Abstract
Gram-positive bacteria are the predominant cause of skin infections. Antimicrobial peptides (AMPs) are believed to be of major importance in skin's innate defense against these pathogens. This study aimed at providing clinical evidence for the contribution of AMP inducibility to determining the severity of Gram-positive skin infection. Using real-time PCR, we determined the induction of human β-defensin 2 (HBD-2), HBD-3, and RNase 7 by comparing healthy and lesional mRNA levels in 32 patients with Gram-positive skin infection. We then examined whether AMP induction differed by disease severity, as measured by number of recurrences and need for surgical drainage in patients with Staphylococcus aureus-positive lesions. We found that HBD-2 and -3, but not RNase 7, mRNA expression was highly induced by Gram-positive bacterial infection in otherwise healthy skin. Less induction of HBD-3, but not HBD-2, was associated with more-severe S. aureus skin infection: HBD-3 mRNA levels were 11.4 times lower in patients with more than 6 recurrences (P = 0.01) and 8.8 times lower in patients reporting surgical drainage (P = 0.01) than in the respective baseline groups. This suggests that inducibility of HBD-3 influences the severity of Gram-positive skin infection in vivo. The physiological function of HBD-2 induction in this context remains unclear.
Gram-positive bacteria, in particular Staphylococcus aureus and group A streptococci (GAS), are by far the most common cause of skin infections (3). Human skin expresses a variety of antimicrobial peptides (AMPs), which are believed to be of major importance in innate defense against pathogens (21, 25). These structurally diverse molecules are currently best categorized into the following two major classes: (i) constitutively expressed AMPs, such as RNase 7, which are present at high levels in healthy skin under physiological conditions; and (ii) inducible AMPs, such as human β-defensin 2 (HBD-2) and HBD-3, which are found at low concentrations in healthy skin but can be upregulated by inflammatory and bacterial stimuli.
Our current understanding of the role of AMPs in preventing and limiting human Gram-positive skin infections is based largely on studies demonstrating their in vitro antibacterial activity, their content in healthy and inflamed skin, and their expression in cultured keratinocytes after exposure to bacteria, bacterial components, and proinflammatory cytokines (1, 4-8, 11, 12, 16, 18, 22, 24). Direct evidence from in vivo studies supporting the involvement of AMPs in defense of human skin against these infections is scarce. Attempts to clarify AMP function by comparing patients with atopic dermatitis (AD) and psoriasis and attributing the known higher propensity of AD patients for S. aureus colonization and infection to differences in AMP expression have led to conflicting results (2, 15). Hence, there is a need to conduct studies of individuals with otherwise healthy skin to elucidate the clinical role of AMPs in Gram-positive skin infection.
The strikingly high in vitro activities of RNase 7 and HBD-3 against S. aureus and GAS (6, 8, 10) suggest a particular function of these AMPs in cutaneous defense against these clinically important pathogens. Recent work by our group provided strong evidence for a key role of constitutively expressed RNase 7 in protecting healthy skin against S. aureus infection in vivo (26). For the inducible AMPs HBD-2 and -3, there was no association of constitutive, noninduced mRNA expression with S. aureus skin infection (26). Whether induced expression of these AMPs is clinically important in defense against Gram-positive skin infections is still not known, although this has been hypothesized widely (1, 25). In a recent review, for instance, Schröder and Harder speculated that “recurrent skin infections may be associated with a dysregulation of antimicrobial peptide and protein production caused by lack of induction” (21). In particular, the high in vitro activity of HBD-3 against S. aureus suggests that interindividual differences in its inducibility may explain some of the variability in presentation and course of skin infections caused by this pathogen, which range from superficial self-limiting disease to deep-seated and recurrent furunculosis (19, 20).
In this study, we investigated the induction of RNase 7, HBD-2, and HBD-3 in previously healthy individuals suffering from skin infection caused by S. aureus and GAS. Inducibility, or the skin's capacity to increase AMP expression in response to infection, was approximated by observed AMP induction and was defined accordingly, as the difference between the lesional mRNA concentration and the baseline concentration in healthy skin. To explore the potential role of AMP inducibility in preventing and limiting S. aureus infection of the skin, we studied whether AMP induction was associated with two clinically relevant measures of disease severity, namely, the need for surgical drainage and the number of recurrences.
MATERIALS AND METHODS
Patients.
Study subjects consisted of otherwise healthy individuals consulting the outpatient clinic at the Institute of Tropical Medicine in Tübingen, Germany, with travel-related conditions of the skin. Enrolment took place between September 2007 and October 2008. Using a 4- by 6-mm biopsy punch, we obtained specimens of lesional and healthy skin from patients presenting with purulent skin lesions. Biopsy specimens were taken from the most-central epithelialized part of the lesion and from the lateral gluteal region, respectively. Some subjects, however, provided only single biopsy specimens of either lesional or healthy skin due to restricted patient consent or anatomical regions unsuitable for biopsy. Before the skin specimens were taken, lesional and nasal swabs were obtained and standard methods were applied for bacterial culture. Only biopsy specimens from individuals with swabs positive for S. aureus or GAS were included in further analyses. Other exclusion criteria were preexisting or concurrent skin disease (e.g., atopic dermatitis or psoriasis), immunodeficiency, and other conditions predisposing patients to skin infection and traumatic or chronic wounds. We used a standardized questionnaire to obtain information on the history of recurrences and need for surgical drainage. Number of recurrences was defined as the number of episodes of similar clinical appearance after complete remission of the primary lesion. Surgical drainage was defined as the use of any instrumentation for the drainage of pus. We also recorded information on allergies, defined as hypersensitivity to an environmental antigen. Patients positive for S. aureus in the nasal swab were considered nasal carriers.
Laboratory procedures.
Skin specimens were stored for 24 h in an RNA stabilization reagent (RNAlater; Ambion) and then frozen at −80°C until analysis. RNA was extracted with an RNeasy lipid tissue miniprep kit (Qiagen) and dissolved in diethyl pyrocarbonate (DEPC)-treated RNase-free water (Invitrogen), and its concentration was measured. Real-time PCR and cDNA synthesis were performed in one step, using a 2× SensiMix one-step kit (Peqlab) and a Rotor-Gene real-time PCR cycler (Corbett). All samples were measured in triplicate, and results were normalized to the expression of the β-actin housekeeping gene. Intron-spanning primers with the following sequences were used for amplification (Biomers.net): RNase 7 forward, 5′-GAAGACCAAGCGCAAAGC-3′; RNase 7 reverse, 5′-CAGCAGAAGCAGCAGAAGG-3′; HBD-2 forward, 5′-TCAGCCATGAGGGTCTTGTA-3′; HBD-2 reverse, 5′-GGATCGCCTATACCACCAAA-3′; HBD-3 forward, 5′-TTGCTCTTCCTGTTTTTGGTG-3′; HBD-3 reverse, 5′-CGCCTCTGACTCTGCAATAA-3′; β-actin forward, 5′-TTGTTACAGGAAGTCCCTTGCC-3′; and β-actin reverse, 5′-ATGCTATCACCTCCCCTGTGTG-3′. Real-time PCR was performed using 100 ng solved RNA in a 25-μl mixture containing reverse transcriptase, Taq polymerase, SYBR green, 5 μmol/liter β-actin primer, and either 7.5 μmol/liter RNase 7 primer or 5 μmol/liter HBD primer. Cycle conditions were 30 min at 49°C, 10 min at 95°C, and then 45 amplification cycles consisting of 15 s at 95°C, 20 s at 58°C or 60°C (for annealing of RNase 7 or HBD primers, respectively), and 15 s at 72°C. The specificity of the amplified product was verified by melting curve analysis. The cycle-to-cycle fluorescence emissions were analyzed using the threshold cycle (CT) method. ΔCT of a specific sample was calculated by subtracting the β-actin CT value from the AMP CT value, thus giving a measure of the relative expression of the target gene (17).
Statistical analysis.
Induction of AMPs was calculated by subtracting relative AMP expression in healthy skin from AMP expression in lesional skin (ΔΔCT inducibility = ΔCT lesional − ΔCT healthy).
Mean ΔΔCT values were converted into ratios that express the mRNA content in lesional skin specimens as a multiple of that in healthy skin. The equation used is based on the doubling of mRNA in each PCR cycle (17), i.e., the ratio was equal to 2−ΔΔCT.
We fitted a random-effects linear regression model that allowed us to include the data from those 15 patients who provided either a healthy or lesional skin sample but not both. Use of a patient-specific random effect takes account of the fact that AMP concentrations in samples from the same patient are likely to be correlated. The random-effects model combines information from comparing AMP levels in healthy and lesional skin within each patient and between patients. In order to examine whether induction differs between patients with more- and less-severe disease, we fitted an interaction term between skin type (lesional or healthy) and severity of disease, as measured by recurrences (three similarly sized categories) and need for surgical drainage (two categories). Age, nasal carriage of S. aureus, RNase 7 expression in healthy skin, and history of allergy were examined as potential confounders of the associations between AMP induction and disease severity. These analyses were carried out using the Stata xtmixed command (Stata software package, version 10.1; StataCorp) and were repeated, as a sensitivity analysis, with SAS proc mixed, using the small-sample correction (SAS software package, version 9.1; SAS).
Ethics.
The study protocol was reviewed and approved by the Ethics Committee, Faculty of Medicine, Eberhard Karls Universität, Tübingen, Germany. All individuals who participated gave written informed consent.
RESULTS
Thirty-two patients suffering from Gram-positive skin infection were enrolled in the study. Seventeen patients provided specimens of both healthy and lesional skin, 5 of lesional skin only, and 10 of healthy skin only. Six lesions were positive for GAS, and 26 lesions were positive for S. aureus. Furuncles and abscesses were the most frequent clinical presentation of S. aureus-positive lesions, while GAS were isolated exclusively from ecthyma. Information on recurrences and surgical drainage was missing for one and two patients, respectively. Table 1 summarizes clinical characteristics and number of biopsy specimens obtained by type of Gram-positive skin infection.
TABLE 1.
Characteristics of patients, stratified by type of Gram-positive bacterial skin infection
| Characteristic | Value |
||
|---|---|---|---|
| All patients with Gram-positive skin infection (n = 32) | Patients with GAS-positive skin infection (n = 6) | Patients with S. aureus-positive skin infection (n = 26) | |
| No. (%) of females | 16 (50) | 4 (67) | 12 (46) |
| Median (interquartile range) age (yr) | 28.5 (21-36.5) | 31 (24-34) | 27.5 (20-37) |
| No. (%) of patients with presentation of: | |||
| Furuncle/abscess | 19 (59) | 0 (0) | 19 (73) |
| Folliculitis | 3 (9) | 0 (0) | 3 (12) |
| Impetigo | 3 (9) | 0 (0) | 3 (12) |
| Ecthyma | 6 (19) | 6 (100) | 0 (0) |
| Cellulitis | 1 (3) | 0 (0) | 1 (4) |
| No. (%) of recurrences | 21 (68) | 0 (0) | 21 (84) |
| Mean (SD) no. of recurrencesa | 3.8 (3.9) | 0 (0) | 4.8 (3.8) |
| No. (%) of patients with history of: | |||
| Surgical drainageb | 16 (53) | 0 (0) | 16 (67) |
| S. aureus nasal carriagea | 22 (71) | 2 (33) | 20 (80) |
| Allergya | 4 (13) | 0 (0) | 4 (16) |
| No. of patients providing biopsy specimen(s) | |||
| Healthy and lesional skin | 17 | 5 | 12 |
| Healthy skin only | 10 | 0 | 10 |
| Lesional skin only | 5 | 1 | 4 |
Missing data for 1 subject.
Missing data for 2 subjects.
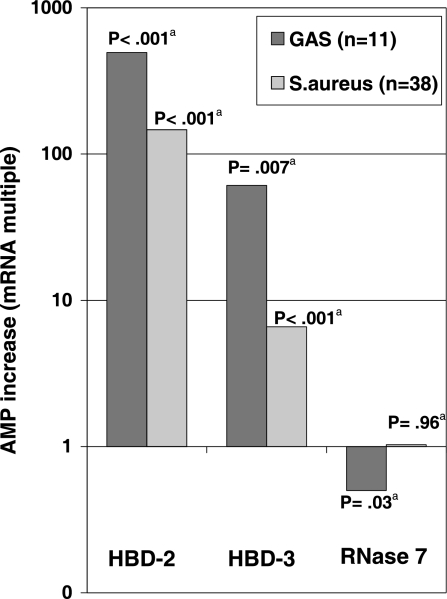
The concentrations of mRNA coding for HBD-2 were 496- and 147-fold higher in lesions caused by GAS and S. aureus, respectively, than in healthy skin. A less-pronounced induction was observed for HBD-3, with 60.8- and 6.6-fold increases in lesions. There was a tendency toward higher induction of β-defensins in GAS-positive lesions than in S. aureus-positive lesions. In contrast to the increase in expression found for HBD-2 and -3, the concentration of mRNA coding for RNase 7 remained unchanged in lesions caused by S. aureus and was only half the concentration in ecthymas caused by GAS compared to that in healthy skin. Table 2 and Fig. 1 present the mean induction of AMP expression by type of Gram-positive skin infection. It should be noted that data on AMP induction for 12 of 26 patients with S. aureus-positive lesions have been presented previously (26).
TABLE 2.
Induction of AMPs in Gram-positive bacterial skin infection
| AMP | mRNA ratio (95% CI) for GAS-positive infectiona | P valuec | mRNA ratio (95% CI) for S. aureus-positive infectionb | P valuec | P valued | P valuee |
|---|---|---|---|---|---|---|
| HBD-2 | 495.9 (202.6-1,213.8) | <0.001 | 147.0 (46.6-463.3) | <0.001 | 0.22 | <0.001 |
| HBD-3 | 60.8 (3.1-1,189.4) | 0.007 | 6.6 (3.0-14.2) | <0.001 | 0.043 | |
| RNase 7 | 0.50 (0.27-0.92) | 0.026 | 1.02 (0.51-1.90) | 0.96 | 0.36 |
Ratio = 2−meanΔΔCT for comparison of mRNA expression levels in 6 biopsy specimens of lesional skin versus 5 biopsy specimens of healthy skin.
Ratio = 2−meanΔΔCT for comparison of mRNA expression levels in 16 biopsy specimens of lesional skin versus 22 biopsy specimens of healthy skin.
Null hypothesis (H0), no induction of the AMP.
H0, inducibility of the AMP is the same for GAS and S. aureus.
H0, inducibility is the same for HBD-2 and HBD-3.
FIG. 1.
Induction of AMPs in Gram-positive bacterial skin infection. HBD, human β-defensin; GAS, group A streptococci; n, number of skin samples; a, test of null hypothesis of no difference in mRNA expression between healthy and lesional skin.
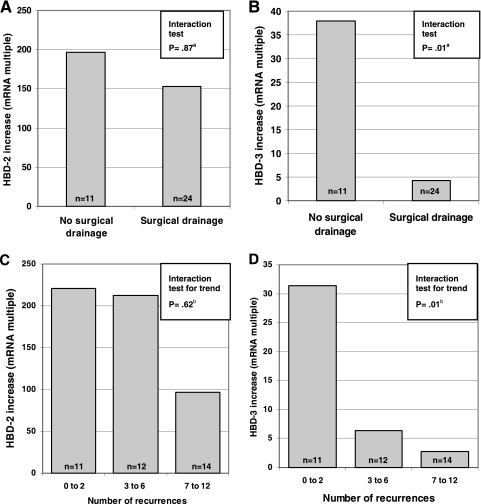
In comparing levels of AMP induction in skin specimens from patients with S. aureus-positive lesions by whether they had required surgical drainage, we found significantly lower mean HBD-3 mRNA levels among those who had undergone an intervention (P = 0.01): lesional HBD-3 mRNA concentrations were raised 4.3-fold, on average, above those in healthy skin for patients who had required drainage, while mean induction was 38-fold for patients without such a history (Fig. 2B). Despite a much more pronounced induction, no such association was present for HBD-2 (P = 0.87) (Fig. 2A). Examining differences in HBD-3 induction by number of recurrences showed a clear trend over the three categories (P = 0.01): induction was >30-fold among patients reporting 2 or fewer recurrences, 6.4-fold among those with 3 to 6 recurrences, and only 2.8-fold among patients reporting more than 6 recurrences (Fig. 2D). In contrast, induction of HBD-2 was not associated with the number of reported disease episodes (Fig. 2C). Table 3 summarizes AMP induction in S. aureus-positive skin infection by number of recurrences and history of surgical drainage. These results remained virtually unchanged when we repeated the analyses using the small-sample size correction and adjusting for potential confounders (data not shown).
FIG. 2.
Induction of antimicrobial peptides by history of surgical drainage (A and B) and recurrence group (C and D) in patients with S. aureus skin infection. n, number of skin samples; a, test of null hypothesis of induction being the same in both groups against alternative hypothesis of a difference between groups; b, test of null hypothesis of induction being the same in each group against alternative hypothesis of a linear trend over groups.
TABLE 3.
Induction of AMPs in S. aureus-positive skin infection, stratified by severity
| Clinical characteristic | HBD-3 |
HBD-2 |
RNase 7 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ratioa | 95% CI | P valueb | P valuec | Ratioa | 95% CI | P valueb | P valuec | Ratioa | 95% CI | P valueb | P valuec | |
| No. of recurrences | ||||||||||||
| 0-2 | 31.4 | 8.5-116.4 | <0.001 | 0.012 | 220.9 | 19.6-2,487.5 | <0.001 | 0.62 | 1.96 | 0.50-7.66 | 0.33 | 0.36 |
| 3-6 | 6.4 | 2.1-19.3 | 0.001 | 212.6 | 27.3-1,653.1 | <0.001 | 0.70 | 0.22-2.20 | 0.54 | |||
| >6 | 2.8 | 1.0-7.7 | 0.050 | 96.6 | 14.3-653.6 | <0.001 | 1.31 | 0.26-2.23 | 0.63 | |||
| Drainage | ||||||||||||
| No | 37.9 | 8.9-161.1 | <0.001 | 0.011 | 196.4 | 15.6-2476.9 | <0.001 | 0.87 | 1.69 | 0.45-6.33 | 0.44 | 0.42 |
| Yes | 4.3 | 1.8-10.2 | 0.001 | 152.9 | 33.3-701.5 | <0.001 | 1.11 | 0.41-1.99 | 0.80 | |||
Ratio = 2−meanΔΔCT for comparison of mRNA expression levels in 15 biopsy specimens of lesional skin versus 22 biopsy specimens of healthy skin (for number of recurrences) and in 14 biopsy specimens of lesional skin versus 21 biopsy specimens of healthy skin (drainage).
H0, no induction in this group.
H0, induction is the same in each group, tested against alternative hypothesis of a linear trend over groups (recurrences) or a difference between groups (drainage).
For clarification, note that the statistically nonsignificant decrease in HBD-2 induction among patients reporting more than 6 recurrences (Fig. 2C) was the consequence of an increase in baseline HBD-2 expression in healthy skin in this group. The absolute mean lesional HBD-2 concentrations were approximately the same in all three recurrence categories.
DISCUSSION
This study demonstrates that infection with Gram-positive bacteria induces the transcription of genes coding for HBD-2 and HBD-3 in human skin. HBD-2 shows a more pronounced relative increase, which is at least in part a consequence of its much lower baseline transcription. These findings confirm results from various in vitro studies of cultured keratinocytes exposed to live and heat-inactivated S. aureus (5, 13) or to cell wall components of Gram-positive bacteria (12, 23) but are contradictory to results from one in vitro study that found GAS to be a poor inducer of HBD-2 (5). Our study adds to the evidence from other in vivo studies demonstrating a strong induction of HBD-2 by Gram-positive bacterial infection (16, 24). Our findings, in part presented earlier for a subgroup of patients with S. aureus skin infection (26), are the first, to our knowledge, to demonstrate induction of HBD-3 in response to Gram-positive skin infection in vivo.
We could not observe any induction of RNase 7 mRNA in skin by Gram-positive bacterial infection in vivo, in contrast to findings from in vitro studies describing its induction in keratinocytes (8) and, more recently, in cultured human skin (18) after stimulation with bacterial components or heat-inactivated Gram-positive bacteria. This indicates that these in vitro findings are unlikely to reflect AMP regulation under physiological conditions. Comparing levels of induction by type of infection showed that concentrations of mRNA coding for RNase 7 were virtually identical in S. aureus-positive lesions and healthy skin, while lesions caused by GAS showed AMP expression even below that of healthy skin. The reasons for the latter observation are unclear but may be attributable to the ulcerative character of ecthyma, the sole clinical presentation of GAS-positive lesions in this study. In summary, these results are in line with the primarily constitutive expression of RNase 7 (21) and its hypothesized main role in preventing clinically apparent infection by early elimination of inoculated pathogens (26).
We found a considerably lower induction of HBD-3 in patients suffering from deep-seated lesions that had required surgical drainage. Upregulation of HBD-3 was also less pronounced in patients who had experienced a large number of recurrent skin infections in the past. These results, in conjunction with its high activity against Gram-positive bacteria as well as previous findings from cultured keratinocytes and epidermis (6, 9, 10), suggest that HBD-3 plays an important role in defense against S. aureus. To our knowledge, this is the first study providing clinical evidence for the widely hypothesized function of inducible AMPs in determining the severity of Gram-positive skin infection (1, 25).
In contrast, the striking upregulation of HBD-2 in S. aureus-positive lesions did not differ by history of surgical drainage or recurrences, suggesting that induction of this AMP does not have a substantial influence on the clinical outcome of infection. This indicates that HBD-2 may have a function in response to S. aureus infection that is unrelated to direct pathogen elimination, a notion in line with its low in vitro activity against S. aureus (22) and with recent findings that β-defensins also act as proinflammatory mediators and promote wound healing (14). It follows that mere inducibility of an AMP by a pathogen or its components is not sufficient to support conclusions about its physiological importance in innate defense against that particular pathogen. Rather, an approach using information on the occurrence and clinical course of infection is required to answer this question.
While it is clear that the need for surgical drainage reflects the depth of a lesion and is thus a measure of the skin's capacity to prevent pathogen invasion into deeper layers, it is less intuitive to understand what recurrences represent. Recurrences are typical for lesions caused by S. aureus but not GAS. They usually occur at new sites after complete resolution of the primary lesion and are thought to be reinfections by manual spread of bacteria from colonized areas such as the nares into casual epidermal microlesions. Accordingly, decolonization is an advocated measure for treatment of recurrent furunculosis (3). Hence, in the context of this study, the number of recurrences is likely to be a measure of the skin's ability to prevent new infection by controlling small inocula of pathogens.
It seems paradoxical that inducibility in lesions should matter for protection from symptomatic infection in areas of healthy skin where expression has not been induced (and would not be induced fast enough in response to pathogen invasion to prevent clinically apparent infection). However, the existence of an HBD-3-dependent mechanism capable of eliminating S. aureus within 1 h of contact with keratinocytes could be demonstrated in vitro. It was proposed that liberation of HBD-3 from stores could explain its apparent independence from time-consuming gene transcription and translation (9, 10). It is thus conceivable that the amount of stored and quickly releasable HBD-3 peptide in healthy skin is somehow linked to the tissue's ability to induce HBD-3 mRNA transcription during symptomatic infection. Following this rationale, inducibility not only would be a direct measure of the skin's ability to increase AMP expression in lesions but also would be an indirect measure of its constitutive presence in healthy skin. The hypothesized concept of AMP stores would also explain the lack of association between constitutive HBD-3 mRNA levels in healthy skin and an individual's propensity toward S. aureus infection (26). To date, however, there have been neither studies further characterizing the proposed stores nor investigations into their relationship to gene expression.
Unlike other studies in the field, we considered a range of factors as potential confounders, including nasal carriage of S. aureus (a known risk factor for recurrent skin infections), history of allergy (to account for the recently identified influence of Th2 cytokines on HBD-3 expression [8a, 9]), RNase 7 expression in healthy skin (shown to be protective against S. aureus infection [26]), and age. None of these factors could explain the associations found, which supports the presence of a true link between HBD-3 inducibility and severity of S. aureus skin infections.
Exposure and outcome measurements were done simultaneously, thus raising the question of reverse causality. In fact, it has been shown for HBD-2 that a lesion can cause upregulation even in nonaffected skin via systemic release of inflammatory mediators (24). In our study, such an effect may be present to some degree for HBD-2, as expression in healthy skin was highest in patients with more than 6 recurrences. This may explain why this group showed a somewhat lower level of HBD-2 induction (Fig. 2C), despite having similar levels of absolute expression in lesional skin. For HBD-3 and RNase 7, however, we could not observe any systemic upregulation in healthy skin.
Recording the number of recurrences at a single visit is likely to have underestimated their number for some patients, as they may have experienced further episodes at a later time. Falsely including patients with a larger number of recurrences in a low-recurrence category would have made categories more similar in terms of AMP expression than they should be, which in turn would have led to an underestimation of the associations reported. Eliminating this misclassification would thus not invalidate our results but rather would strengthen them.
Finally, it would have been desirable to confirm our results on the peptide level. However, we were not able to perform such studies due to the scarcity of material and the need for immediate stabilization of RNA after biopsy in a reagent rendering the skin unsuitable for quantitative immunohistochemistry studies.
Summary and conclusions.
In summary, our findings indicate that (i) RNase 7 is not inducible by Gram-positive skin infection in vivo, (ii) HBD-2 and HBD-3 are inducible by Gram-positive skin infection in vivo, and (iii) higher inducibility of HBD-3, but not HBD-2, is associated with a more favorable clinical course and outcome of S. aureus skin infection. Combining these with other recent findings (9, 10, 26), we conclude that both RNase 7 and HBD-3 appear to be vital components of the skin's response repertoire against Gram-positive bacterial infection. The evidence to date suggests that limiting the extent of clinically overt disease involves HBD-3 induction, while susceptibility to infection is substantially influenced by constitutively expressed levels of RNase 7 as well as a constitutive mechanism related to the inducibility of HBD-3. The physiological function of HBD-2, however, remains to be elucidated. Further studies using clinical outcomes are warranted to clarify the role of inducible AMPs in defense against other pathogens.
Acknowledgments
Financial support was provided by the Programm für Angewandte Klinische Forschung (AKF), Eberhard Karls Universität, Tübingen, Germany (grant 204-0-0), the Interdisziplinäres Zentrum für Klinische Forschung (IZKF), Eberhard Karls Universität, Tübingen, Germany (grant 1596), and the Deutsche Forschungsgemeinschaft (SFB766).
We thank Karen Petersen, Ulrike Müller-Pinau, Ines Wanke, and Walter Deschle for their excellent technical assistance. Many thanks also go to Anna Goodman and Peter Kremsner for their helpful comments on the manuscript.
All authors declare that they have no commercial or other association that might pose a conflict of interest with regard to the content of the manuscript.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 19 April 2010.
REFERENCES
- 1.Ali, R. S., A. Falconer, M. Ikram, C. E. Bissett, R. Cerio, and A. G. Quinn. 2001. Expression of the peptide antibiotics human beta defensin-1 and human beta defensin-2 in normal human skin. J. Invest. Dermatol. 117:106-111. [DOI] [PubMed] [Google Scholar]
- 2.Asano, S., Y. Ichikawa, T. Kumagai, M. Kawashima, and G. Imokawa. 2008. Microanalysis of an antimicrobial peptide, beta-defensin-2, in the stratum corneum from patients with atopic dermatitis. Br. J. Dermatol. 159:97-104. [DOI] [PubMed] [Google Scholar]
- 3.Bernard, P. 2008. Management of common bacterial infections of the skin. Curr. Opin. Infect. Dis. 21:122-128. [DOI] [PubMed] [Google Scholar]
- 4.Chronnell, C. M., L. R. Ghali, R. S. Ali, A. G. Quinn, D. B. Holland, J. J. Bull, W. J. Cunliffe, I. A. McKay, M. P. Philpott, and S. Muller-Rover. 2001. Human beta defensin-1 and -2 expression in human pilosebaceous units: upregulation in acne vulgaris lesions. J. Invest. Dermatol. 117:1120-1125. [DOI] [PubMed] [Google Scholar]
- 5.Dinulos, J. G., L. Mentele, L. P. Fredericks, B. A. Dale, and G. L. Darmstadt. 2003. Keratinocyte expression of human beta defensin 2 following bacterial infection: role in cutaneous host defense. Clin. Diagn. Lab. Immunol. 10:161-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harder, J., J. Bartels, E. Christophers, and J. M. Schröder. 2001. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 276:5707-5713. [DOI] [PubMed] [Google Scholar]
- 7.Harder, J., J. Bartels, E. Christophers, and J. M. Schröder. 1997. A peptide antibiotic from human skin. Nature 387:861. [DOI] [PubMed] [Google Scholar]
- 8.Harder, J., and J. M. Schröder. 2002. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J. Biol. Chem. 277:46779-46784. [DOI] [PubMed] [Google Scholar]
- 8a.Howell, M. D., M. Boguniewicz, S. Pastore, N. Novak, T. Bieber, G. Girolomoni, and D. Y. Leung 2006. Mechanism of HBD-3 deficiency in atopic dermatitis. Clin. Immunol. 121:332-338. [DOI] [PubMed] [Google Scholar]
- 9.Kisich, K. O., C. W. Carspecken, S. Fieve, M. Boguniewicz, and D. Y. Leung. 2008. Defective killing of Staphylococcus aureus in atopic dermatitis is associated with reduced mobilization of human beta-defensin-3. J. Allergy Clin. Immunol. 122:62-68. [DOI] [PubMed] [Google Scholar]
- 10.Kisich, K. O., M. D. Howell, M. Boguniewicz, H. R. Heizer, N. U. Watson, and D. Y. Leung. 2007. The constitutive capacity of human keratinocytes to kill Staphylococcus aureus is dependent on beta-defensin 3. J. Invest. Dermatol. 127:2368-2380. [DOI] [PubMed] [Google Scholar]
- 11.Menzies, B. E., and A. Kenoyer. 2006. Signal transduction and nuclear responses in Staphylococcus aureus-induced expression of human beta-defensin 3 in skin keratinocytes. Infect. Immun. 74:6847-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menzies, B. E., and A. Kenoyer. 2005. Staphylococcus aureus infection of epidermal keratinocytes promotes expression of innate antimicrobial peptides. Infect. Immun. 73:5241-5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Midorikawa, K., K. Ouhara, H. Komatsuzawa, T. Kawai, S. Yamada, T. Fujiwara, K. Yamazaki, K. Sayama, M. A. Taubman, H. Kurihara, K. Hashimoto, and M. Sugai. 2003. Staphylococcus aureus susceptibility to innate antimicrobial peptides, beta-defensins and CAP18, expressed by human keratinocytes. Infect. Immun. 71:3730-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niyonsaba, F., H. Ushio, N. Nakano, W. Ng, K. Sayama, K. Hashimoto, I. Nagaoka, K. Okumura, and H. Ogawa. 2007. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J. Invest. Dermatol. 127:594-604. [DOI] [PubMed] [Google Scholar]
- 15.Ong, P. Y., T. Ohtake, C. Brandt, I. Strickland, M. Boguniewicz, T. Ganz, R. L. Gallo, and D. Y. Leung. 2002. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 347:1151-1160. [DOI] [PubMed] [Google Scholar]
- 16.Oono, T., W. K. Huh, Y. Shirafuji, H. Akiyama, and K. Iwatsuki. 2003. Localization of human beta-defensin-2 and human neutrophil peptides in superficial folliculitis. Br. J. Dermatol. 148:188-191. [DOI] [PubMed] [Google Scholar]
- 17.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reithmayer, K., K. C. Meyer, P. Kleditzsch, S. Tiede, S. K. Uppalapati, R. Glaser, J. Harder, J. M. Schröder, and R. Paus. 2009. Human hair follicle epithelium has an antimicrobial defence system that includes the inducible antimicrobial peptide psoriasin (S100A7) and RNase 7. Br. J. Dermatol. 161:78-89. [DOI] [PubMed] [Google Scholar]
- 19.Roberts, S., and S. Chambers. 2005. Diagnosis and management of Staphylococcus aureus infections of the skin and soft tissue. Intern. Med. J. 35(Suppl. 2):S97-S105. [DOI] [PubMed] [Google Scholar]
- 20.Schleucher, R. D., M. Gaessler, and J. Knobloch. 2008. Panton-Valentine leukocidin-producing methicillin-sensitive Staphylococcus aureus as a cause for recurrent, contagious skin infections in young, healthy travelers returned from a tropical country: a new worldwide public health problem? J. Travel Med. 15:137-139. [DOI] [PubMed] [Google Scholar]
- 21.Schröder, J. M., and J. Harder. 2006. Antimicrobial skin peptides and proteins. Cell. Mol. Life Sci. 63:469-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schröder, J. M., and J. Harder. 1999. Human beta-defensin-2. Int. J. Biochem. Cell Biol. 31:645-651. [DOI] [PubMed] [Google Scholar]
- 23.Sørensen, O. E., D. R. Thapa, A. Rosenthal, L. Liu, A. A. Roberts, and T. Ganz. 2005. Differential regulation of beta-defensin expression in human skin by microbial stimuli. J. Immunol. 174:4870-4879. [DOI] [PubMed] [Google Scholar]
- 24.Stryjewski, M. E., R. P. Hall, V. H. Chu, Z. A. Kanafani, W. D. O'Riordan, M. S. Weinstock, R. S. Stienecker, R. Streilein, R. A. Dorschner, V. G. Fowler, Jr., G. R. Corey, and R. L. Gallo. 2007. Expression of antimicrobial peptides in the normal and involved skin of patients with infective cellulitis. J. Infect. Dis. 196:1425-1430. [DOI] [PubMed] [Google Scholar]
- 25.Yamasaki, K., and R. L. Gallo. 2008. Antimicrobial peptides in human skin disease. Eur. J. Dermatol. 18:11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zanger, P., J. Holzer, R. Schleucher, H. Steffen, B. Schittek, and S. Gabrysch. 2009. Constitutive expression of the antimicrobial peptide RNase 7 is associated with Staphylococcus aureus infection of the skin. J. Infect. Dis. 200:1907-1915. [DOI] [PubMed] [Google Scholar]