Abstract
Aspergillus fumigatus is a pathogenic mold which causes invasive, often fatal, pulmonary disease in immunocompromised individuals. Recently, proteins involved in the biosynthesis of trehalose have been linked with virulence in other pathogenic fungi. We found that the trehalose content increased during the developmental life cycle of A. fumigatus, throughout which putative trehalose synthase genes tpsA and tpsB were significantly expressed. The trehalose content of A. fumigatus hyphae also increased after heat shock but not in response to other stressors. This increase in trehalose directly correlated with an increase in expression of tpsB but not tpsA. However, deletion of both tpsA and tpsB was required to block trehalose accumulation during development and heat shock. The ΔtpsAB double mutant had delayed germination at 37°C, suggesting a developmental defect. At 50°C, the majority of ΔtpsAB spores were found to be nonviable, and those that were viable had severely delayed germination, growth, and subsequent sporulation. ΔtpsAB spores were also susceptible to oxidative stress. Surprisingly, the ΔtpsAB double mutant was hypervirulent in a murine model of invasive aspergillosis, and this increased virulence was associated with alterations in the cell wall and resistance to macrophage phagocytosis. Thus, while trehalose biosynthesis is required for a number of biological processes that both promote and inhibit virulence, in A. fumigatus the predominant effect is a reduction in pathogenicity. This finding contrasts sharply with those for other fungi, in which trehalose biosynthesis acts to enhance virulence.
Aspergillus fumigatus is a ubiquitous thermotolerant mold which plays an important role in the recycling of environmental carbon and nitrogen (32, 38). A. fumigatus is also an important opportunistic human pathogen that invades the lungs of immunocompromised patients, causing a progressive pneumonia that can disseminate to the heart, brain, and other organs (21). Invasive aspergillosis is a leading cause of death in transplant and leukemic patients, with a mortality rate exceeding 50% despite the best available antifungal therapy (19). This poor response to existing antifungal therapy has led to a great interest in novel therapeutic approaches directed against this organism.
In both the environment and the host, A. fumigatus is exposed to a variety of stressors. While growing in compost, A. fumigatus is commonly found at temperatures exceeding 50°C (5). Similarly, in human tissues, hyphae are subjected to nutrient deprivation and oxidative stress from host immune cells (24, 31). Little is known about the mechanisms by which A. fumigatus survives under these conditions. One possible mechanism by which A. fumigatus adapts to environmental stress is via the biosynthesis of the carbohydrate trehalose, which is involved in mediating the stress response and virulence of other pathogenic fungi such as Candida albicans and Cryptococcus neoformans (1, 22, 30, 48). The trehalose content of these fungal cells may increase up to 50-fold in response to oxidative stress, osmotic stress, and heat shock (27).
Trehalose is a nonreducing disaccharide of glucose that is synthesized by bacteria, plants, insects, and fungi. In fungi, trehalose functions both as a reserve carbohydrate and as a stress metabolite (37, 39, 40). As a reserve carbohydrate, trehalose is found in vegetative resting cells and spores, where it can constitute up to 15% of the dry weight of these structures (46). It is an important source of energy in fungal development, as it is utilized in cell processes such as glycolysis, sporulation, and germination. In addition, trehalose helps the cell withstand environmental stress and nutrient limitation. Trehalose molecules protect the cell by preventing aggregation of denatured proteins and scavenging free radicals (37). Interestingly, trehalose is absent from mammalian cells, and therefore enzymes involved in trehalose biosynthesis have been considered as potential targets for antifungal therapy.
In medically relevant fungi, trehalose is synthesized from glucose. First, the enzyme hexokinase converts a molecule of glucose into glucose-6-phosphate. Then, trehalose-6-phosphate synthase (Tps) catalyzes the production of trehalose-6-phosphate (T6P) from glucose-6-phosphate and a molecule of UDP-glucose. Finally, a phosphate group is removed from trehalose-6-phosphate by trehalose-6-phosphate phosphatase (Tpp) to yield trehalose (29, 42). When trehalose is required for energy, it can be recycled back to glucose through the universal enzyme trehalase (20). The enzymes involved in fungal trehalose biosynthesis have been well characterized in Saccharomyces cerevisiae. These proteins are encoded by four genes: TPS1, which encodes trehalose-6-phosphate synthase; TPS2, which encodes trehalose-6-phosphate phosphatase; and TPS3 and TSL1, redundant genes which encode a large regulatory subunit of the synthase complex (6, 15, 28, 29, 42, 44). All four proteins are tightly regulated both at the genetic level and at the protein level, and they form a protein complex to catalyze the synthesis of trehalose (6, 45).
In other fungi, trehalose biosynthesis appears to play similar but not identical roles in governing growth, stress response, and virulence. In the pathogenic yeasts C. albicans and C. neoformans, the single-copy trehalose-6-phosphate synthase Tps1 is required for normal oxidative stress response and hyphal development and has a role in mediating virulence (1, 22, 30, 48). In Aspergillus nidulans, the trehalose-6-phosphate synthase TpsA is necessary for normal fungal development, thermosensitive growth and response to sublethal exposure to oxidative stress (16). In Aspergillus niger, two putative trehalose-6-phosphate synthases, TpsA and TpsB, have been identified. Inactivation of tpsA resulted in a reduction in T6P activity; however, the role of TpsA and TpsB in trehalose metabolism has not been examined (47). Importantly, trehalose biosynthesis has not been studied in A. fumigatus, which is the most important filamentous fungus causing human disease.
We undertook the study of the role of trehalose biosynthesis in A. fumigatus development, stress response, and virulence. We found that the trehalose content increased in A. fumigatus hyphae throughout development and when actively growing hyphae were exposed to heat shock, but not when they were exposed to other environmental stresses. Increases in trehalose content directly correlated with elevated expression levels of two putative trehalose-6-phosphate synthase genes, tpsA and tpsB. Disruption of both tpsA and tpsB was required to prevent trehalose biosynthesis. Abolition of trehalose biosynthesis via disruption of both tpsA and tpsB affected conidial germination, thermotolerance, and high-level oxidative stress response in vitro. Surprisingly, disruption of tpsA and tpsB resulted in a strain that was hypervirulent by several measures in a murine model of invasive aspergillosis.
MATERIALS AND METHODS
Strains and growth conditions.
Molecular manipulations were done using the A. fumigatus strain Af293 (a generous gift from P. Magee, University of Minnesota). Mutant Af293 ΔtpsA, ΔtpsB, and ΔtpsAB strains, as well as complemented ΔtpsA::tpsA, ΔtpsB::tpsB, and ΔtpsAB::tpsA strains, were used in this study. The identities of genes deleted in the A. fumigatus mutant strains used in this study are Afu6g12950 for tpsA, Afu2g04010for tpsB, Afu4g03190 for tpsC, and Afu5g14300 for tpsD. For most experiments, strains were grown on YEPD agar (1% yeast extract, 2% peptone, 2% dextrose, 1.5% agar) at 37°C. Some experiments required the use of Aspergillus minimal medium containing trace elements (Na2B4O7·10H2O, CuSO4·5H2O, FePO4·4H2O, MnSO4·H2O, and Na2MoO4·2H2O), salts (KCl, MgSO4·7H2O, and KH2PO4), 1% glucose, and NaNO3 adjusted to pH 6.5. Media used in specific experiments included Aspergillus minimal medium containing 0.5% glucose (Aspergillus minimal glucose medium), Aspergillus minimal glucose medium supplemented with 0.5% trehalose, and Aspergillus minimal glucose medium supplemented with 1 M sorbitol. To obtain hyphae, conidia were inoculated at 5 × 105 cells/ml into liquid YEPD medium and allowed to germinate and grow in a 37°C incubator with shaking at 200 rpm. Hyphae were isolated as described previously (25). When developmental time courses were performed, the organisms were collected after 8 h, 12 h, 18 h, and 24 h of incubation to sample various stages in the A. fumigatus life cycle. To test the effects of environmental stressors on hyphae, strains were grown for 12 h at 37°C in liquid YEPD and then subjected for 1 h to oxidative shock with 100 mM H2O2, osmotic shock with 0.5 M NaCl, or heat shock at 50°C. For the effects of stress on conidia, suspensions of 5 × 105 conidia/ml were grown in Aspergillus minimal glucose medium at 37°C for 5.5 h. Samples were exposed either to 1.5 mM H2O2 to test prolonged exposure to sublethal oxidative stress or to 100 mM H2O2 for 10 min to test severe oxidative shock.
Determination of fungal trehalose content.
An enzymatic assay was used to determine the trehalose content of the organisms. Trehalose was extracted from 30 mg of hyphae or 107 conidia in water by heating at 95 to 98°C for 3.5 h. Samples were then treated with 1 M acetic acid and 0.2 M sodium acetate. Following centrifugation, supernatants were treated with 0.05 U/ml of trehalase from porcine kidney (Sigma) or left untreated as a control. Supernatants were incubated overnight at 37°C to allow for the conversion of trehalose to glucose. The glucose concentration in all samples was assayed using a glucose (GO) assay kit (Sigma) following the manufacturer's instructions. The glucose concentration in all test samples was calculated from a standard curve after subtraction of untreated control sample values. The trehalose content of these samples was then determined from the glucose concentration.
Real-time reverse transcription-PCR (RT-PCR).
Total RNA was extracted from hyphae using the Nucleospin RNA plant mini kit (Machery-Nagel). The RNA was reverse transcribed into cDNA using the Moloney murine leukemia virus (M-MuLV) reverse transcription kit (Fermentas). After reverse transcription, quantitative real-time PCR was performed as previously described (17). Expression of all genes was normalized to that of the endogenous housekeeping gene TEF1 (10, 17, 34, 41). The primers for real-time PCR are listed in Table 1.
TABLE 1.
Primers used in this study
| Primer | Target gene | Sequence (5′ → 3′) |
|---|---|---|
| TpsA RT sense | tpsA | CAATCCTTGGAATACGGAAGAG |
| TpsA RT antisense | tpsA | GTCCAGTTTGGAGAAGTTGAGG |
| TpsB RT sense | tpsB | GGGTGGTCTGGTAACTGGTTT |
| TpsB RT antisense | tpsB | ACCGATCCAAGTTCATCCTCT |
| TpsC RT sense | tpsC | TACGTTGCAGAAGCAAGAGGT |
| TpsC RT antisense | tpsC | AGGCCCTTGATGTAGTCCAGT |
| TpsD RT sense | tpsD | TGTCGGATTGGGTTTACTCTG |
| TpsD RT antisense | tpsD | AACACCCTTGAGCAGTTCCTT |
| TEF1 RT sense | TEF1 | CCATGTGTGTCGAGTCCTTC |
| TEF1 RT antisense | TEF1 | GAACGTACAGCAACAGTCTGG |
| F1-TpsA | tpsA | TACAAGCACCGACCAGACGA |
| F1-TpsA+HindIII | tpsA | CCCAAGCTTACAAGCACCGACCAGACGA |
| F2-TpsA | tpsA | TTGGAGTATGCCGAGAAGGT |
| F2-TpsA+SpeI | tpsA | GACTAGTCTTGGAGTATGCCGAGAAGGT |
| F3-TpsA | tpsA | GAGAATGCAGAGCTGGGATG |
| F3-TpsA+XhoI | tpsA | CCGCTCGAGCTCGGATAGGCATGACAGGT |
| F4-TpsA | tpsA | TGGTTTCCCTTGGGCTAGAAA |
| F4-TpsA+SpeI | tpsA | GACTAGTGGTTTCCCTTGGGCTAGAAA |
| F4-TpsA+XbaI | tpsA | GCTCTAGAGCTGGTTTCCCTTGGGCTAGAAA |
| O1-TpsA | tpsA | CCTCAAGATTTGTCCGCAAG |
| O2-TpsA | tpsA | TAGGCATGGAGTTGGTTTCC |
| TpsA first gene | tpsA | GAGAACTCCACCCAAAACGA |
| TpsA last gene | tpsA | TCAGCATCAGCACTTTCAGG |
| F1-TpsB | tpsB | GCAGTTTGGCTGAGAGATGAC |
| F2-TpsB | tpsB | AAGCTGGTGATGCTACCCTTT |
| F3-TpsB | tpsB | CCTCTGGGGTATGGATTCAGG |
| F4-TpsB | tpsB | TCAGGGCGACTGTTTTCTATG |
| O1-TpsB | tpsB | GACTTATTGTGGCGTCTCTGG |
| O2-TpsB | tpsB | ACCCTGGCAGTCACACAAGA |
| TpsB first gene | tpsB | GTCGAAAACAACCACTTTCCA |
| TpsB last gene | tpsB | ATACCAGGTTCATCCCGTCTC |
| M13F | hph | CGCCAGGGTTTTCCCAGTCACGAC |
| M13F RC | hph | GTCGTGACTGGGAAAACCCTGGCG |
| M13R | hph | AGCGGATAACAATTTCACACAGGA |
| M13R RC | hph | TCCTGTGTGAAATTGTTATCCGCT |
| HY | hph | GGATGCCTCCGCTCGAAGTA |
| YG | hph | CGTTGCAAGACCTGCCTGAA |
| BL | ble | AAGTTGACCAGTGCCGTTC |
| LE | ble | TGATGAACAGGGTCACGTC |
| Prom-Ble | ble | GTTTTCCCAGTCACGACGTT |
| Term-Ble | ble | TTTCACACAGGAAACAGCTATGAC |
Strain construction.
The genomic sequences and flanking regions of the putative tps genes were retrieved from GenBank. Analysis of sequences, including homology, was done using DNAMAN software. To obtain gene disruption constructs for single ΔtpsA and ΔtpsB null mutants, the split-marker strategy for molecular cloning was used to increase the rate of targeted homologous integration (9, 34). In this strategy, two incomplete but overlapping fragments of the hygromycin resistance cassette (hph) fused to ∼2 kb of flanking sequences of the tpsA and tpsB genes were generated. These constructs were obtained by fusion PCR as previously described (41), using the Expand high-fidelity PCR system (Roche). The primer sets F1-F2 and F3-F4 for each tps gene were used to amplify flanking sequences from total Af293 DNA, and the M13F-M13R primer set was used to amplify hph from the pAN-71 plasmid for a first round of PCR; constructs were then generated using primer sets F1-YG and F4-HY in a second round of PCR using products from the first round of PCR as templates (Table 1). The resulting constructs were then integrated into the A. fumigatus genome at the tpsA or the tpsB locus by protoplast transformation, replacing the entire protein-coding regions of these genes with an intact hph.
Constructs for the complementation of ΔtpsA and ΔtpsB mutants with tpsA or tpsB were generated using standard molecular cloning techniques. These constructs contained an intact copy of tpsA or tpsB, including promoter and terminator sequences for complementation, and the phleomycin resistance gene (ble) as a selection marker. High-fidelity PCR was used to amplify the tpsA and tpsB genes, including upstream and downstream sequences, using the F1-F4 primer sets from total Af293 DNA, and ble was amplified using the Prom Ble-Term Ble primer sets from the p402 plasmid. Restriction sites were added to these primers where applicable (Table 1). The ΔtpsA complementation cassette was constructed by cloning the tpsA gene within plasmid p402 at the HindIII and SpeI restriction sites. This construct was linearized with ClaI to direct integration of tpsA into its native locus. Likewise, the ΔtpsB complementation cassette was constructed by cloning of the tpsB and ble genes at the NotI restriction sites of the pGEM-T plasmid (Promega). This construct was linearized with SacI to direct integration of tpsB into its native locus.
To create a double disruption of both tpsA and tpsB, tpsA was disrupted in the ΔtpsB mutant background using phleomycin as the second selection marker. The tpsA disruption cassette was constructed within the plasmid p402 in which ∼2 kb of 5′ and 3′ flanking sequences of tpsA was cloned into the HindIII-SpeI and XhoI-XbaI sites, respectively. Flanking sequences were amplified by high-fidelity PCR using the F1-F2 and F3-F4 primer sets with generated restriction sites HindIII, SpeI, XhoI, and XbaI, respectively (Table 1). Finally, to construct ΔtpsAB strains complemented with tpsA, an intact tpsA gene including 1 to 2 kb of flanking sequences was amplified by high-fidelity PCR from whole genomic Af293 DNA using the F1-F4 primer sets, and these products were used directly for fungal transformation.
A. fumigatus was transformed by spheroplasting as described previously (7, 41). Transformants were selected on hygromycin- or phleomycin-containing plates where applicable. Since the ΔtpsAB mutant was resistant to both phleomycin and hygromycin, selection of ΔtpsAB strains complemented with tpsA was performed using growth at 50°C. Successful integration of all constructs was confirmed at the DNA level by PCR, and RNA expression levels of the tpsA gene were confirmed using real-time RT-PCR.
In vitro characterization of tps mutants.
The size of conidia was assessed using flow cytometry. For preparation, 106 freshly harvested conidia were killed with 3% formaldehyde for 1 h, washed three times with phosphate-buffered saline (PBS) plus 0.1% Tween 80, and resuspended in 300 μl of PBS plus 0.1% Tween 80. Samples were then analyzed with a FACScalibur analyzer (BD biosciences). The estimated volume of mutant conidia was compared to that of wild-type conidia using forward scatter (FSC).
Germination time courses were performed using six-well plates in which 5 ml of medium in each well was inoculated with 105 conidia and incubated at 37°C. To assess germination, conidia were monitored microscopically and 100 cells of each strain were counted every hour. Germination was scored based on the detection of visible germ tubes elongating from conidia. Growth and development of each strain were assessed by spot inoculating 10-fold serial dilutions of conidia onto agar plates in triplicate and incubating at 37°C and at 50°C for 2 to 5 days. Conidial viability of each strain at 50°C compared to that at 37°C was determined by quantitative culture. The percent viability at 50°C was determined by expressing the CFU at 50°C as a percentage of counts at 37°C for the same strain. The viability of swollen conidia exposed to oxidative stress was also assessed by quantitative culture and compared with that of untreated controls.
Transmission electron microscopy.
A. fumigatus conidia were grown on YPD agar (Difco) plates at 37°C and harvested at day 6 with PBS plus 0.1% Tween 80 before fixing. Hyphae were obtained by incubating conidia for 24 h in 24-well culture plates containing serum-free RPMI 1640 without phenol red. All specimens were fixed overnight at 4°C with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer. Samples were then washed three times with 0.1 M sodium cacodylate buffer and postfixed with 1% osmium tetroxide and 1.5% potassium ferrocyanide in double-distilled water (ddH2O) for 45 min at 4°C. Washed conidia and hyphae were then dehydrated with increasing concentrations of acetone or ethanol (30%, 50%, 70%, 80%, 90%, and 100%), respectively, followed by embedding in Epon 812. Ultrathin sections were cut using an ultramicrotome (Reichert-Jung ultra cut E), stained with uranyl acetate and lead citrate, and viewed under an FEI Tecnai T12 transmission electron microscope.
Macrophage phagocytosis assays.
RAW264.7 cells were seeded at a density of 5 × 106 cells per well in a six-well plate (3 ml RPMI-10% fetal bovine serum [FBS] with penicillin-streptomycin) and allowed to adhere for 24 h. Cells were then infected with conidia at a multiplicity of infection (MOI) of 10:1. The plates were centrifuged at 800 × g for 5 min to bring the conidia to the bottoms of the wells. After 4 h of coincubation at 37°C and 5% CO2, cells and conidia were scraped from the wells and fixed with 4% paraformaldehyde for 30 min. The cell pellets were washed twice and resuspended in PBS plus 0.1% Tween 80. Samples were then analyzed with a FACScalibur flow cytometer (BD Biosciences, Mississauga, Ontario, Canada). Analysis was done using the FlowJo v8.8.6 program (Tree Star, Ashland, OR).
Antifungal susceptibility testing.
The susceptibilities of all strains to amphotericin B, voriconazole, and caspofungin were assessed using a broth microdilution method based on the recommendations in the CLSI (formerly NCCLS) document M38-A (26). To assess metabolic activity in each drug well quantitatively, an XTT [sodium 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide inner salt] metabolic assay was performed (2-4). Inhibition was compared to that in control wells for each strain in which no drug was added. For the determination of susceptibility to calcofluor, serial dilutions of conidia of each strain were plated on Aspergillus minimal medium with or without calcofluor and incubated at 37°C overnight.
In vivo characterization of tps mutants.
The virulence of tps mutants was tested in a murine intranasal model of invasive aspergillosis (36). In this model, male BALB/c mice (Taconic Labs, Germantown, NY) were immunosuppressed with cortisone acetate (Sigma-Aldrich) as previously described (17). Groups of 11 mice were infected with each A. fumigatus strain using an aerosol chamber. Three mice were used for verification of fungal inocula, and the remaining eight were monitored for survival. To monitor for toxicity of the immunosuppression, eight additional mice were immunosuppressed but uninfected. All procedures involving mice were approved by the Institutional Animal Use and Care Committee according to the National Institutes of Health guidelines for animal housing and care.
In separate experiments, 8 to 10 mice were immunosuppressed and then infected with each strain as described above. After 3 days of infection, the mice were sacrificed, after which their lungs were harvested and homogenized as previously described (13). The pulmonary galactomannan content was measured using the Platelia Aspergillus kit (Bio-Rad). A standard curve was established using serial dilutions of a pool of lung homogenates from 12 immunosuppressed mice heavily infected with the Af293 wild-type strain for 6 and 8 days (35). The lung myeloperoxidase content, which is a measure of phagocyte accumulation, was determined using an enzyme immunoassay (Cell Sciences) (35). To avoid the healthy-survivor bias, galactomannan and myeloperoxidase studies were performed on the third day of infection, at which point at least 90% of infected animals remained alive.
Statistical analysis.
For experiments comparing two groups, single-factor analysis of variance (ANOVA) was performed. Survival curves were analyzed using the log rank test. When comparing nonparametric groups, the Wilcoxon rank sum test was performed. A P value of <0.05 was defined as statistically significant.
RESULTS
Identification of genes encoding trehalose-6-phosphate synthase in A. fumigatus.
Analysis of the available whole-genome sequence of A. fumigatus identified four putative genes that were annotated as encoding trehalose-6-phosphate synthases based on homology to other fungi (Afu6g12950, Afu2g04010, Afu4g03190, and Afu5g14300). At the protein level, Afu6g12950 was most significantly homologous to A. niger TpsA and A. nidulans TpsA. We therefore named this protein TpsA. Similarly, Afu2g04010 was most significantly homologous to A. niger TpsB. We therefore named this protein TpsB. Afu4g03190 and Afu5g14300, TpsC and TpsD, respectively, were not closely related to any of the trehalose synthases in other yeasts and fungi. At the DNA level, tpsA and tpsB as well as tpsC and tpsD were highly homologous, with the areas of sequence divergence interspersed throughout the gene.
Trehalose content and expression of tps genes during A. fumigatus development and in response to stress.
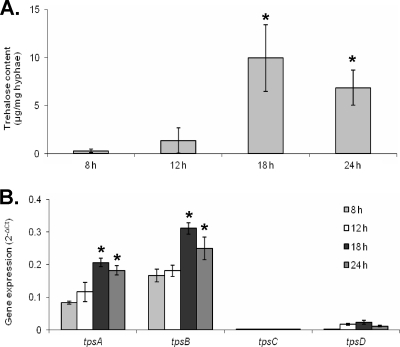
In A. nidulans the trehalose content is low in actively growing hyphae, accumulates during or shortly before conidiation, and remains high in conidia (11). To test if trehalose content was similar in A. fumigatus, we measured the trehalose content and trehalose synthase gene expression in wild-type A. fumigatus during development. Germlings and immature hyphae had low trehalose contents. However, after 18 to 24 h, there was a significant increase in fungal trehalose levels (Fig. 1 A). These time points correspond to the onset of developmental competence and subsequent conidiation, respectively (34). Conidia also had a high trehalose content (data now shown). To determine if the increase in fungal trehalose might correspond to changes in trehalose synthase gene transcription, we examined the mRNA levels of the putative trehalose-6-phosphate synthase genes tpsA, tpsB, tpsC, and tpsD using real-time RT-PCR. tpsA and tpsB mRNAs were expressed throughout the course of development, and their levels increased in parallel with the accumulation of trehalose in maturing hyphae (Fig. 1B). In contrast, expression of tpsC and tpsD remained low at all time points. Collectively, these results suggest that transcriptional upregulation of tpsA and tpsB may contribute to trehalose accumulation during hyphal development and that these two genes may play a dominant role in trehalose biosynthesis during development.
FIG. 1.
Trehalose levels increase in mature hyphae and correlate directly with expression of tpsA and tpsB. Developmental time courses were performed with wild-type A. fumigatus grown in YEPD medium. Samples of hyphae were isolated at the indicated time points. (A) Trehalose content of hyphae at the indicated time points. (B) RNA expression of trehalose synthase genes during development as assessed by real-time RT-PCR. Data were normalized to TEF1 expression. All results are expressed as mean ± standard error and represent at least three different experiments performed on different days. *, statistically significant difference relative to 8-h hyphae (P < 0.05).
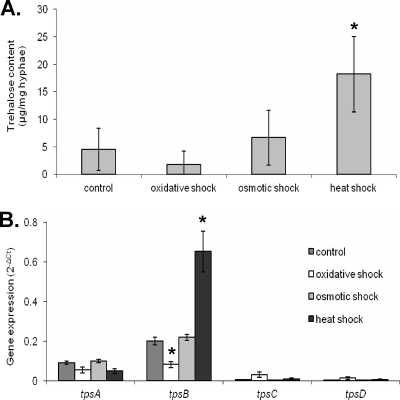
To determine the effects of environmental stressors on trehalose content in A. fumigatus, actively growing exponential-phase hyphae were subjected for 1 h to oxidative shock with 100 mM H2O2, osmotic shock with 0.5 M, NaCl or heat shock at 50°C. Only heat shock at 50°C was associated with an increase in hyphal trehalose content (Fig. 2 A). Real-time RT-PCR assays of tps gene expression under these conditions showed that this increase correlated with increased expression of tpsB but not the other tps genes (Fig. 2B). Interestingly, oxidative stress caused a significant decrease in tpsB mRNA levels and was not associated with an increase in trehalose content. However, unlike the other shock conditions, exposure to peroxide resulted in a growth arrest in hyphae as determined by a reduction in galactomannan release and stable dry weight for 12 h after exposure (data not shown), and this may explain the lack of induction of trehalose biosynthesis under these conditions. Collectively, these results suggest that trehalose may play an important role in protecting the fungus from heat stress. Furthermore, tpsB is the trehalose synthase gene with the highest expression levels under these conditions, suggesting that it may play a dominant role in trehalose biosynthesis in A. fumigatus under conditions of thermal stress.
FIG. 2.
The trehalose content of hyphae increases in response to heat shock and directly correlates with an increase in expression of tpsB. Wild-type hyphae were grown for 12 h and then exposed to shock with either 100 mM H2O2, 0.5 M NaCl, or at 50°C for 1 h. (A) Trehalose content of hyphae at the indicated time points. (B) RNA expression of trehalose synthase genes during development as assessed by real-time RT-PCR. Data were normalized to TEF1 expression. All results are expressed as mean ± standard error and represent at least three different experiments performed on different days. *, statistically significant difference relative to control hyphae (P < 0.05).
Since tpsA and tpsB were considerably homologous to tps genes of other fungi and were significantly expressed during stress and development, we constructed mutant strains in which tpsA and tpsB were deleted both singly and in combination. To verify the specificity of our findings, each mutant was complemented with a wild-type allele of the disrupted gene, and the ΔtpsAB double mutant was complemented with a wild-type allele of tpsA. These strain sets were then used to determine the roles of TpsA and TpsB in fungal development, stress response, and virulence.
Either tpsA or tpsB is sufficient for trehalose accumulation in hyphae and conidia.
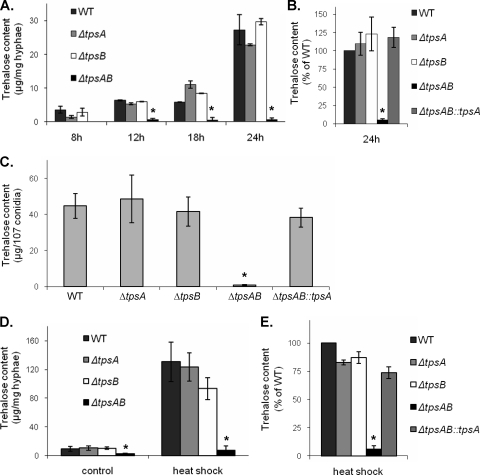
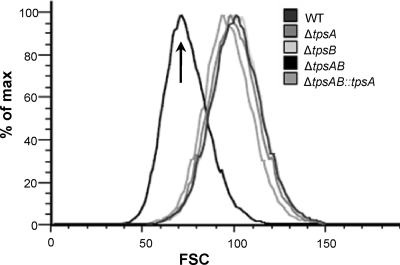
We first tested the effects of deletion of tpsA, tpsB, or both genes on trehalose levels during development. No significant difference in trehalose content between wild-type A. fumigatus and ΔtpsA and ΔtpsB single mutant strains was observed at any time point (Fig. 3 A). In contrast, the ΔtpsAB double mutant strain produced no detectable trehalose, even after prolonged incubation. Complementation of the ΔtpsAB mutant with tpsA restored production of trehalose to wild-type levels (Fig. 3B). Similarly, the trehalose contents of ΔtpsA, ΔtpsB, and ΔtpsAB::tpsA conidia were similar to that of the wild-type strain, while that of the ΔtpsAB mutant was very low (Fig. 3C). Since trehalose normally accounts for up to 15% of the biomass of conidia (46), we investigated whether the absence of trehalose in the ΔtpsAB conidia might result in a reduction of their size. By flow cytometric analysis, we observed a 30% reduction in the forward scatter (FSC) of conidia from the ΔtpsAB mutant compared with those isolated from the wild-type, single mutant, and complemented strains (Fig. 4). This reduction in size is likely in part due to trehalose deficiency, as complementing the ΔtpsAB mutant with tpsA restored both the trehalose content and the size of the conidia.
FIG. 3.
Trehalose production is abrogated in ΔtpsAB hyphae and conidia. WT, wild type. (A) Trehalose content of hyphae isolated from the indicated strains during a developmental time course. Note that data from the ΔtpsAB mutant are omitted at 8 h since this organism had not produced hyphae at this time point. (B) Complementation of the ΔtpsAB mutant restores trehalose content during development. (C) Trehalose content of conidia of the indicated strains. (D) The trehalose content was measured in hyphae grown for 12 h and then incubated at 50°C for 1 h. Control hyphae were incubated at 37°C for 1 h. To compensate for the germination delay of the ΔtpsAB mutant, hyphae were pregrown for 15 h before heat shock. (E) Complementation of the ΔtpsAB mutant restores trehalose content during heat shock. All experiments were repeated in triplicate on three separate days and are presented as mean ± standard error. *, statistically significant difference (P < 0.05) compared to the wild-type strain at any time point.
FIG. 4.
Conidia from the ΔtpsAB mutant are smaller than those from other strains. Conidial size of the indicated strains was assessed by flow cytometry. About 106 conidia of each strain was chemically fixed for analysis, and the estimated volume of conidia was measured by forward scatter (FSC) using the wild-type strain as a reference.
Since an increase in trehalose levels was also observed in wild-type A. fumigatus in response to heat stress, we next compared the trehalose responses of all strains during heat shock. When actively growing hyphae were exposed to heat shock at 50°C for 1 h, similar increases in trehalose content were found in wild-type, ΔtpsA, and ΔtpsB hyphae. In contrast, no trehalose was detectable in ΔtpsAB hyphae (Fig. 3D). Complementation of the ΔtpsAB mutant with tpsA restored production of trehalose under these and all other conditions (Fig. 3E).
These results suggest that tpsA and tpsB have redundant roles in trehalose biosynthesis in A. fumigatus. We therefore tested the ΔtpsB single mutant for a compensatory increase in expression of tpsA or the other putative trehalose-6-phosphate synthase genes tpsC and tpsD. Expression of tpsA, tpsC, and tpsD was measured in the ΔtpsB mutant under the condition of heat shock, a condition which resulted in a significant increase in tpsB expression and trehalose production in the wild type and the ΔtpsB mutant of A. fumigatus. Under these conditions, deletion of tpsB was not associated with the upregulation of tpsA, tpsC, or tpsD mRNA expression compared with untreated controls and the wild-type strain (data not shown). The absence of compensatory upregulation of tpsA, tpsC, or tpsD suggests that although transcriptional regulation of tps gene function may contribute to trehalose synthase regulation in development and in shock, there are likely posttranslational mechanisms of regulation that allow the TpsA protein to functionally compensate for the lack of TpsB and increased trehalose production.
Normal germination requires either tpsA or tpsB.
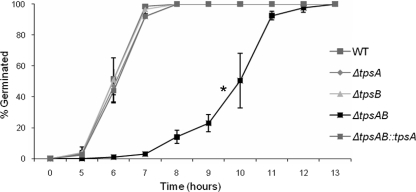
A. nidulans mutants deficient in tpsA, a single-copy trehalose-6-phosphate synthase gene, have a germination delay at lower temperatures (16). We therefore examined the A. fumigatus wild-type strain and the ΔtpsA, ΔtpsB, and ΔtpsAB mutant strains for their ability to germinate at 37°C. In YEPD medium, we observed at least a 2- to 3-h germination delay for the ΔtpsAB mutant compared to the wild-type and single mutant strains, which was reversed by complementation of ΔtpsAB with an intact copy of tpsA (Fig. 5). Supplementation of the medium with exogenous trehalose had no effect on the delayed germination of the ΔtpsAB mutant (data not shown).
FIG. 5.
The ΔtpsAB mutant is delayed in germination at 37°C. Germination of wild-type, ΔtpsA, ΔtpsB, ΔtpsAB, and ΔtpsAB::tpsA conidia was monitored hourly in YEPD medium. For each time point, 100 cells were scored for each strain and percent germination was assessed. Data represent the means from two independent experiments ± standard error.
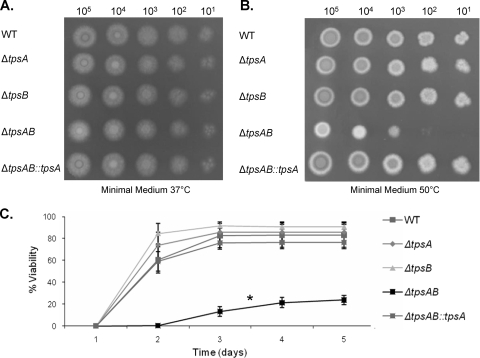
tpsA or tpsB is required for A. fumigatus thermotolerance.
We found that the ΔtpsAB mutant had undetectable trehalose content, even when exposed to high temperature. To determine the functional consequences of this deficiency, we compared the growth of wild-type, ΔtpsA, ΔtpsB, ΔtpsAB, and ΔtpsAB::tpsA strains at 37°C and 50°C. Growth of all strains was indistinguishable at 37°C (Fig. 6 A). However, at 50°C the ΔtpsAB mutant was severely delayed in colony development and had reduced radial growth and conidiation relative to the other strains (Fig. 6B). Moreover, radial growth and conidiation of the ΔtpsAB mutant did not reach wild-type levels despite prolonged incubation (data not shown). Microscopic examination confirmed that ΔtpsAB conidia were able to germinate, grow, and produce phenotypically normal reproductive structures at 50°C; however, this process was markedly delayed compared to that in the wild-type or single mutant strains (data not shown). In addition, at 50°C, only 20% of the conidia of the ΔtpsAB mutant were viable, compared with over 80% of the wild-type or single mutant conidia (Fig. 6C). Furthermore, ΔtpsAB conidia that were viable were delayed in growth and conidiation by about 1 day compared to other strains. Supplementation of the medium with trehalose, sorbitol, or glycerol was unable to reverse these thermosensitive phenotypes (data not shown). Globally, these results suggest that tpsA and tpsB are redundant genes which have a role in thermotolerance.
FIG. 6.
The ΔtpsAB mutant is delayed in growth and development and significantly less viable at 50°C. Serial dilutions of conidia of the indicated strains were plated on Aspergillus minimal glucose medium and grown at 37°C or 50°C. (A) Photograph of colonial morphology of strains grown for 2 days at 37°C. (B) Photograph of colonial morphology of strains grown for 3 days at 50°C. (C) Conidia of the indicated strains were plated on Aspergillus minimal glucose medium at 37°C and at 50°C, and fungal colonies were counted daily for 5 days. The percent viability was measured by expressing counts at 50°C as a percentage of counts at 37°C for the same strain. Experiments were repeated in triplicate on three independent occasions. Data are presented as the mean ± standard error.
A. fumigatus deficient in tpsA and tpsB is susceptible to oxidative shock.
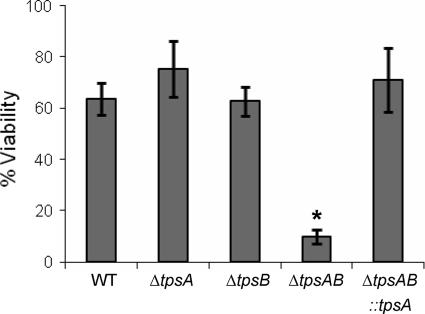
Trehalose is an important antioxidant. Mutant strains of A. nidulans that are deficient in trehalose have increased susceptibility to prolonged sublethal exposure to oxidative stress (16), and similar mutant strains of C. albicans and C. neoformans also have increased susceptibility to oxidative shock (1, 22). Even though trehalose levels in A. fumigatus did not change in response to oxidant stress (Fig. 2A), we tested the viability of wild-type, ΔtpsA, ΔtpsB, ΔtpsAB, and ΔtpsAB::tpsA swollen conidia after sublethal and lethal exposure to oxidative stress. When conidia of the various strains were exposed to a sublethal dose of 1.5 mM H2O2, there was no difference in their viability (data not shown). However, exposure of conidia to 100 mM H2O2 for 10 min resulted in a marked reduction of viability of the ΔtpsAB mutant compared with the single mutants and the wild-type strain (Fig. 7). Again, this phenotype was reversed by complementation of the ΔtpsAB mutant with tpsA. Thus, basal production of trehalose by the products of tpsA and tpsB is likely necessary for protection of A. fumigatus from severe oxidative stress.
FIG. 7.
The ΔtpsAB mutant is highly susceptible to oxidative shock. Swollen conidia of the indicated strains were exposed to 100 mM H2O2 for 10 min and then recovered on Aspergillus minimal glucose medium. Fungal colonies were then enumerated after 2 days of incubation. Viability was expressed as the mean number of fungal colonies for each strain on plates inoculated with conidia exposed to oxidative shock as a percentage of those on plates inoculated with untreated conidia. Data are presented as the mean ± standard error. All experiments were repeated in triplicate on three separate occasions. *, statistically significant (P < 0.05) difference in survival relative to the wild-type strain.
A. fumigatus mutants deficient in tpsA and tpsB are hypervirulent.
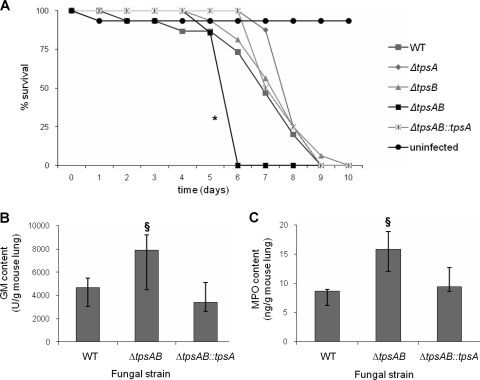
Since the ΔtpsAB mutant had delayed germination and was more susceptible to oxidative stress than the wild-type or single mutant strains, we hypothesized that this strain might also be impaired in virulence. To test this hypothesis, we compared the virulence of wild-type, ΔtpsA, ΔtpsB, ΔtpsAB, and ΔtpsAB::tpsA strains in a corticosteroid-treated murine model of invasive aspergillosis. The survival of mice infected with either the ΔtpsA or ΔtpsB mutant was indistinguishable from that of mice infected with the wild-type strain (Fig. 8 A). Surprisingly, the survival of mice infected with the ΔtpsAB double mutant strain was significantly shorter than that of mice infected with the wild-type strain. The hypervirulence of the ΔtpsAB strain was abrogated when the ΔtpsAB double mutant was complemented with a single copy of tpsA.
FIG. 8.
The ΔtpsAB mutant is hypervirulent in a murine model of invasive aspergillosis. (A) Survival of mice infected with the indicated strains. Mice were immunosuppressed with cortisone acetate and infected intranasally with A. fumigatus wild-type, ΔtpsA, ΔtpsB, ΔtpsAB and ΔtpsAB::tpsA, strains. Eight infected mice per strain were monitored for survival relative to a group of eight uninfected mice. Data represent combined results from two independent experiments. *, statistically significant difference (P < 0.05) relative to the wild-type and ΔtpsAB::tpsA strains using the log rank test. (B and C) The concentrations of galactomannan (GM) (B) and myeloperoxidase (MPO) (C) were measured from the lungs of mice infected with A. fumigatus wild-type, ΔtpsAB, and ΔtpsAB::tpsA strains after 4 days of infection. Results represent the median ± interquartile range for five to eight mice per strain. §, statistically significant difference (P < 0.05) relative to the wild-type strain using the Wilcoxon rank sum test.
To confirm these results, the fungal burden and pulmonary inflammatory response to infection with the wild-type, ΔtpsAB, and ΔtpsAB::tpsA strains were examined in an independent set of experiments. Consistent with the survival studies, mice infected with the ΔtpsAB mutant displayed an increased fungal burden as measured by galactomannan content (Fig. 8B), as well as increased pulmonary inflammation as measured by myeloperoxidase levels (Fig. 8C). As in the survival studies, complementation of the ΔtpsAB mutant with an intact allele of tpsA reduced pulmonary fungal burden and pulmonary inflammation.
Finally, to test if the hypervirulence of the ΔtpsAB mutant strain was a consequence of increased pulmonary delivery of the smaller conidia of this mutant, we examined the pulmonary fungal burden of the mice sacrificed immediately postinhalation in the previous experiments. Mice infected with the ΔtpsAB mutant strain did not have a higher pulmonary fungal burden immediately after inhalation than those infected with the wild-type strain (5.4 × 103 CFU/mouse for the ΔtpsAB mutant strain versus 7.5 × 103 CFU/mouse for strain Af293; n = 8, P > 0.3). Thus, the hypervirulence of the ΔtpsAB strain is unlikely to be due to higher levels of pulmonary infection during inhalation.
Collectively these data strongly suggest that tpsA and tpsB are not required for virulence and may indeed moderate the virulence of A. fumigatus in vivo.
The hypervirulence of the ΔtspAB mutant strain may result from changes in cell wall composition leading to altered host cell interactions.
The majority of hypervirulent A. fumigatus mutants that have been reported to date have displayed alterations in cell wall architecture and composition (13, 23, 33). To determine if the hypervirulent phenotype of the ΔtpsAB double mutant might be related to changes in cell wall architecture, transmission electron microscopy was performed to examine the cell wall of the ΔtpsAB mutant strain. Consistent with the observations from other hypervirulent strains, the cell walls of both hyphae and conidia of the ΔtpsAB mutant strain exhibited a loss of the electron-dense outer layer, and conidia had an increased electrolucent zone compared with wild-type A. fumigatus (Fig. 9 A). In addition, the ΔtpsAB mutant had increased susceptibility to calcofluor white and caspofungin compared with the wild-type or ΔtpsAB::tpsA complemented strain (Fig. 9B and C). This effect was specific to cell wall-active compounds, as the ΔtpsAB strain displayed normal susceptibility to voriconazole and amphotericin B (data not shown).
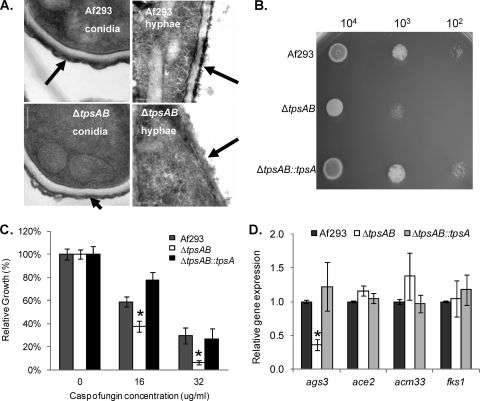
FIG. 9.
Either tpsA or tpsB is required for normal cell wall architecture in A. fumigatus. (A) Transmission electron microscopy of conidia and hyphae of the A. fumigatus strains demonstrates a loss of the electron-dense outer layer (arrows) in both conidia and hyphae of the ΔtpsAB mutant. Scale bars indicate 200 nm. (B) Tenfold serial dilutions of Af293, ΔtpsAB, and ΔtpsAB::tpsA conidia were spot inoculated on plates of Aspergillus minimal glucose medium containing 75 μg/ml of calcofluor white. Photographs of each plate were taken after strains were grown for 2 days at 37°C. (C) Relative growth of individual strains in the presence of various concentrations of caspofungin. (D) RNA expression of cell wall-active genes as assessed by real-time RT-PCR. Data were normalized to TEF1 expression. All results are expressed as mean ± standard error and represent at least three different experiments performed on different days. *, statistically significant difference relative to wild-type strain Af293 (P < 0.05).
To determine if these changes in the cell wall were due to altered expression of known virulence-modulating factors, we performed a real-time RT-PCR analysis of the expression of the A. fumigatus ags3, ace2, and ecm33 genes. Deletion of each of these genes has been associated with alterations in cell wall architecture and increased virulence in murine models of invasive pulmonary aspergillosis (13, 23, 33). Expression of fks1 was also examined in light of the hypersusceptibility to caspofungin seen with the ΔtpsAB strain. Real-time RT-PCR analysis of these genes revealed a statistically significant reduction in the expression of ags3, which encodes an α-glucan synthase, but not any of the other three genes (Fig. 9D). These results suggest that a decrease in ags3 activity may contribute to alterations in the cell wall composition of the ΔtpsAB mutant.
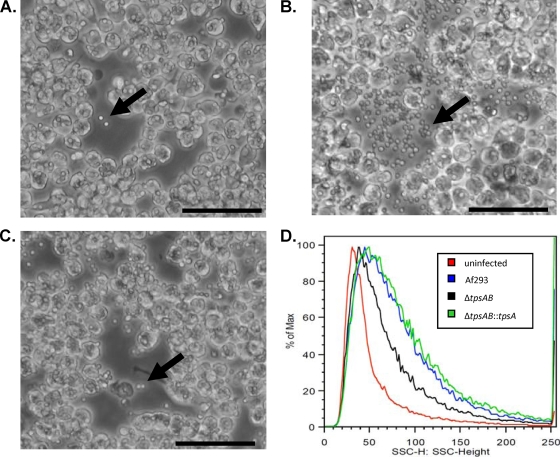
Finally, to determine if these alterations in cell wall composition may influence interactions with immune cells, we examined the effect of tpsAB deletion on the phagocytosis of conidia by the RAW264.7 macrophage cell line. Upon microscopic examination, macrophages infected with conidia of the ΔtpsAB mutant were found to bind and phagocytose conidia much less efficiently than those of the wild-type and ΔtpsAB::tpsA complemented strains (Fig. 10 A to C). To confirm these results, we performed flow cytometric analysis of macrophages after 4 h of infection with conidia of each of the strains. Macrophages infected with the ΔtpsAB mutant strain exhibited significantly lower complexity than those infected with the wild-type or ΔtpsAB::tpsA strain, confirming a reduction in the number of ΔtpsAB conidia that were cell associated (adherent or phagocytosed) with the macrophages (Fig. 10D). Collectively these results suggest that alterations in the cell wall of the ΔtpsAB mutant result in adherence and phagocytosis of the fungus, leading to hypervirulence.
FIG. 10.
Either tpsA or tpsB is required for normal phagocytosis by macrophages. Cultures of RAW264.7 cells were infected with conidia of the indicated A. fumigatus strains and incubated for 4 h. (A to C) Photomicrographs of RAW264.7 cultures infected with conidia of wild-type Af293 (A) or the ΔtpsAB (B) or ΔtpsAB::tpsA (C) strain. Arrows indicated nonadherent, unphagocytosed, extracellular conidia. (D) Flow cytometric analysis of macrophage cells infected as for panels A to C. Side-scatter kinetics were determined as a measure of the number of cell-associated conidia. Bars, 50 μm.
DISCUSSION
Trehalose is an important reserve carbohydrate in fungal development and metabolism, contributing to energy requirements in cell processes such as glycolysis, sporulation, and germination. As a result, trehalose-deficient mutants of multiple fungal species have notable developmental defects. S. cerevisiae deficient in TPS1 sporulates poorly, while C. albicans deficient in TPS1 is impaired in yeast-to-hypha transformation, an important virulence factor in this pathogenic yeast (14, 48). Similarly, A. nidulans ΔtpsA mutants are delayed in germination when glucose or fructose is the sole carbon source (16). We found that A. fumigatus mutants deficient in both tpsA and tpsB were also delayed in germination. However, the germination delay of the A. fumigatus ΔtpsAB mutant was unaffected by trehalose supplementation or growth in rich media. These results suggest the possibility that in addition to mediating the synthesis of trehalose, A. fumigatus TpsA and TpsB may play a more complex role in governing fungal metabolism and development. It has been hypothesized that the S. cerevisiae Tps1 protein could have a direct regulatory role in glycolysis by interacting with glucose transport and sugar kinase mechanisms in the cell (12). Therefore, it is possible that A. fumigatus TpsA and TpsB may also be involved in regulation of glycolysis or other crucial cell processes.
In addition to serving as a reserve carbohydrate in development, trehalose protects actively growing cells from environmental injury. Thermosensitivity is a common defect of trehalose-deficient fungi, including S. cerevisiae (18), C. albicans (1, 43), and A. nidulans (8, 16). In wild-type A. fumigatus, trehalose levels increased during exposure to 50°C, and the ΔtpsAB double mutant had a severe defect in growth and viability at this temperature. Also, conidia of the ΔtpsAB mutant were markedly less viable at 50°C, with only 20% of ΔtpsAB conidia able to undergo germination. Those ΔtpsAB conidia that were able to germinate developed phenotypically normal hyphae and conidiophores; however, the developmental process was delayed by about 1 day. This germination block was insensitive to supplementation with glycerol, contrasting with findings for A. nidulans. Thus, TpsA and TpsB have an important role in the thermostability of A. fumigatus.
In A. nidulans, trehalose levels increase with oxidant stress and trehalose-deficient ΔtpsA mutants have increased susceptibility to this stress condition (16). We found that trehalose levels in A. fumigatus were unchanged by oxidant stress. Despite this finding, the ΔtpsAB double mutant had increased susceptibility to oxidant stress. Thus, while inducible trehalose synthesis plays an important role in mediating thermotolerance in A. fumigatus, basal trehalose levels proved resistance to oxidative stress.
Our results suggest that regulation of tps activity in A. fumigatus occurs by both transcriptional and posttranslational mechanisms. Increases in trehalose levels in the wild-type strain correlated directly with tpsA and tpsB mRNA levels during development and with tpsB transcript levels during heat shock. These results suggest that transcriptional regulation is important for governing trehalose metabolism in A. fumigatus, in contrast with findings for other fungi, where posttranslational modifications of the trehalose synthase complex are thought to govern trehalose homeostasis (45, 49). On the other hand, our studies with the ΔtpsB single mutant suggest that posttranslational regulation of trehalose-6-phosphate synthase activity can also occur in A. fumigatus. In this mutant strain, trehalose levels increased normally during heat shock and development, despite an absence of TpsB, and without any detectable increase in the mRNA levels of tpsA (or tpsC or -D). Since deletion of tpsA in addition to tpsB abrogated the increase in trehalose content under these conditions, these findings suggest that a posttranslational modification of TpsA activity mediates compensation for a lack of TpsB.
In most fungi, including A. nidulans, a single-copy trehalose-6-phosphate synthase is required for trehalose biosynthesis (16). In A. niger, two trehalose-6-phosphate synthase genes have been reported: tpsA, which is more highly expressed during vegetative growth, and tpsB, whose expression increased significantly during heat shock and was barely detectable during vegetative growth (47). The contribution of each of these genes to trehalose biosynthesis has not been studied. Our results show that, like in A. niger, two genes encode trehalose-6-phosphate synthase in A. fumigatus and have significant protein homology to the tpsA and tpsB counterparts in A. niger and A. nidulans. We found that expression patterns of tpsA and tpsB in A. fumigatus were similar to those in A. niger except that A. fumigatus tpsB was highly expressed during both development and heat shock. Disruption of both tpsA and tpsB in A. fumigatus was required to block trehalose biosynthesis under all conditions, indicating redundancy between these two genes. This finding is consistent with our observed high level of sequence conservation between tpsA and tpsB. Differences in sequence between these two genes were distributed uniformly along their length, suggesting that these genes might represent a remote gene duplication event. This redundancy of Tps function may reflect the critical importance that thermotolerance plays in the life cycle of A. fumigatus, which commonly grows in high-temperature compost.
During infection, A. fumigatus is exposed to conditions of both oxidative stress and nutrient depletion. We found that A. fumigatus conidia deficient in tpsA and tpsB had increased susceptibility to severe oxidative shock with hydrogen peroxide as well as delayed germination, suggesting that this strain might be impaired in virulence. Surprisingly, we found that the trehalose-deficient ΔtpsAB mutant strain did not display a reduction in virulence in a murine model of invasive aspergillosis and was in fact hypervirulent. Mice infected with the ΔtpsAB mutant strain had a shorter survival and a higher fungal burden than mice infected with the wild-type parent strain. Furthermore, the higher fungal burden was associated with higher levels of pulmonary inflammation as measured by total pulmonary myeloperoxidase. Complementation of the ΔtpsAB mutant with a single copy of tpsA resulted in lower fungal burdens and a prolongation of survival to wild-type levels. These results contrast sharply with the role of trehalose-6-phosphate synthases in other fungi such as C. albicans and C. neoformans, in which Tps activity is required for normal virulence (30, 48).
The majority of hypervirulent strains of A. fumigatus that have been reported display abnormalities in cell wall content and architecture which have been hypothesized to lead to altered interactions with host cells (13, 23, 33). Consistent with these reports, we also observed that the hypervirulent phenotype of the tpsAB strain was associated with alterations in the cell wall and that these changes were associated with a significant reduction in macrophage adherence and phagocytosis. Similar results were observed with Candida albicans mutants deficient in tps1, in which alterations in cell wall composition were visible by transmission electron microscopy (22). In C. albicans, these changes were accompanied by a reduction in adherence to and phagocytosis by macrophages, although the magnitude of these effects was less marked than what we found with A. fumigatus and did not result in an overall increase in virulence.
Although the mechanism by which A. fumigatus TpsA and TpsB govern cell wall synthesis is not yet elucidated, we observed a reduction in mRNA expression of the putative glucan synthase gene ags3 in the ΔtpsAB mutant strain. Deletion of ags3 has been associated with hypervirulence in a nonneutropenic model of invasive aspergillosis very similar to the one used in this study. However, a decrease in Ags3 expression is likely not the only mechanism by which trehalose influences the cell wall, since the conidia of the ΔtpsAB mutant display a cell wall architecture different from that of conidia deficient in Ags3. Ags3-deficient conidia were found to have an increase in the outer electron-dense cell layer of the cell wall, in contrast to the decrease seen with the ΔtpsAB mutant strain. Further, Ags3-deficient conidia were more resistant to oxidative shock, unlike conidia of the ΔtpsAB mutant. Thus, it is likely that additional genes that govern cell wall synthesis, or their protein products, are dysregulated in the tpsAB mutant.
Collectively, our results indicate that TpsA and TpsB are redundant trehalose-6-phosphate synthase proteins, both of which have a role in development, thermosensitive growth and oxidative stress, and cell wall architecture of A. fumigatus. Furthermore, our results suggest that trehalose is linked to a variety of processes in fungi that both promote and reduce virulence. While in other fungi such as C. albicans and C. neoformans the overall effect of trehalose is to promote virulence, the opposite seems to be true in A. fumigatus, where an absence of Tps activity promotes virulence, likely through altered cell wall synthesis. Although the mechanism by which TpsA and TpsB modulate virulence in A. fumigatus is not yet completely defined, our results suggest that these alterations in cell wall composition lead to enhanced evasion of host immune responses. The mechanism by which alterations in trehalose biosynthesis affect cell wall synthesis are under active study by our group.
Acknowledgments
This work was supported by a Canadian Institutes of Health Research (CIHR) operating grant to D.C.S. Additional funding was provided by grant R01AI073829 and contract N01-AI-30041 from the National Institutes of Health to S.G.F. and D.C.S. D.C.S. is supported by a Clinician-Scientist Award from CIHR, and N.A. was supported by a fellowship from the Research Institute of the McGill University Health Centre.
Editor: G. S. Deepe, Jr.
Footnotes
Published ahead of print on 3 May 2010.
REFERENCES
- 1.Alvarez-Peral, F. J., O. Zaragoza, Y. Pedreno, and J. C. Arguelles. 2002. Protective role of trehalose during severe oxidative stress caused by hydrogen peroxide and the adaptive oxidative stress response in Candida albicans. Microbiology 148:2599-2606. [DOI] [PubMed] [Google Scholar]
- 2.Antachopoulos, C., J. Meletiadis, E. Roilides, T. Sein, and T. J. Walsh. 2006. Rapid susceptibility testing of medically important zygomycetes by XTT assay. J. Clin. Microbiol. 44:553-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antachopoulos, C., J. Meletiadis, T. Sein, E. Roilides, and T. J. Walsh. 2008. Comparative in vitro pharmacodynamics of caspofungin, micafungin, and anidulafungin against germinated and nongerminated Aspergillus conidia. Antimicrob. Agents Chemother. 52:321-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antachopoulos, C., J. Meletiadis, T. Sein, E. Roilides, and T. J. Walsh. 2007. Concentration-dependent effects of caspofungin on the metabolic activity of Aspergillus species. Antimicrob. Agents Chemother. 51:881-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beffa, T., F. Staib, J. Lott Fischer, P. F. Lyon, P. Gumowski, O. E. Marfenina, S. Dunoyer-Geindre, F. Georgen, R. Roch-Susuki, L. Gallaz, and J. P. Latge. 1998. Mycological control and surveillance of biological waste and compost. Med. Mycol. 36(Suppl. 1):137-145. [PubMed] [Google Scholar]
- 6.Bell, W., W. Sun, S. Hohmann, S. Wera, A. Reinders, C. De Virgilio, A. Wiemken, and J. M. Thevelein. 1998. Composition and functional analysis of the Saccharomyces cerevisiae trehalose synthase complex. J. Biol. Chem. 273:33311-33319. [DOI] [PubMed] [Google Scholar]
- 7.Bhabhra, R., M. D. Miley, E. Mylonakis, D. Boettner, J. Fortwendel, J. C. Panepinto, M. Postow, J. C. Rhodes, and D. S. Askew. 2004. Disruption of the Aspergillus fumigatus gene encoding nucleolar protein CgrA impairs thermotolerant growth and reduces virulence. Infect. Immun. 72:4731-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borgia, P. T., Y. Miao, and C. L. Dodge. 1996. The orlA gene from Aspergillus nidulans encodes a trehalose-6-phosphate phosphatase necessary for normal growth and chitin synthesis at elevated temperatures. Mol. Microbiol. 20:1287-1296. [DOI] [PubMed] [Google Scholar]
- 9.Catlett, N. L., B.-N. Lee, O. C. Yoder, and B. G. Turgeon. 2003. Split-marker recombination for efficient targeted deletion and fungal genes. Fungal Genet. News 50:9-11. [Google Scholar]
- 10.Chiang, L. Y., D. C. Sheppard, F. N. Gravelat, T. F. Patterson, and S. G. Filler. 2008. Aspergillus fumigatus stimulates leukocyte adhesion molecules and cytokine production by endothelial cells in vitro and during invasive pulmonary disease. Infect. Immun. 76:3429-3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.d'Enfert, C., and T. Fontaine. 1997. Molecular characterization of the Aspergillus nidulans treA gene encoding an acid trehalase required for growth on trehalose. Mol. Microbiol. 24:203-216. [DOI] [PubMed] [Google Scholar]
- 12.dos Passos, J. B., M. Vanhalewyn, R. L. Brandao, I. M. Castro, J. R. Nicoli, and J. M. Thevelein. 1992. Glucose-induced activation of plasma membrane H(+)-ATPase in mutants of the yeast Saccharomyces cerevisiae affected in cAMP metabolism, cAMP-dependent protein phosphorylation and the initiation of glycolysis. Biochim. Biophys. Acta 1136:57-67. [DOI] [PubMed] [Google Scholar]
- 13.Ejzykowicz, D. E., M. M. Cunha, S. Rozental, N. V. Solis, F. N. Gravelat, D. C. Sheppard, and S. G. Filler. 2009. The Aspergillus fumigatus transcription factor Ace2 governs pigment production, conidiation and virulence. Mol. Microbiol. 72:155-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira, J. C., and A. D. Panek. 1993. Trehalose metabolism during sporulation in Saccharomyces cerevisiae. Biochem. Mol. Biol. Int. 31:1081-1090. [PubMed] [Google Scholar]
- 15.Ferreira, J. C., J. T. Silva, and A. D. Panek. 1996. A regulatory role for TSL1 on trehalose synthase activity. Biochem. Mol. Biol. Int. 38:259-265. [PubMed] [Google Scholar]
- 16.Fillinger, S., M. K. Chaveroche, P. van Dijck, R. de Vries, G. Ruijter, J. Thevelein, and C. d'Enfert. 2001. Trehalose is required for the acquisition of tolerance to a variety of stresses in the filamentous fungus Aspergillus nidulans. Microbiology 147:1851-1862. [DOI] [PubMed] [Google Scholar]
- 17.Gravelat, F. N., T. Doedt, L. Y. Chiang, H. Liu, S. G. Filler, T. F. Patterson, and D. C. Sheppard. 2008. In vivo analysis of Aspergillus fumigatus developmental gene expression determined by real-time reverse transcription-PCR. Infect. Immun. 76:3632-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross, C., and K. Watson. 1998. De novo protein synthesis is essential for thermotolerance acquisition in a Saccharomyces cerevisiae trehalose synthase mutant. Biochem. Mol. Biol. Int. 45:663-671. [DOI] [PubMed] [Google Scholar]
- 19.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, W. V. Kern, K. A. Marr, P. Ribaud, O. Lortholary, R. Sylvester, R. H. Rubin, J. R. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. R. Hodges, H. T. Schlamm, P. F. Troke, and B. de Pauw. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 20.Jorge, J. A., M. L. Polizeli, J. M. Thevelein, and H. F. Terenzi. 1997. Trehalases and trehalose hydrolysis in fungi. FEMS Microbiol. Lett. 154:165-171. [DOI] [PubMed] [Google Scholar]
- 21.Latge, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez-Esparza, M., A. Aguinaga, P. Gonzalez-Parraga, P. Garcia-Penarrubia, T. Jouault, and J. C. Arguelles. 2007. Role of trehalose in resistance to macrophage killing: study with a tps1/tps1 trehalose-deficient mutant of Candida albicans. Clin. Microbiol. Infect. 13:384-394. [DOI] [PubMed] [Google Scholar]
- 23.Maubon, D., S. Park, M. Tanguy, M. Huerre, C. Schmitt, M. C. Prevost, D. S. Perlin, J. P. Latge, and A. Beauvais. 2006. AGS3, an alpha(1-3)glucan synthase gene family member of Aspergillus fumigatus, modulates mycelium growth in the lung of experimentally infected mice. Fungal Genet. Biol. 43:366-375. [DOI] [PubMed] [Google Scholar]
- 24.McDonagh, A., N. D. Fedorova, J. Crabtree, Y. Yu, S. Kim, D. Chen, O. Loss, T. Cairns, G. Goldman, D. Armstrong-James, K. Haynes, H. Haas, M. Schrettl, G. May, W. C. Nierman, and E. Bignell. 2008. Sub-telomere directed gene expression during initiation of invasive aspergillosis. PLoS Pathog. 4:e1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monroy, F., and D. C. Sheppard. 2005. Taf1: a class II transposon of Aspergillus fumigatus. Fungal Genet. Biol. 42:638-645. [DOI] [PubMed] [Google Scholar]
- 26.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard. NCCLS document M38-A. NCCLS, Wayne, PA.
- 27.Nwaka, S., and H. Holzer. 1998. Molecular biology of trehalose and the trehalases in the yeast Saccharomyces cerevisiae. Prog. Nucleic Acid Res. Mol. Biol. 58:197-237. [DOI] [PubMed] [Google Scholar]
- 28.Panek, A. C., P. S. Araujo, S. C. Poppe, and A. D. Panek. 1990. On the determination of trehalose-6-phosphate synthase in Saccharomyces. Biochem. Int. 21:695-704. [PubMed] [Google Scholar]
- 29.Paschoalin, V. M., J. T. Silva, and A. D. Panek. 1989. Identification of an ADPG-dependent trehalose synthase in Saccharomyces. Curr. Genet. 16:81-87. [DOI] [PubMed] [Google Scholar]
- 30.Petzold, E. W., U. Himmelreich, E. Mylonakis, T. Rude, D. Toffaletti, G. M. Cox, J. L. Miller, and J. R. Perfect. 2006. Characterization and regulation of the trehalose synthesis pathway and its importance in the pathogenicity of Cryptococcus neoformans. Infect. Immun. 74:5877-5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Philippe, B., O. Ibrahim-Granet, M. C. Prevost, M. A. Gougerot-Pocidalo, M. Sanchez Perez, A. Van der Meeren, and J. P. Latge. 2003. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect. Immun. 71:3034-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhodes, J. C. 2006. Aspergillus fumigatus: growth and virulence. Med. Mycol. 44(Suppl. 1):S77-S81. [DOI] [PubMed] [Google Scholar]
- 33.Romano, J., G. Nimrod, N. Ben-Tal, Y. Shadkchan, K. Baruch, H. Sharon, and N. Osherov. 2006. Disruption of the Aspergillus fumigatus ECM33 homologue results in rapid conidial germination, antifungal resistance and hypervirulence. Microbiology 152:1919-1928. [DOI] [PubMed] [Google Scholar]
- 34.Sheppard, D. C., T. Doedt, L. Y. Chiang, H. S. Kim, D. Chen, W. C. Nierman, and S. G. Filler. 2005. The Aspergillus fumigatus StuA protein governs the up-regulation of a discrete transcriptional program during the acquisition of developmental competence. Mol. Biol. Cell 16:5866-5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheppard, D. C., K. A. Marr, D. N. Fredricks, L. Y. Chiang, T. Doedt, and S. G. Filler. 2006. Comparison of three methodologies for the determination of pulmonary fungal burden in experimental murine aspergillosis. Clin. Microbiol. Infect. 12:376-380. [DOI] [PubMed] [Google Scholar]
- 36.Sheppard, D. C., G. Rieg, L. Y. Chiang, S. G. Filler, J. E. Edwards, Jr., and A. S. Ibrahim. 2004. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 48:1908-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singer, M. A., and S. Lindquist. 1998. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol. Cell 1:639-648. [DOI] [PubMed] [Google Scholar]
- 38.Tekaia, F., and J. P. Latge. 2005. Aspergillus fumigatus: saprophyte or pathogen? Curr. Opin. Microbiol. 8:385-392. [DOI] [PubMed] [Google Scholar]
- 39.Thevelein, J. M. 1984. Regulation of trehalose mobilization in fungi. Microbiol. Rev. 48:42-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thevelein, J. M., and S. Hohmann. 1995. Trehalose synthase: guard to the gate of glycolysis in yeast? Trends Biochem. Sci. 20:3-10. [DOI] [PubMed] [Google Scholar]
- 41.Twumasi-Boateng, K., Y. Yu, D. Chen, F. N. Gravelat, W. C. Nierman, and D. C. Sheppard. 2009. Transcriptional profiling identifies a role for BrlA in the response to nitrogen depletion and for StuA in the regulation of secondary metabolite clusters in Aspergillus fumigatus. Eukaryot. Cell 8:104-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vandercammen, A., J. Francois, and H. G. Hers. 1989. Characterization of trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase of Saccharomyces cerevisiae. Eur. J. Biochem. 182:613-620. [DOI] [PubMed] [Google Scholar]
- 43.Van Dijck, P., L. De Rop, K. Szlufcik, E. Van Ael, and J. M. Thevelein. 2002. Disruption of the Candida albicans TPS2 gene encoding trehalose-6-phosphate phosphatase decreases infectivity without affecting hypha formation. Infect. Immun. 70:1772-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vuorio, O. E., N. Kalkkinen, and J. Londesborough. 1993. Cloning of two related genes encoding the 56-kDa and 123-kDa subunits of trehalose synthase from the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 216:849-861. [DOI] [PubMed] [Google Scholar]
- 45.Winderickx, J., J. H. de Winde, M. Crauwels, A. Hino, S. Hohmann, P. Van Dijck, and J. M. Thevelein. 1996. Regulation of genes encoding subunits of the trehalose synthase complex in Saccharomyces cerevisiae: novel variations of STRE-mediated transcription control? Mol. Gen. Genet. 252:470-482. [DOI] [PubMed] [Google Scholar]
- 46.Winkler, K., I. Kienle, M. Burgert, J. C. Wagner, and H. Holzer. 1991. Metabolic regulation of the trehalose content of vegetative yeast. FEBS Lett. 291:269-272. [DOI] [PubMed] [Google Scholar]
- 47.Wolschek, M. F., and C. P. Kubicek. 1997. The filamentous fungus Aspergillus niger contains two “differentially regulated” trehalose-6-phosphate synthase-encoding genes, tpsA and tpsB. J. Biol. Chem. 272:2729-2735. [DOI] [PubMed] [Google Scholar]
- 48.Zaragoza, O., M. A. Blazquez, and C. Gancedo. 1998. Disruption of the Candida albicans TPS1 gene encoding trehalose-6-phosphate synthase impairs formation of hyphae and decreases infectivity. J. Bacteriol. 180:3809-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaragoza, O., P. Gonzalez-Parraga, Y. Pedreno, F. J. Alvarez-Peral, and J. C. Arguelles. 2003. Trehalose accumulation induced during the oxidative stress response is independent of TPS1 mRNA levels in Candida albicans. Int. Microbiol. 6:121-125. [DOI] [PubMed] [Google Scholar]