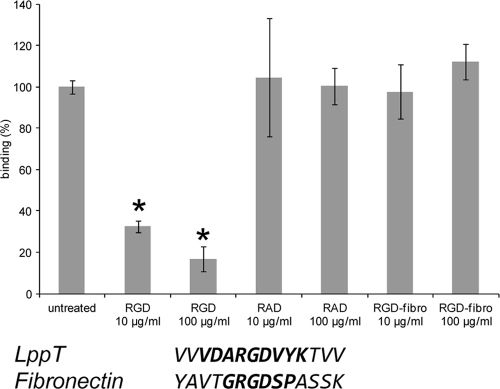
In order to study the role of RGD-promoted binding of LppT in the overall adherence of M. conjunctivae directly to LSM 192 cells, an assay on the binding of C14-labeled M. conjunctivae to confluent LSM 192 cells either in the absence or the competitive presence of LppT-derived RGD and RAD analogous nonapeptides, respectively, was performed. The RGD nonapeptide reduced the binding of M. conjunctivae to LSM 192 cells by 80% (P < 0.001) at a concentration of 100 μg/ml and by 75% (P < 0.001) at a concentration of 10 μg/ml, showing that RGD-promoted binding has a significant impact on the adherence of M. conjunctivae directly to LSM 192 cells (Fig. 4). The analogous RAD nonapeptide did not inhibit binding. The fibronectin-derived RGDfibr hexapeptide had virtually no inhibitory effect on the binding of M. conjunctivae to LSM 192 cells (Fig. 4).
FIG. 4.
(Top) Binding of labeled M. conjunctivae to LSM 192 cells without competing peptides (untreated), in the presence of peptide VDARGDVYK (RGD), in the presence of peptide VDARADVYK (RAD), and in the presence of fibronectin-derived peptide GRGDSP (RGD-fibro) at two different concentrations. Binding of M. conjunctivae to LSM 192 cells in the absence of peptides is referred to as 100%. The data shown are mean values from 5 independent measurements. Standard errors calculated are indicated by error bars. Statistically significant differences between the groups and the untreated sample are marked with asterisks, and P values are given in the text. Standard errors are indicated by error bars. (Bottom) A comparison of the RGD-flanking amino acids between LppT and fibronectin is shown (the sequences of the peptides used in this experiment are in boldface).
DISCUSSION
Integrins constitute a large family of 24 αβ heterodimeric members of cell adhesion receptors that mediate the attachment of cells to the extracellular matrix and also participate in specialized cell-cell interactions (3, 18, 32). They are evolutionarily old proteins, and hence a variety of microorganisms, including pathogenic parasites, bacteria, and viruses, as well as bacterial toxins, have developed lectins to bind to integrins as a prerequisite for causing infections or toxic activity in the host cells, respectively (21, 35, 39, 40). A subset of integrins that recognized the specific RGD (Arg-Gly-Asp) sequence in the native ligand was termed the RGD receptor group of integrins (3). These bind, in particular, fibronectin, where the function of the RGD sequence was initially detected (30), vibronectin, fibrillin, fibrinogen, von Willebrand factor, osteopontin, bone sialoprotein (BSP), tenascin, thrombospondin, and milk fat globule-EFG factor 8 (MFG-E8) (18). These findings gave RGD, besides other specific sites, such as LDV, a central role in cell adhesion biology. RGD represents the prototype of an adhesin signal which directs cells to their appropriate locations in the organism, provides anchoring, and controls proliferation, differentiation, and apoptosis (3, 14, 30, 32). While the amino acids RGD are essential for many integrin binding proteins, the specificity of the binding proteins for the various individual RGD group integrins is determined by the amino acids flanking the RGD site, thus determining the specific functions of the various adhesin proteins in the cellular network. Among the microbial pathogens that mediate their cell adhesion via RGD binding integrins, Bordetella pertussis expresses the protein pertactin as the central binding protein in which one of the two RGD sites was shown to be necessary for the attachment to mammalian cells (21). Diverse viruses are known to use integrins as host cell receptors, among them certain herpesviruses, picornaviruses, and reoviruses (35). Interactions between viral proteins and integrins are also involved in viral entry, the step following binding to cell surface receptors. Entry is a complex process associated with structural changes in the virus as well as in the host cell membrane and, in some cases, in the endosomal and nuclear membranes (22). For example, rotaviruses, which belong to the reoviruses, use no fewer than 5 different integrins in a finely tuned sequential process to gain access to their host cells through the cell membrane (2). The interaction of adenovirus types 2 and 12 with the αVβ5 integrin has been characterized in detail and was shown to be promoted via long, flexible RGD loops on the surface of the penton base, which binds four to five integrin molecules per penton base (34). The ligation of integrins induces receptor clustering and ensuing activation of the focal adhesion kinase, which has been linked to tumor necrosis factor alpha (TNF-α) induction in dendritic cells (24, 35). Very recently, Chintakuntlawar and colleagues (11) reported that the empty capsid of human adenovirus 37, a virus causing epidemic keratoconjunctivitis, directly activated early events of inflammation. They concluded that the capsid was in fact a pathogen-associated molecular pattern (PAMP) triggering leukocyte infiltration and chemokine expression in an RGD-dependent fashion (11). This remarkable finding highlights the possibility that some of the signals triggered by binding of viral ligands to integrins may directly contribute to the pathogenesis of epidemic keratoconjunctivitis in humans, which is clinically similar to mycoplasmal keratoconjunctivitis in ruminants.
The LppT-promoted adhesion process to LSM 192 primary lamb synovial cells involves the participation of an RGD-containing sequence in LppT. This was shown by assays on binding of LSM 192 cells to slides coated with recombinant LppT and LppT-derived RGD-noncontaining and RGE-containing proteins and by the specific inhibition of M. conjunctivae to LSM 192 cells by the LppT-derived RGD-containing nonapeptide. Lipoprotein LppT is currently the first mycoplasmal adhesion protein found to contain an RGD site that is directly involved in binding to eukaryotic host cells. Our results show that the deletion of the RGD site in LppT or competition with synthetic, LppT-derived RGD-containing nonapeptides results in a drastic reduction of the binding capacity of LppT for LSM 192 cells. Direct competitive inhibition experiments using the same RGD nonapeptides, however, reduced binding of the entire M. conjunctivae bacteria to LSM 192 cells by 80%. This result indicates that RGD-mediated binding of LppT is not the sole factor but a major factor in the adhesion mechanism of M. conjunctivae to LSM 192 cells. The fibronectin-derived RGD hexapeptide had no inhibitory effect on the binding of M. conjunctivae to LSM 192 cells, indicating that the amino acids flanking RGD play an important role in defining the specificity of the RGD binding protein. In this respect, it has to be noted that the fibronectin-derived hexapeptide GRGDSP strongly inhibited binding to fibronectin-coated slides but also partially inhibited binding of LSM 192 cells to slides coated with recombinant LppT, while the LppT-derived nonapeptide VDARGDVYK was specific to LppT in this assay and had no effect on binding to fibronectin. In contrast, GRGDSP was not inhibiting M. conjunctivae binding to LSM 192 cells directly. We explain this discrepancy to be due to the fact that the “coated slides” method is a strict in vitro method, in contrast to binding of M. conjunctivae to LSM 192 cells, which mimics more binding under natural conditions. Furthermore, LppT used for coating the slides was a recombinant protein, while fibronectin was purified from bovine plasma. However, we conclude from our results that LppT of M. conjunctivae binds to an integrin different than fibronectin. In this respect, it must be noted that in LppT of M. conjunctivae, the RGD site is flanked by several valine residues, which appears to play a role in the specificity of the various subclasses of integrins (26), whereas fibronectin has only one valine residue preceding RGD (Fig. 4). In this context, it is also interesting to note that selective binding of Mycoplasma penetrans to fibronectin was not inhibited by the RGD tripeptide (15), but no experiments with longer and more specific RGD-containing peptides have been performed. On the other hand, an RGD tripeptide at the very C-terminal end of the putative cytoskeleton-forming proline-rich P65 of M. pneumoniae was noticed (28). However, the role of this RGD residue in adherence to cell tissues was not investigated and seems to be less likely in the view of the role of P65 as a cytoskeleton-forming protein. In this respect, it must be noted that RGD is a motif that is found in about 3% of bacterial (including mycoplasmal) proteins deposited in the Swiss-Prot database (1) but that only a very small number of these proteins have a function as adhesion or integrin binding proteins.
The adhesion mechanism of M. conjunctivae is expected to be multifactorial, despite the fact that anti-LppT antibodies may inhibit adhesion to a major extent. In this respect, it is important to note that both Fab fragments from anti-LppT IgG and Fab from anti-LppS IgG have an inhibitory effect on the binding of M. conjunctivae to LSM 192 cells (6; this work). Furthermore, since LppS and LppT are encoded on the same operon, we expect these two proteins to interact in the adhesion process of M. conjunctivae to host cells. Hence, blocking one of the proteins with Fab fragments from specific IgG would lead to the functional inactivation of the LppS-LppT-mediated adhesion process, similar to that of the analogues proteins P146 and mhp683 of Mycoplasma hyopneumoniae strain 232, which are also encoded on the same operon (10). The strong impact of the RGD locus in LppT of M. conjunctivae on cell adhesion indicates that it constitutes an important lectin that potentially binds to α integrins in the adhesion process, a mechanism that may well also be found in several other Mycoplasma species.
Acknowledgments
We are grateful to Pierre Vaudaux, Benedicte Fleury, and Adriana Renzoni from the Department of Medicine, University Hospital of Geneva, Switzerland, for their help with the coated slide binding technique. We thank Thomas Jungi and Antoinette Golomingi from the Institute for Veterinary Virology, Berne, Switzerland; Heinz Sager from the Institute of Parasitology, Berne, Switzerland; Yvonne Schlatter, Sonja Kittl, and Edy Vilei from the Institute for Veterinary Bacteriology, Berne, Switzerland; and Marco Giacometti from Wildvet Projects, Stampa, Switzerland, for their excellent support and advice.
This project was granted by the fund for research on infectious keratoconjunctivitis (Gemsblindheit), Naturforschende Gesellschaft Graubünden, Chur, Switzerland, and by a research grant from the Institute for Veterinary Bacteriology, Berne, Switzerland.
Footnotes
Published ahead of print on 21 May 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Bairoch, A., P. Bucher, and K. Hofmann. 1996. The PROSITE database, its status in 1995. Nucleic Acids Res. 24:189-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, M., and B. V. Prasad. 14 April 2010. Rotavirus cell entry. Curr. Top. Microbiol. Immunol. [Epub ahead of print.] doi: 10.1007/82_2010_34. [DOI] [PubMed]
- 3.Barczyk, M., S. Carracedo, and D. Gullberg. 2010. Integrins. Cell Tissue Res. 339:269-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barile, M. F., R. A. Del Giudice, and J. G. Tully. 1972. Isolation and characterization of Mycoplasma conjunctivae sp. n. from sheep and goats with keratoconjunctivitis. Infect. Immun. 5:70-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belloy, L., M. Janovsky, E. M. Vilei, P. Pilo, M. Giacometti, and J. Frey. 2003. Molecular epidemiology of Mycoplasma conjunctivae in caprinae: transmission across species in natural outbreaks. Appl. Environ. Microbiol. 69:1913-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belloy, L., E. M. Vilei, M. Giacometti, and J. Frey. 2003. Characterization of LppS, an adhesin of Mycoplasma conjunctivae. Microbiology 149:185-193. [DOI] [PubMed] [Google Scholar]
- 7.Bischof, D. F., C. Janis, E. M. Vilei, G. Bertoni, and J. Frey. 2008. Cytotoxicity of Mycoplasma mycoides subsp. mycoides small colony type to bovine epithelial cells. Infect. Immun. 76:263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bordier, C. 1981. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 256:1604-1607. [PubMed] [Google Scholar]
- 9.Braman, J., C. Papworth, and A. Greener. 1996. Site-directed mutagenesis using double-stranded plasmid DNA templates. Methods Mol. Biol. 57:31-44. [DOI] [PubMed] [Google Scholar]
- 10.Burnett, T. A., K. Dinkla, M. Rohde, G. S. Chhatwal, C. Uphoff, M. Srivastava, S. J. Cordwell, S. Geary, X. Liao, F. C. Minion, M. J. Walker, and S. P. Djordjevic. 2006. P159 is a proteolytically processed, surface adhesin of Mycoplasma hyopneumoniae: defined domains of P159 bind heparin and promote adherence to eukaryote cells. Mol. Microbiol. 60:669-686. [DOI] [PubMed] [Google Scholar]
- 11.Chintakuntlawar, A. V., X. Zhou, J. Rajaiya, and J. Chodosh. 2010. Viral capsid is a pathogen-associated molecular pattern in adenovirus keratitis. PLoS Pathog. 6:e1000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dallo, S. F., A. Chavoya, and J. B. Baseman. 1990. Characterization of the gene for a 30-kilodalton adhesion-related protein of Mycoplasma pneumoniae. Infect. Immun. 58:4163-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeBey, M. C., and R. F. Ross. 1994. Ciliostasis and loss of cilia induced by Mycoplasma hyopneumoniae in porcine tracheal organ cultures. Infect. Immun. 62:5312-5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giancotti, F. G., and E. Ruoslahti. 1999. Integrin signaling. Science 285:1028-1032. [DOI] [PubMed] [Google Scholar]
- 15.Giron, J. A., M. Lange, and J. B. Baseman. 1996. Adherence, fibronectin binding, and induction of cytoskeleton reorganization in cultured human cells by Mycoplasma penetrans. Infect. Immun. 64:197-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hochuli, E., H. Dobeli, and A. Schacher. 1987. New metal chelate adsorbent selective for proteins and peptides containing neighboring histidine residues. J. Chromatogr. 411:177-184. [DOI] [PubMed] [Google Scholar]
- 17.Hsu, T., S. Artiushin, and F. C. Minion. 1997. Cloning and functional analysis of the P97 swine cilium adhesin gene of Mycoplasma hyopneumoniae. J. Bacteriol. 179:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humphries, J. D., A. Byron, and M. J. Humphries. 2006. Integrin ligands at a glance. J. Cell Sci. 119:3901-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkins, C., J. L. Wilton, F. C. Minion, L. Falconer, M. J. Walker, and S. P. Djordjevic. 2006. Two domains within the Mycoplasma hyopneumoniae cilium adhesin bind heparin. Infect. Immun. 74:481-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krause, D. C., and M. F. Balish. 2004. Cellular engineering in a minimal microbe: structure and assembly of the terminal organelle of Mycoplasma pneumoniae. Mol. Microbiol. 51:917-924. [DOI] [PubMed] [Google Scholar]
- 21.Leininger, E., M. Roberts, J. G. Kenimer, I. G. Charles, N. Fairweather, P. Novotny, and M. J. Brennan. 1991. Pertactin, an Arg-Gly-Asp-containing Bordetella pertussis surface protein that promotes adherence of mammalian cells. Proc. Natl. Acad. Sci. U. S. A. 88:345-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsh, M., and A. Helenius. 2006. Virus entry: open sesame. Cell 124:729-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minion, F. C., C. Adams, and T. Hsu. 2000. R1 region of P97 mediates adherence of Mycoplasma hyopneumoniae to swine cilia. Infect. Immun. 68:3056-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philpott, N. J., M. Nociari, K. B. Elkon, and E. Falck-Pedersen. 2004. Adenovirus-induced maturation of dendritic cells through a PI3 kinase-mediated TNF-alpha induction pathway. Proc. Natl. Acad. Sci. U. S. A. 101:6200-6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierschbacher, M. D., and E. Ruoslahti. 1984. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309:30-33. [DOI] [PubMed] [Google Scholar]
- 26.Pierschbacher, M. D., and E. Ruoslahti. 1984. Variants of the cell recognition site of fibronectin that retain attachment-promoting activity. Proc. Natl. Acad. Sci. U. S. A. 81:5985-5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pilo, P., E. M. Vilei, E. Peterhans, L. Bonvin-Klotz, M. H. Stoffel, D. Dobbelaere, and J. Frey. 2005. A metabolic enzyme as a primary virulence factor of Mycoplasma mycoides subsp. mycoides small colony. J. Bacteriol. 187:6824-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Proft, T., H. Hilbert, G. Layh-Schmitt, and R. Herrmann. 1995. The proline-rich P65 protein of Mycoplasma pneumoniae is a component of the Triton X-100-insoluble fraction and exhibits size polymorphism in the strains M129 and FH. J. Bacteriol. 177:3370-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rottem, S. 2003. Interaction of mycoplasmas with host cells. Physiol. Rev. 83:417-432. [DOI] [PubMed] [Google Scholar]
- 30.Ruoslahti, E. 1996. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 12:697-715. [DOI] [PubMed] [Google Scholar]
- 31.Ruoslahti, E. 2003. The RGD story: a personal account. Matrix Biol. 22:459-465. [DOI] [PubMed] [Google Scholar]
- 32.Ruoslahti, E., and M. D. Pierschbacher. 1987. New perspectives in cell adhesion: RGD and integrins. Science 238:491-497. [DOI] [PubMed] [Google Scholar]
- 33.Schaller, A., R. Kuhn, P. Kuhnert, J. Nicolet, T. J. Anderson, J. I. MacInnes, R. P. A. M. Segers, and J. Frey. 1999. Characterization of apxIVA, a new RTX determinant of Actinobacillus pleuropneumoniae. Microbiology 145:2105-2116. [DOI] [PubMed] [Google Scholar]
- 34.Stewart, P. L., C. Y. Chiu, S. Huang, T. Muir, Y. Zhao, B. Chait, P. Mathias, and G. R. Nemerow. 1997. Cryo-EM visualization of an exposed RGD epitope on adenovirus that escapes antibody neutralization. EMBO J. 16:1189-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart, P. L., and G. R. Nemerow. 2007. Cell integrins: commonly used receptors for diverse viral pathogens. Trends Microbiol. 15:500-507. [DOI] [PubMed] [Google Scholar]
- 36.Studier, F. W., A. H. Rosenberg, J. J. Dunn, and J. W. Dubendorff. 1990. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185:60-89. [DOI] [PubMed] [Google Scholar]
- 37.Washburn, L. R., S. Hirsch, and L. L. Voelker. 1993. Mechanisms of attachment of Mycoplasma arthritidis to host cells in vitro. Infect. Immun. 61:2670-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilton, J., C. Jenkins, S. J. Cordwell, L. Falconer, F. C. Minion, D. C. Oneal, M. A. Djordjevic, A. Connolly, I. Barchia, M. J. Walker, and S. P. Djordjevic. 2009. Mhp493 (P216) is a proteolytically processed, cilium and heparin binding protein of Mycoplasma hyopneumoniae. Mol. Microbiol. 71:566-582. [DOI] [PubMed] [Google Scholar]
- 39.Zecchinon, L., T. Fett, E. Baise, and D. Desmecht. 2004. Characterization of the caprine (Capra hircus) beta-2 integrin CD18-encoding cDNA and identification of mutations potentially responsible for the ruminant-specific virulence of Mannheimia haemolytica. Mol. Membr. Biol. 21:289-295. [DOI] [PubMed] [Google Scholar]
- 40.Zecchinon, L., T. Fett, P. Vanden Bergh, and D. Desmecht. 2006. LFA-1 and associated diseases: the dark side of a receptor. Clin. Appl. Immunol. Rev. 6:201-216. [Google Scholar]
- 41.Zhang, Q., T. F. Young, and R. F. Ross. 1995. Identification and characterization of a Mycoplasma hyopneumoniae adhesin. Infect. Immun. 63:1013-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zielinski, G. C., and R. F. Ross. 1993. Adherence of Mycoplasma hyopneumoniae to porcine ciliated respiratory tract cells. Am. J. Vet. Res. 54:1262-1269. [PubMed] [Google Scholar]
- 43.Zielinski, G. C., T. Young, R. F. Ross, and R. F. Rosenbusch. 1990. Adherence of Mycoplasma hyopneumoniae to cell monolayers. Am. J. Vet. Res. 51:339-343. [PubMed] [Google Scholar]