Abstract
Complement-containing immune complexes can be presented to phagocytes by human erythrocytes bearing complement receptor 1 (CR1). Although this has long been assumed to be a mechanism by which humans are able to protect themselves from “extracellular” bacteria such as pneumococci, there is little direct evidence. In these studies we have investigated this question by comparing results for erythrocytes from transgenic mice expressing human CR1 on their erythrocytes to the results for wild-type mouse erythrocytes that do not express CR1. We demonstrate that human CR1 expression on murine erythrocytes allows immune adherence to beads opsonized with either mouse or human serum as a source of complement. The role of CR1 in immune adherence was supported by studies showing that it was blocked by the addition of antibody to human CR1. Furthermore, human CR1 expression enhances the immune adherence of opsonized pneumococci to erythrocytes in vitro, and the pneumococci attached to erythrocytes via CR1 can be transferred in vitro to live macrophages. Even more importantly, we observed that if complement-opsonized pneumococci are injected intravenously with CR1+ mouse erythrocytes into wild-type mice (after a short in vitro incubation), they are cleared faster than opsonized pneumococci similarly injected with wild-type mouse erythrocytes. Finally, we have shown that the intravenous (i.v.) injection of pneumococci into CR1+ mice also results in more rapid blood clearance than in wild-type mice. These data support that immune adherence via CR1 on erythrocytes likely plays an important role in the clearance of opsonized bacteria from human blood.
Streptococcus pneumoniae (pneumococcus) is a major pathogen causing bacteremia in young children and the elderly (22). Pneumococci in the blood are cleared mainly through complement- and antibody (Ab)-dependent opsonization and phagocytosis (8). Previous studies have shown that pneumococci can attach to erythrocytes through immune adherence (IA), which facilitates the clearance of pneumococci by increasing the transfer of pneumococci from erythrocytes to macrophages (20, 24). IA is mediated by complement receptor 1 (CR1) (or CD35) on erythrocytes interacting with C3b, C1q, C4b, and mannose-binding lectin (MBL) on the immune complexes (13, 14, 28). Factors that influence complement deposition on pneumococci thus also affect the IA of pneumococci.
For example, the expression of pneumococcal surface protein A (PspA) and PspC can protect pneumococci from IA. PspA interferes with C1q binding and the classical pathway of complement activation, and PspC interferes with the amplification of the alternative pathway of complement activation that is triggered by the absence of PspA (20, 26). In addition, anticapsule antibody induced by immunization with a 23-valent pneumococcal polysaccharide vaccine can enhance the IA of pneumococci and the subsequent transfer of pneumococci from erythrocytes to macrophages by promoting classical pathway C3 activation (21). Once complement is deposited on pneumococci, through either the absence of PspA and PspC or the presence of antibody to capsule, the pneumococci are able to show IA to CR1 of human erythrocytes and can be readily transferred to macrophages (20, 21).
Human CR1 is a single-chain transmembrane protein expressed on erythrocytes, most white blood cells, tissue phagocytes, and glomerular podocytes (17). Levels of CR1 are variable between individuals, ranging from ∼100 to over 1,000 per human erythrocyte (30). The clustered expression of CR1 on human erythrocytes results in the high-affinity binding of immune complexes (11). In contrast to human erythrocytes, those of mice do not express CR1 (18). In the present paper we utilize transgenic mice expressing human CR1 on mouse erythrocytes (27) to examine the role of erythrocyte CR1 in immune adherence. In this work, we first establish that mouse complement C3b supports IA to human CR1 expressed by transgenic mouse erythrocytes, before investigating the role that human CR1 could play in the clearance of pneumococci from the blood.
MATERIALS AND METHODS
Pneumococcal strains.
Pneumococcal strains BG7322 (6), TIGR4 (1), and EF3030 (2) were grown, as previously described (4), in Todd-Hewitt broth supplemented with 0.5% yeast extract or on a blood-agar plate containing 3% defibrinated sheep erythrocytes. Bacterial stocks were frozen at −80°C in Todd-Hewitt broth containing 10% glycerol.
Mice.
Transgenic mice expressing human CR1 on erythrocytes (CR1+) were generated on the C57BL/6J genetic background as previously described (27). Human CR1 expression on murine erythrocytes was confirmed by flow cytometric staining with an anti-CR1 monoclonal antibody (gift from R. Taylor, University of Virginia). Mice were backcrossed to C57BL/6J (N10) mice by Taconic Farms (Germantown, NY). Age- and gender-matched, wild-type (WT) control C57BL/6J mice were purchased from Taconic Farms.
Cells.
Erythrocytes were separated from WT mouse, CR1+ mouse, and human as previously described (21). Purified erythrocytes were preserved in Alsever's solution (MP Biomedicals Inc., Aurora, OH) and stored at 4°C. The murine macrophage cell line J774A.1 was cultured in Dulbecco modified Eagle medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (HyClone, Logan, UT) and 1% gentamicin (10 mg/ml; Invitrogen, Carlsbad, CA). The cells were split every 3 days to maintain a viability of no less than 90% as judged by trypan blue exclusion.
Sera.
Serum was obtained from blood of WT C57BL/6, CR1+, or C3-deficient mice (12). Human serum was obtained from healthy donors with informed consent and Institutional Review Board approval through the University of Alabama at Birmingham. All sera were stored at −80°C as single-use aliquots of 50 to 100 μl.
Complement-opsonized particles.
The preparation of complement-opsonized particles was performed by using latex beads as previously described (15). Briefly, 100 μl of 200-nm latex beads (Invitrogen) was coated with Alexa Fluor 488-labeled bovine serum albumin (BSA) (Invitrogen) for 10 min at room temperature and washed twice in phosphate-buffered saline (PBS). Next, beads were incubated with 10 μg/ml anti-BSA rabbit polyclonal antibodies (Invitrogen) for an additional 10 min at room temperature and then with either mouse or human fresh serum for 10 min at 37°C in the presence of Ca2+ and Mg2+. As controls, beads were treated as described above in the presence of 2.5 mM EDTA to chelate Ca2+ and Mg2+. Beads were sonicated for 10 min and spun at 9,000 × g for an additional 5 min to pellet the bead aggregates. Ten microliters of the supernatants was incubated with WT or CR1+ erythrocytes for 15 min at 37°C. For some conditions, erythrocytes were preincubated with 10 μg/ml of mouse anti-human CR1 monoclonal antibody 3D9 (gift from R. Taylor, University of Virginia) or mouse IgG control (eBioscience, San Diego, CA) for 60 min. Erythrocytes were washed twice and analyzed by flow cytometry using an LSR II machine (Becton Dickinson). The results were analyzed with FlowJo 8.8.6 software (TreeStar, OR).
Erythrocyte IA of pneumococci and transfer to phagocytes.
Assays of erythrocyte IA of pneumococci and transfer to phagocytes were carried out as previously described (21), where live bacteria were labeled with fluorescein isothiocyanate (FITC) and stored frozen in aliquots of determined numbers of CFU/ml. The FITC-labeled bacteria were opsonized with normal or complement-deficient sera as indicated prior to incubation with erythrocytes from WT mouse, CR1+ mouse, or normal human blood. For some experiments with pneumococci, erythrocytes were preincubated with mouse anti-CR1 monoclonal antibody 3D9 or the mouse IgG control (10 μg/ml) for 60 min and then washed once in PBS. The attachment of labeled bacteria to erythrocytes from different sources was assessed by using flow cytometry and determined by mean fluorescence. The transfer of bacteria to macrophages was evaluated by the addition of bacteria adherent to erythrocytes to the mouse macrophage cell line J774A.1. After a 30-min incubation, the erythrocytes were lysed with BD FACS lysing solution (BD Biosciences, San Jose, CA) for 10 min at room temperature. After washing with 0.1% BSA-Hanks' buffered salt solution (HBSS), the macrophages were fixed with 1% paraformaldehyde and analyzed by flow cytometry. Macrophages were gated, and 15,000 events were collected. The mean fluorescence of macrophages was used to measure the transfer reaction. The natural fluorescence of macrophages was subtracted from each sample.
Blood clearance of pneumococci combined with different erythrocytes.
Frozen stocks of BG7322 containing known concentrations of viable bacteria were thawed and resuspended in 5% bovine serum albumin-Hanks' buffered salt solution with Ca2+ and Mg2+ at a concentration of 4 × 106 bacteria/ml. Twenty microliters of WT mouse serum was added to each 500 μl of bacterial suspension. The mixture was incubated at 37°C for 30 min for opsonization. Next, 500 μl of erythrocytes from either a WT mouse or a CR1+ mouse was added at a concentration of 1 × 108 erythrocytes/ml. After incubation at 37°C for 30 min, the bacteria combined with erythrocytes were injected intravenously (i.v.) into groups of five WT mice through the tail vein. For each mouse, 2 × 105 CFU of bacteria in a volume of 100 μl of the bacterium-erythrocyte suspension was injected. Mice were bled retroorbitally (75 μl) at 1 h and at subsequent time points as indicated. Serial dilutions of the blood were made before subculture on a blood agar plate. After overnight incubation at 37°C, bacteria were enumerated and calculated for the evaluation of CFU in blood in each group of mice (29).
Clearance of bacteria from WT C57BL/6 and CR1+ mice.
The number of bacteria injected into mice was confirmed by plating residual inocula onto blood agar. To assess the net clearance/growth of pneumococci in the circulation, mice were challenged i.v. with 2 × 105 CFU/mouse, and 30 μl of blood was collected from the tail at 10 min, 1 h, 4 h, 6 h, and 24 h postinoculation. Blood was serially diluted and plated onto blood agar plates with overnight incubation at 37°C to determine the number of viable S. pneumoniae bacteria recovered.
Statistical analysis.
To compare CFU levels of bacteremia, Student's t tests were applied to log-transformed data. To compare survival times of WT and transgenic mice infected with S. pneumoniae, the Mann-Whitney U test was used.
RESULTS
Immune adherence to human CR1 on mouse erythrocytes can be mediated by either murine or human complement.
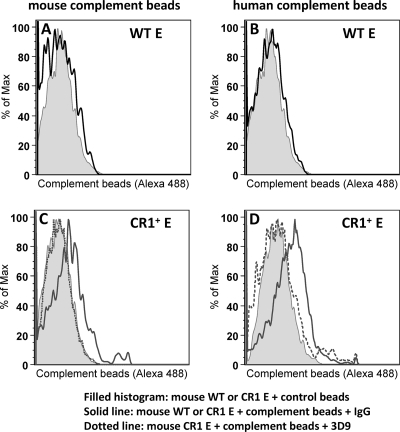
Our first aim was to ascertain if mouse complement supports IA to human CR1. Latex beads coated with fluorescent (Alexa Fluor 488) bovine serum albumin (BSA) and anti-BSA antibodies were allowed to react with either fresh mouse or human serum as a complement source. These antibody-coated and complement-opsonized particles were then incubated with either WT mouse or CR1+ mouse erythrocytes prior to analysis by flow cytometry (Fig. 1). WT erythrocytes did not bind to the anti-BSA-coated particles opsonized with either fresh mouse or human complement (Fig. 1A and B). However, CR1+ mouse erythrocytes bound to particles opsonized with either mouse (Fig. 1C) or human (Fig. 1D) complement, as represented by the right shifts of the solid-line histograms. CR1+ mouse erythrocyte binding to human complement particles was overall greater than that to mouse complement particles, as the CR1+-dependent enhancement of mean fluorescence was 2.78-fold greater than the control for human complement and 1.78-fold greater than the control for mouse complement. Regardless, the cross-species recognition of mouse complement by human CR1 was clearly present. In addition, the specificity of the interaction was further confirmed by using inhibitory anti-CR1 monoclonal Ab (3D9) that completely prevented the binding of both human and mouse complement-opsonized beads to CR1 on either human or mouse red blood cells (RBCs). When CR1+ mouse erythrocytes were preincubated with anti-human CR1 monoclonal antibody 3D9, the binding of the mouse erythrocytes to particles opsonized with either mouse (Fig. 1C) or human (Fig. 1D) complement, as represented by the dotted-line histograms, was largely abrogated.
FIG. 1.
Mouse erythrocytes expressing human CR1 bind mouse complement-opsonized particles and human complement-opsonized particles. Mouse (A and C) or human (B and D) complement-opsonized beads were incubated with WT mouse erythrocytes (E) (A and B) or CR1+ mouse erythrocytes (C and D) for 15 min at 37°C. Cells were washed twice and analyzed by flow cytometry. Shifts in the CR1+ erythrocyte population in the presence of Ca2+ and Mg2+ were observed, in comparison to the absence of Ca2+ and Mg2+ to block complement activation. Some CR1+ erythrocytes were pretreated with anti-human CR1 monoclonal antibody 3D9, which led to an inhibition of the shift.
Transgenic expression of CR1 on mouse erythrocytes supports immune adherence of pneumococci to erythrocytes.
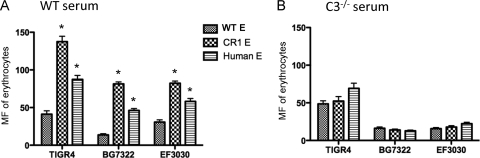
To evaluate the role of CR1 in IA, we opsonized FITC-labeled pneumococci using fresh WT mouse serum, followed by incubation with WT mouse, CR1+ mouse, or normal human erythrocytes. We observed that the adherence of opsonized TIGR4 (capsular type 4), BG7322 (capsular type 6B), and EF3030 (capsular type 19F) pneumococci, assessed by fluorescence, to CR1+ mouse erythrocytes was 2.5- to 5-fold greater than adherence to WT mouse erythrocytes (P < 0.01 by a Student's two-tailed t test). We also observed 2- to 3-fold-more adherence to human erythrocytes than to WT mouse erythrocytes (P < 0.01 by a Student's two-tailed t test) (Fig. 2 A). The fact that all three pneumococcal strains examined exhibited greater IA to CR1+ than to CR1− erythrocytes even though these strains were selected to have high, medium, and low virulence in mice suggests that CR1-dependent IA is likely to be widely distributed among pneumococci.
FIG. 2.
Immune adherence of pneumococci to erythrocytes obtained from WT mouse, CR1+ mouse, or human erythrocytes. The bacteria were opsonized with WT mouse serum (A) or C3-deficient mouse serum (B), followed by incubation with WT mouse, CR1+ mouse, or human erythrocytes (E). (A) The immune adherence of pneumococci to either CR1+ mouse or human erythrocytes, assessed as the mean fluorescence (MF) of erythrocytes, was significantly higher than that to WT mouse erythrocytes (*, P < 0.01 by a Student's two-tailed t test). (B) The increased immune adherence exhibited with CR1+ mouse and human erythrocytes was C3 dependent. Error bars indicate the standard deviations (SD) of data from triplicate samples.
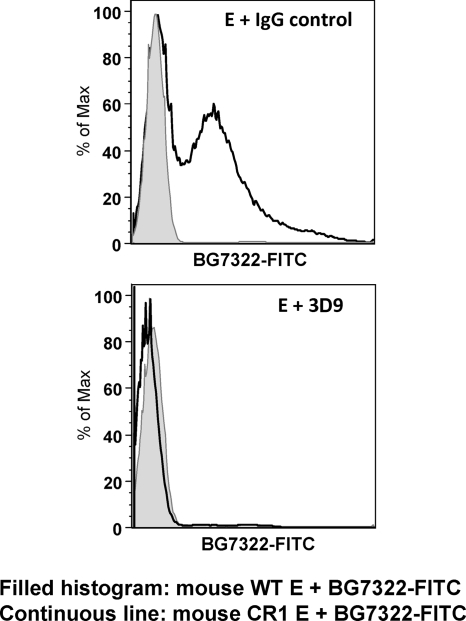
To determine whether the increase in adherence mediated by transgenic or natural CR1 was dependent on mouse C3, the experiment was also carried out by incubating FITC-labeled pneumococci in C3-deficient mouse serum before adding them to the erythrocytes. In the absence of C3, the adherence of the pneumococci to the erythrocytes was very similar regardless of which type of erythrocytes was used (Fig. 2B). These combined data demonstrate that in these studies, the IA of pneumococci to CR1+ transgenic mouse erythrocytes is dependent on both human CR1 and mouse C3. To confirm that the binding of pneumococci was dependent on CR1, we used anti-CR1 monoclonal antibody 3D9 to block CR1 on erythrocytes. The preincubation of erythrocytes with 3D9, but not control IgG, caused a complete inhibition of binding to pneumococcus strain BG7322 (Fig. 3).
FIG. 3.
Adherence of pneumococci to CR1+ erythrocytes is inhibited in the presence of anti-CR1 antibody. FITC-labeled Streptococcus pneumoniae BG7322 was opsonized in mouse serum and then incubated with WT mouse erythrocytes (E) or CR1+ mouse erythrocytes that had been pretreated with either anti-CR1 antibody 3D9 or control IgG for 1 h at room temperature. While a shift in the CR1+ population was present in comparison to the WT population, the shift was not observed for CR1+ erythrocytes pretreated with monoclonal antibody 3D9 to human CR1.
Expression of CR1 on mouse erythrocytes facilitates the transfer of pneumococci from erythrocytes to macrophages.
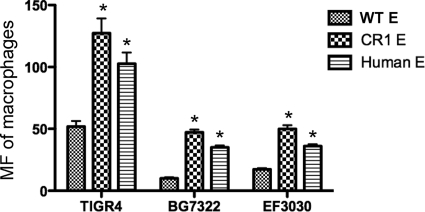
Previous studies have shown that the level of IA of pneumococci to erythrocytes correlates with the level of the subsequent transfer of pneumococci from erythrocytes to macrophages (20). We therefore established that the FITC-labeled pneumococci from the erythrocytes shown in Fig. 2A could be transferred to macrophages. For each of the pneumococcal strains examined, significantly more bacteria were transferred from CR1+ mouse erythrocytes and human erythrocytes to macrophages than from the WT mouse erythrocytes (Fig. 4) (P < 0.01 by a Student's two-tailed t test). The pattern of pneumococcal acquisition by macrophages from WT mouse, CR1+ mouse, and human erythrocytes duplicated the pattern of IA of the opsonized pneumococci to these three types of erythrocytes (Fig. 2A).
FIG. 4.
Macrophage transfer reaction of pneumococci from WT mouse, CR1+ mouse, or human erythrocytes. The transfer reaction was conducted by incubating J774A.1 cells with pneumococci adherent to erythrocytes for 30 min prior to fixation and subsequent analysis by flow cytometry. The amount of bacteria transferred to macrophages was measured as the mean fluorescence (MF) acquired by the macrophages. The asterisk indicates that significantly more pneumococci were transferred from erythrocytes (E) of CR1+ mouse and human than from WT mouse (P < 0.01 by a Student's two-tailed t test). Error bars indicate the SD of data from triplicate samples.
Blood clearance of BG7322 and TIGR4 in C57BL/6J mice was facilitated by expression of CR1 on mouse erythrocytes.
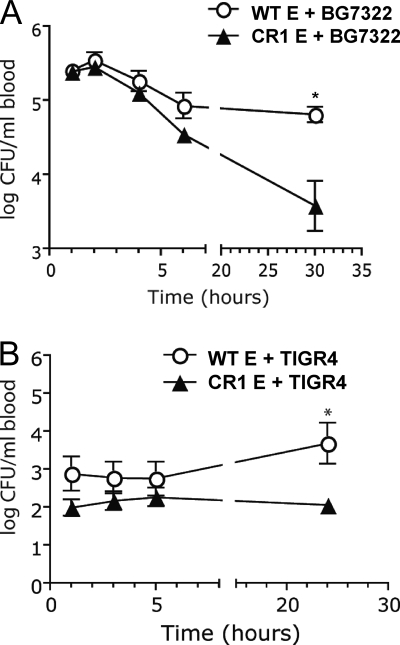
To determine if the effects of CR1 expression on mouse erythrocytes could enhance the in vivo clearance of pneumococci, BG7322 pneumococci were opsonized with WT mouse serum and then incubated for 30 min with WT or CR1+ mouse erythrocytes prior to i.v. injection into WT mice. Mice were injected with 100 μl of a mixture containing 2 × 105 opsonized bacteria and 5 × 106 erythrocytes (n = 5 per group). Within the first few hours postinjection we observed more clearance of pneumococci in mice receiving CR1+ mouse erythrocytes than in mice receiving wild-type erythrocytes, but the difference was not statistically significant until the 30-h time point, when the P value was 0.006 (Fig. 5 A). By 48 h postinjection, four of the five mice receiving BG7322 and WT erythrocytes were dead. The remaining mouse lived until the experiment was terminated at 21 days postinfection. In contrast, all five mice receiving BG7322 survived all 21 days (P < 0.05). This study was also conducted by using S. pneumoniae TIGR4 (also preopsonized in fresh WT mouse serum). As with BG7322, a more rapid clearance of TIGR4 from the blood of the mice was observed when opsonized TIGR4 bacteria were injected with CR1+ mouse erythrocytes than with WT mouse erythrocytes. The difference showed up as early as 1 h postinjection but was not statistically significant (P = 0.02) until 24 h postinjection (Fig. 5B). Thus, with both BG7322 and TIGR4, we observed lower levels of CFU in the blood in the presence of the adoptively transferred CR1+ erythrocytes from the transgenic mice.
FIG. 5.
Blood clearance of BG7322 (A) and TIGR4 (B) combined with erythrocytes. Pneumococci were opsonized with WT mouse serum, followed by incubation with WT or CR1+ murine erythrocytes. A mixture containing 2 × 105 bacteria and 5 × 106 erythrocytes (E) was injected i.v. into WT mice (n = 5). Blood was drawn at different time intervals to evaluate the bacterial clearance. The asterisk indicates that BG7322 or TIGR4 combined with CR1+ mouse erythrocytes was cleared from blood significantly faster than when combined with WT mouse erythrocytes (P = 0.006 [A] and P = 0.02 [B] by a Student's two-tailed t test). Error bars indicate the standard errors of the means (SEM).
The numbers of CFU of TIGR4 in the mice were never more than 1/10 as much as those of BG7322, and none of the mice infected with TIGR4 died. The fact that the differences in numbers of CFU were still increasing after the 5- and 6-h time points suggests that the majority of the opsonized bacteria did not get presented to phagocytes on the first few passes through the capillary beds of the spleen and liver and that significant time was required for them to either bind to the CR1 erythrocytes or be properly presented to phagocytes. We did not examine strain EF3030 because our prior data had established that it was essentially avirulent when given i.v. and that bacteremia was not observed following i.v. inoculation (7).
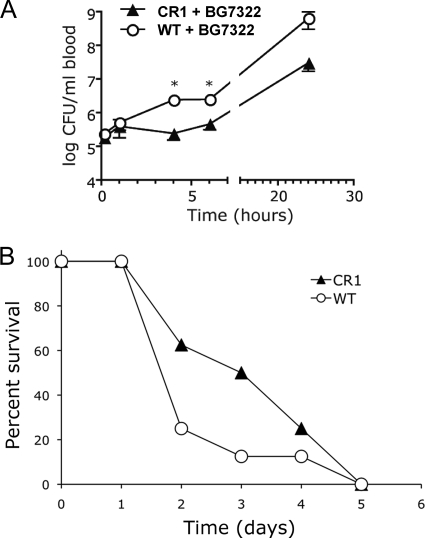
Clearance and/or killing of blood-borne pneumococci is more rapid in CR1+ mice than in wild-type mice.
Since the preincubation of pneumococci with CR1+ erythrocytes resulted in more efficient clearance than with WT erythrocytes, it seemed likely that CR1+ transgenic mice would be more resistant to pneumococci than WT mice. To test this, 2 × 105 BG7322 bacteria were injected i.v. into CR1+ transgenic mice (n = 8) and into WT mice (n = 8). At 10 min and 1 h postinfection, no significant differences in blood clearance of pneumococci were observed between CR1+ and WT mice (Fig. 6 A). However, at 4 h and 6 h postinfection, 10-fold-more pneumococci were recovered from the blood of WT mice than from the blood of CR1+ mice (P < 0.001). This 10-fold difference was also observed at 24 h, although the difference did not meet statistical significance (P = 0.07 by a Student's two-tailed t test). We also assessed the abilities of CR1+ mice and WT mice to survive pneumococcal infection. Each of the eight mice in each group became moribund over a course of 5 days. The median survival time for CR1+ mice was 3.5 days, compared to 2 days for WT mice. This difference, however, was not statistically significant (P = 0.2 by two-tailed Mann-Whitney U test) (Fig. 6B). The blood clearance and mortality studies were repeated a second time, with similar results (data not shown). In the second experiment almost 30% of mice in both groups failed to die, thus complicating a combined analysis of the survival data from both studies. However, for the mice that died in the second study, the median number of days until they became moribund was 3 days for wild-type mice and 5.5 days for CR1 mice. If we pooled “time-to-moribund” data from the first 6 days postinfection for both experiments, the P value determined by a Mann-Whitney U test was 0.022.
FIG. 6.
Bacterial clearance and survival of WT and CR1+ transgenic mice infected with Streptococcus pneumoniae BG7322. (A) Blood clearance and/or growth of pneumococci in WT and CR1+ transgenic mice. WT mice and transgenic mice were infected i.v. with 2 × 105 CFU of pneumococci. Bacteremia was determined at different time points. Data are presented as the mean CFU/ml of blood ± SEM for eight mice in each group. The asterisks indicate that bacteremia in deficient mice is significantly different (P < 0.001 by a Student's two-tailed t test). (B) Number of mice not yet moribund at each day following their infection as described above. It took a day longer for 50% of the CR1+ mice to become moribund compared to the C57BL/6 mice. This difference, however, was not statistically significant (P = 0.2 by two-tailed Mann-Whitney U test).
DISCUSSION
These studies provide the first in vivo evidence that CR1 expression on erythrocytes has an actual, and not just a theoretical (24), effect on the clearance of pneumococci from the blood. We have demonstrated that human CR1 expression on mouse erythrocytes enhances the immune adherence of pneumococci to erythrocytes, facilitates the transfer of pneumococci to phagocytes, and improves the clearance of pneumococci from the blood of infected mice.
Prior studies demonstrated the activity of human soluble CR1 (sCR1) in mouse models of disease and showed that sCR1 and its fragments are active against the mouse alternative pathway of complement activation, which suggests at least some interaction between sCR1 and mouse C3b (25). However, the behavior of sCR1 may differ from that of membrane-bound CR1. Our data establish both in vitro and in vivo that mouse complement can interact with human CR1 expressed on murine erythrocytes in the transgenic model. This was demonstrated by comparisons of the results obtained with wild-type erythrocytes to those obtained with CR1+ transgenic erythrocytes. It was also demonstrated by showing that the CR1-dependent enhancement of the binding of complement-coated pneumococci or beads to erythrocytes could be blocked by the use of a monoclonal antibody to CR1.
Moreover, the CR1 transgenic mouse was also shown to clear bacteriophage φX174 in the presence of a bispecific monoclonal antibody heteropolymer that can bind both CR1 and bacteriophage φX174 (27). This CR1-dependent clearance of bacteriophage φX174 in the mouse was similar to what was observed previously for baboons, which have erythrocyte-based immune adherence mediated by CR1-like molecules that are glycosylphosphatidylinositol (GPI) anchored (5). These combined findings suggest that the CR1 transgenic mouse is a useful surrogate animal model for studying the clearance of immune complexes through IA.
Our in vitro data using blocking antibody against human CR1 demonstrate that transgenic CR1 RBCs bind to the pneumococcus in a manner that is dependent on CR1 and C3b. Nearly all of the pneumococcal association with CR1+ RBCs was inhibited by 3D9, an antibody that inhibits the binding of CR1 to C3b/C4b (19). Also, data from the use of serum from C3-deficient mice as a complement source suggest that mouse C1q, C4b, and MBL play a minimal role of the attachment to CR1 in IA in our model since the absence of C3 by itself largely abrogated IA. The serum used as a complement source for the opsonization of bacteria was obtained from C57BL/6 mice that lacked significant antibody against pneumococci. Therefore, the deposition of C1q, C4, and MBL on the pneumococcal strains in our experimental system is expected to be low. However, C3 deposition can still be achieved because even in the presence of normal serum lacking detectable antibody to pneumococci, there is a measurable but low-level activation of C3 through the classical pathway, which is greatly amplified by the alternative pathway (9, 20), thus making the total amount of C3 products available to bind CR1 much greater than that of C1q or C4. Previous studies using human complement indicated that the interaction between CR1 and the immune complexes is made largely through C3b (13). Although it is possible that C3b fragments generated through the activation of mouse complement C3 might play a critical role in the CR1-dependent IA that we have observed, our studies have not experimentally examined this issue.
We observed a significantly greater virulence of our BG7322 infections shown in Fig. 6A than those shown in Fig. 5A. This was reflected by the over 1,000-fold-more CFU at 24 h in C57BL/6 wild-type mice. This difference could be due to inadvertent differences in the phases of growth of the bacterial stock between the experiments, which were performed at two different sites. Although the difference makes interpretations of the data more complex, it also provides additional strength to the findings, since the presence of CR1 was associated with greater protection against bacteremia in the experiments using mice regardless of the experimental site.
In the studies shown in Fig. 5A, BG7322 pneumococci injected with CR1+ erythrocytes were cleared significantly faster than in mice where there were no CR1+ cells. Moreover, four of the five mice given BG7322 and WT cells died within 48 h, and all five mice given bacteria and CR1+ cells lived. In the studies shown in Fig. 6A, where the BG7322 infections rapidly progressed in wild-type mice, nearly 10-fold-lower levels of CFU were present in CR1+ mice than in wild-type mice at early time points, but no difference in the rates of survival of the mice was observed.
Another interesting observation was that although the 10-fold effect of CR1 expression on CFU levels in transgenic mice (Fig. 6A) occurred within 4 to 6 h of infection, the difference in CFU levels showed no further increase at 24 h postinfection. This finding was in contrast to the data shown in Fig. 5A, where the greatest differences in circulating pneumococci in the absence and presence of CR1 occurred at 22 to 30 h postinfection. This difference in the kinetics of clearance may be a reflection of the 10- to 1,000-fold-higher bacterial burden observed for C57BL/6 mice (Fig. 6A) from the 6-h time point on. Previous studies demonstrated that when the blood levels of pneumococci in mice exceed 106, as occurred in the studies where CR1+ and WT mice were injected with pneumococci, a very protective inflammatory response was elicited (3). It is possible that once this inflammatory response developed, CR1 expression no longer provided an advantage for the clearance of pneumococci from blood.
Our finding that pneumococcal clearance can be enhanced by CR1 is consistent with the work of others who have shown that nonbacterial immune complexes bound to heterologous CR1-expressing erythrocytes can be removed by phagocytes in the liver (16, 23). Even so, the ability of our studies and previous studies to see CR1-dependent clearance in the mouse seemed remarkable considering the fact that since the mouse host lacks CR1, it thus has lacked evolutionary pressure to maintain or develop a CR1-dependent clearance mechanism.
The findings presented here support the possibility that pathogens, in addition to pneumococci, that have been opsonized with complement may be cleared from the blood of humans more rapidly because of the presence of CR1 on human erythrocytes. We are actively investigating this possibility with bacterial, fungal, and viral pathogens. Interestingly, we have observed an increase in the binding of adenovirus with transgenic CR1 erythrocytes in vivo, indicating that CR1 affects viral pathogenesis by altering the delivery of the virus to different organs (10). Future studies with the CR1 transgenic mouse will allow a greater understanding of the role of IA in vivo.
Acknowledgments
We thank Janice King for technical assistance and Gabriel Hendricks for assistance with flow cytometry.
This work was supported by National Institutes of Health grants AI21548 (to D.E.B.), AI64349 (to R.W.F.), and AI053542 (to J.P.W.); Juvenile Diabetes Research Foundation grant 24-2008-950 (to R.W.F. and J.P.W.); MRMC grant W81XWH-07-1-0286 (to I.G.); the New England Center for Excellence in Biodefense and Emerging Diseases (to R.W.F.); and National Research Council of Korea grant WCU R33-10045 (D.E.B.).
Editor: J. N. Weiser
Footnotes
Published ahead of print on 3 May 2010.
REFERENCES
- 1.Aaberge, I. S., J. Eng., G. Lermark, and M. Lovik. 1995. Virulence of Streptococcus pneumoniae in mice: a standardized method for preparation and frozen storage of the experimental bacterial inoculum. Microb. Pathog. 18:141-152. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, B., J. Dahmen, T. Frejd, H. Leffler, G. Magnusson, G. Noori, and C. S. Eden. 1983. Identification of an active disaccharide unit of a glycoconjugate receptor for pneumococci attaching to human pharyngeal epithelial cells. J. Exp. Med. 158:559-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benton, K. A., M. P. Everson, and D. E. Briles. 1995. A pneumolysin-negative mutant of Streptococcus pneumoniae causes chronic bacteremia rather than acute sepsis in mice. Infect. Immun. 63:448-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benton, K. A., J. C. Paton, and D. E. Briles. 1997. Differences in virulence for mice among Streptococcus pneumoniae strains of capsular types 2, 3, 4, 5, and 6 are not attributable to differences in pneumolysin production. Infect. Immun. 65:1237-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birmingham, D. J., and L. A. Hebert. 2001. CR1 and CR1-like: the primate immune adherence receptors. Immunol. Rev. 180:100-111. [DOI] [PubMed] [Google Scholar]
- 6.Briles, D. E., M. J. Crain, B. M. Gray, C. Forman, and J. Yother. 1992. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect. Immun. 60:111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briles, D. E., S. K. Hollingshead, J. C. Paton, E. W. Ades, L. Novak, F. W. van Ginkel, and W. H. Benjamin, Jr. 2003. Immunizations with pneumococcal surface protein A and pneumolysin are protective against pneumonia in a murine model of pulmonary infection with Streptococcus pneumoniae. J. Infect. Dis. 188:339-348. [DOI] [PubMed] [Google Scholar]
- 8.Brown, E. J., S. W. Hosea, and M. M. Frank. 1983. The role of antibody and complement in the reticuloendothelial clearance of pneumococci from the bloodstream. Rev. Infect. Dis. 5(Suppl. 4):S797-S805. [DOI] [PubMed] [Google Scholar]
- 9.Brown, J. S., T. Hussell, S. M. Gilliland, D. W. Holden, J. C. Paton, M. R. Ehrenstein, M. J. Walport, and M. Botto. 2002. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc. Natl. Acad. Sci. U. S. A. 99:16969-16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlisle, R. C., Y. Di, A. M. Cerny, A. F. Sonnen, R. B. Sim, N. K. Green, V. Subr, K. Ulbrich, R. J. Gilbert, K. D. Fisher, R. W. Finberg, and L. W. Seymour. 2009. Human erythrocytes bind and inactivate type 5 adenovirus by presenting coxsackie virus-adenovirus receptor and complement receptor 1. Blood 113:1909-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chevalier, J., and M. D. Kazatchkine. 1989. Distribution in clusters of complement receptor type one (CR1) on human erythrocytes. J. Immunol. 142:2031-2036. [PubMed] [Google Scholar]
- 12.Circolo, A., G. Garnier, W. Fukuda, X. Wang, T. Hidvegi, A. J. Szalai, D. E. Briles, J. E. Volanakis, R. A. Wetsel, and H. R. Colten. 1999. Genetic disruption of the murine complement C3 promoter region generates deficient mice with extrahepatic expression of C3 mRNA. Immunopharmacology 42:135-149. [DOI] [PubMed] [Google Scholar]
- 13.Fearon, D. T. 1980. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J. Exp. Med. 152:20-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghiran, I., S. F. Barbashov, L. B. Klickstein, S. W. Tas, J. C. Jensenius, and A. Nicholson-Weller. 2000. Complement receptor 1/CD35 is a receptor for mannan-binding lectin. J. Exp. Med. 192:1797-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghiran, I., A. M. Glodek, G. Weaver, L. B. Klickstein, and A. Nicholson-Weller. 2008. Ligation of erythrocyte CR1 induces its clustering in complex with scaffolding protein FAP-1. Blood 112:3465-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson, A. L., M. A. Lindorfer, A. D. Kennedy, P. L. Foley, and R. P. Taylor. 2002. Concerted clearance of immune complexes bound to the human erythrocyte complement receptor: development of a heterologous mouse model. J. Immunol. Methods 270:183-197. [DOI] [PubMed] [Google Scholar]
- 17.Hess, C., and J. A. Schifferli. 2003. Immune adherence revisited: novel players in an old game. News Physiol. Sci. 18:104-108. [DOI] [PubMed] [Google Scholar]
- 18.Kinoshita, T., J. Takeda, K. Hong, H. Kozono, H. Sakai, and K. Inoue. 1988. Monoclonal antibodies to mouse complement receptor type 1 (CR1). Their use in a distribution study showing that mouse erythrocytes and platelets are CR1-negative. J. Immunol. 140:3066-3072. [PubMed] [Google Scholar]
- 19.Krych, M., D. Hourcade, and J. P. Atkinson. 1991. Sites within the complement C3b/C4b receptor important for the specificity of ligand binding. Proc. Natl. Acad. Sci. U. S. A. 88:4353-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, J., D. T. Glover, A. J. Szalai, S. K. Hollingshead, and D. E. Briles. 2007. PspA and PspC minimize immune adherence and transfer of pneumococci from erythrocytes to macrophages through their effects on complement activation. Infect. Immun. 75:5877-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, J., A. J. Szalai, S. K. Hollingshead, M. H. Nahm, and D. E. Briles. 2009. Antibody to the type 3 capsule facilitates immune adherence of pneumococci to erythrocytes and augments their transfer to macrophages. Infect. Immun. 77:464-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musher, D. M. 1992. Infections caused by Streptococcus pneumoniae: clinical spectrum, pathogenesis, immunity, and treatment. Clin. Infect. Dis. 14:801-807. [DOI] [PubMed] [Google Scholar]
- 23.Nardin, A., M. A. Lindorfer, and R. P. Taylor. 1999. How are immune complexes bound to the primate erythrocyte complement receptor transferred to acceptor phagocytic cells? Mol. Immunol. 36:827-835. [DOI] [PubMed] [Google Scholar]
- 24.Nelson, R. A., Jr. 1953. The immune-adherence phenomenon; an immunologically specific reaction between microorganisms and erythrocytes leading to enhanced phagocytosis. Science 118:733-737. [DOI] [PubMed] [Google Scholar]
- 25.Pemberton, M., G. Anderson, V. Vetvicka, D. E. Justus, and G. D. Ross. 1993. Microvascular effects of complement blockade with soluble recombinant CR1 on ischemia/reperfusion injury of skeletal muscle. J. Immunol. 150:5104-5113. [PubMed] [Google Scholar]
- 26.Ren, B., A. J. Szalai, S. K. Hollingshead, and D. E. Briles. 2004. Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface. Infect. Immun. 72:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Repik, A., S. E. Pincus, I. Ghiran, A. Nicholson-Weller, D. R. Asher, A. M. Cerny, L. S. Casey, S. M. Jones, S. N. Jones, N. Mohamed, L. B. Klickstein, G. Spitalny, and R. W. Finberg. 2005. A transgenic mouse model for studying the clearance of blood-borne pathogens via human complement receptor 1 (CR1). Clin. Exp. Immunol. 140:230-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tas, S. W., L. B. Klickstein, S. F. Barbashov, and A. Nicholson-Weller. 1999. C1q and C4b bind simultaneously to CR1 and additively support erythrocyte adhesion. J. Immunol. 163:5056-5063. [PubMed] [Google Scholar]
- 29.Tu, A. H., R. L. Fulgham, M. A. McCrory, D. E. Briles, and A. J. Szalai. 1999. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect. Immun. 67:4720-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson, J. G., E. E. Murphy, W. W. Wong, L. B. Klickstein, J. H. Weis, and D. T. Fearon. 1986. Identification of a restriction fragment length polymorphism by a CR1 cDNA that correlates with the number of CR1 on erythrocytes. J. Exp. Med. 164:50-59. [DOI] [PMC free article] [PubMed] [Google Scholar]