Abstract
Lipopolysaccharide (LPS), a major component of the meningococcal outer membrane, is sensed by the host through activation of Toll-like receptor 4 (TLR4). Recently, we demonstrated that a surprisingly large fraction of Neisseria meningitidis disease isolates are lipid A mutants, due to inactivating mutations in the lpxL1 gene. The lpxL1 mutants activate human TLR4 much less efficiently than wild-type bacteria, which may be advantageous by allowing them to escape from the innate immune system. Here we investigated the influence of lipid A structure on virulence in a mouse model of meningococcal sepsis. One limitation, however, is that murine TLR4 recognizes lpxL1 mutant bacteria much better than human TLR4. We show that an lpxL2 mutant, another lipid A mutant lacking an acyl chain at a different position, activates murine TLR4 less efficiently than the lpxL1 mutant. Therefore, the lpxL2 mutant in mice might be a better model for infections with lpxL1 mutants in humans. Interestingly, we found that the lpxL2 mutant is more virulent in mice than the wild-type strain, whereas the lpxL1 mutant is actually much less virulent than the wild-type strain. These results demonstrate the crucial role of N. meningitidis lipid A structure in virulence.
Lipopolysaccharide (LPS) is a major component of the outer membranes of virtually all Gram-negative bacteria (12). Many species contain a common form that consists of a highly variable surface-exposed polysaccharide and a more conserved lipid A portion that anchors the molecule to the bacterial outer membrane. It has been known for a long time that LPS has a wide variety of biological activities even at very low concentrations. These properties gave LPS its alternative name, “endotoxin.” The anticipated receptor for mediating the biological effects of LPS remained elusive for many years (6). Now we know that lipid A, the bioactive component of the LPS, is recognized by the innate immune system through Toll-like receptor 4 (TLR4). Lipopolysaccharide binding protein (LBP) and CD14 facilitate the transfer of LPS to the coreceptor MD-2 (18). Binding of LPS to the TLR4/MD-2 receptor complex leads to dimerization of its extracellular domains and recruitment of adaptor proteins to the intracellular domains. This triggers a signaling cascade and subsequent activation of the innate immune system (33). Among the Toll-like receptors, TLR4 is unique in that it utilizes both adaptor proteins MyD88 and TRIF. Activation of these proteins eventually leads to induction of proinflammatory cytokines and type I interferon (IFN), respectively. TLR4 is important for protection against many Gram-negative bacterial pathogens (3, 5, 40, 47, 54).
Several studies have shown that the prototypical Escherichia coli lipid A with six acyl chains of 12 to 14 carbons in length gives optimal activation of human TLR4, whereas changing the number or the length of the acyl chains or altering the charge of the lipid A reduces the activity of LPS (38, 39, 43). In addition, there are species-specific differences in recognition of lipid A by TLR4/MD-2 (1, 22, 27, 35). The murine TLR4/MD-2 complex seems to be more promiscuous than human TLR4/MD-2 in recognizing various lipid A molecules, but also for murine TLR4/MD-2 recognition, lipid A with six acyl chains is optimal.
Lipid A was once thought to be an invariant component, but recent studies have shown that the variety in lipid A structures is actually quite large. These structural alterations have an impact on how well LPS is sensed by the host. Therefore, it seems that bacteria have evolved ways to manipulate the pathogen-associated molecular pattern recognized by the host innate immune system to modulate host interactions to their benefit. For example, Salmonella and Pseudomonas species can modulate their lipid A structure in response to the host environment (13, 14, 20, 22, 24, 25). Other human pathogens, such as Helicobacter pylori, Legionella pneumophila, Yersinia pestis, and Francisella spp., also have lipid A moieties that are poorly recognized by human TLR4, which likely contributes to their ability to cause disease in humans (28).
The Gram-negative bacterium Neisseria meningitidis is a frequent commensal of the human upper respiratory tract (46). Occasionally the bacterium becomes invasive, causing the serious conditions meningitis and/or sepsis in otherwise healthy individuals. Meningococcal lipid A consists of six acyl chains and is highly biologically active. It is a major contributor to the induction of the excessive proinflammatory responses seen in meningococcal disease (23, 51). Moreover, levels of circulating N. meningitidis LPS are directly correlated to the morbidity and mortality of meningococcal sepsis (7, 8).
Recently, we demonstrated that a surprisingly large fraction (about 9%) of meningococcal disease isolates have lipid A with only five acyl chains, due to inactivating mutations in the acyl-transferase gene lpxL1 (17). These LPS mutants induced much lower levels of proinflammatory cytokines in different cell types, and this effect was TLR4 dependent. The high frequency of the lpxL1 mutants suggests there must be a benefit for the bacterium to have underacylated lipid A under certain conditions. A reasonable hypothesis would be that the lpxL1 mutants are better capable of evading the innate immune defenses of the host because of the reduced capability of their lipid A to activate TLR4.
In this study we wanted to compare the virulence of the N. meningitidis lpxL1 mutant with that of the wild-type strain in a mouse model of meningococcal sepsis. However, while lpxL1 LPS is a poor activator of human TLR4/MD-2, it is still a significant activator of murine TLR4/MD-2 (45). Therefore, we also included an lpxL2 mutant in our study; this mutant is defective in the other gene required for the addition of a secondary acyl chain to the lipid A moiety (50). Here we show that the lpxL2 mutant is a weaker activator of murine TLR4/MD-2 than the lpxL1 mutant. Interestingly, the lpxL2 mutant was much more virulent in the mouse model of meningococcal sepsis than the wild-type strain. On the other hand, the lpxL1 mutant was completely avirulent. These results demonstrate the importance of the N. meningitidis lipid A structure for virulence.
MATERIALS AND METHODS
Animals.
Female specific-pathogen-free C57BL/6JIco mice were purchased from Charles River Laboratories and were housed under specific-pathogen-free conditions. Mice were acclimatized for approximately 1 week and were 6 to 8 weeks old at the start of the experiment. Animal experiments were approved by the Netherlands Vaccine Institute's Animal Ethics Committee.
Bacterial strains and growth conditions.
The lpxL1 and lpxL2 mutants were generated from the parent strain H44/76 (immunotype L8) as described previously (50). The construction of the lpxA mutant is also described elsewhere (44). Strains were grown on GC medium base (Difco Laboratories) supplemented with IsoVitaleX (Becton Dickinson) overnight at 37°C in 5% CO2 in a humid atmosphere. For stimulation of cell lines, bacteria were suspended from plates in phosphate-buffered saline (PBS) and the A620 was determined. Next, the bacteria were heat inactivated at 56°C for 30 min. For infection experiments, bacteria were suspended from plates in tryptic soy broth (TSB) (Becton Dickinson) at an A620 of approximately 0.075 and grown at 37°C to log phase (A620 of 0.2 to 0.3). To determine CFU, serial dilutions of bacterial suspensions in PBS were plated on GC agar and grown overnight at 37°C in 5% CO2. The next day, the number of colonies was counted. LPS from these strains was isolated by the hot-phenol extraction method as described previously (53).
Outer membrane profile analysis.
N. meningitidis strains L8 H44/76, L8 lpxL1, and L8 lpxL2 were grown in TSB liquid medium at 37°C to late log phase, and then the bacteria were heat inactivated at 56°C for 1 h. These were used for isolation of outer membrane complexes by sarcosyl extraction as described previously (49). The quantity of protein was determined with the bicinchoninic acid protein assay reagent (Pierce), with bovine serum albumin as a standard. Protein profiles were analyzed by SDS-PAGE. Gels were stained with Coomassie blue.
In vitro growth of bacteria in whole mouse blood.
Whole blood from C57BL/6JIco mice was collected in 1-ml lithium heparin tubes (Greiner Bio-One) and pooled. Strain H44/76 and the lpxL1 and lpxL2 mutants were grown until log phase as described above and diluted to 1 × 108 CFU/ml in RPMI 1640 medium (Gibco BRL). Next, bacteria where suspended in 1 ml whole mouse blood at a final concentration of 1 × 103 CFU/ml or 1 × 105 CFU/ml and incubated for 4 h at 37°C and 80 rpm in a humid atmosphere. Each hour a sample of 10 μl was taken and serial dilutions in RPMI were made, which were plated on GC agar and grown overnight at 37°C in 5% CO2. The next day, the numbers of colonies on the plates were determined to calculate the CFU in the samples. This was also done for the original stock of bacteria at 1 × 108 CFU/ml in RPMI to determine the actual number of bacteria that was used.
Cell lines.
For experiments and/or maintenance, J774A.1 mouse macrophage cells were suspended in Iscove modified Dulbecco medium (IMDM) (Gibco BRL) supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin, 300 μg/ml l-glutamine (Gibco BRL), and 10% heat-inactivated fetal calf serum (FCS) (Gibco BRL). For experiments with and maintenance of HEK-293 cells stably transfected with mouse TLR4A, MD-2, and CD14 (Invivogen), Dulbecco modified Eagle medium (DMEM) (Gibco BRL) was used, supplemented with 10% FCS, 10 μg/ml blasticidin (Invivogen), and 50 μg/ml hygromycin B (Invivogen).
ELISA.
J774A.1 or HEK-293 mTLR4/MD-2/CD14 cells were seeded in 96-well flat-bottom plates at 2 × 105 to 3 × 105 cells in 200 to 300 μl per well. Cells were stimulated with heat-inactivated bacteria or purified LPS and incubated overnight at 37°C in a humidified atmosphere containing 5% CO2. Cytokine concentrations in the culture supernatants were quantified by enzyme-linked immunosorbent assay (ELISA). Mouse interleukin-6 (IL-6) was determined with the mouse IL-6 ELISA set (BD Biosciences), mouse interferon-inducible protein 10 (IP-10) with the mouse IP-10 ELISA kit (R&D Systems), and human IL-8 with PeliPair reagent sets (Sanquin).
Animal model.
Female C57BL/6JIco mice 6 to 8 weeks old were randomly distributed in groups of three. Animals were injected intraperitoneally (i.p.) with 100 μl of 20-mg/ml iron-dextran (Sigma) in PBS a few hours before infection and again 24 h later. Strain H44/76 and the lpxL1 and lpxL2 mutants were grown to log phase as described above and diluted to the desired dose in PBS. Mice were injected i.p. with 100 μl of bacterial suspension. Four different doses of each strain were used: 1 × 104, 1 × 105, 1 × 106, and 1 × 107 CFU per mouse. Animal health was monitored twice a day over a period of 48 h, and a health score was assigned to each mouse at each time point as follows: healthy, 0; slightly ruffled fur, 1; ruffled fur and active, 2; ruffled fur and inactive, 3; ruffled fur, inactive, and crouched, 4; very sick, not eating or drinking, and no movement after stimulation, 5; and death, 6. Mice with a score of 5 were killed to limit suffering. At 2 h and 19 h after infection, blood samples (50 μl) were taken from the tail vein and collected in 1 ml lithium heparin tubes (Greiner Bio-One). Serial dilutions in PBS were plated to determine CFU. The remaining blood was separated by centrifugation to obtain the plasma, which was centrifuged again with a 0.22-μm Ultrafree-MC filter (Millipore) to eliminate possible remaining bacteria. Plasma was stored at −20°C for later use.
Determination of cytokine levels in plasma.
Plasma samples of mice at 2 h and 19 h after infection were analyzed using a Bio-Plex system (Bio-Rad) for determination of cytokines. A six-plex Bio-Plex assay (Bio-Rad) containing beads for mouse IL-1β, IL-6, IL-10, IL-12p70, RANTES, and tumor necrosis factor alpha (TNF-α) was used.
Statistical analysis.
Before statistical analysis, data from CFU values were log10 converted, which normalized their distribution. One-way analysis of variance (ANOVA) was performed, followed by the post hoc Bonferroni multiple-comparison test to analyze differences in means. Differences in survival were tested with the Fischer exact test (GraphPad Prism 4). For each dose, H44/76 was compared with the lpxL1 and lpxL2 mutants. Differences were considered significant at a P value of <0.05.
RESULTS
Characterization of the lpxL mutants.
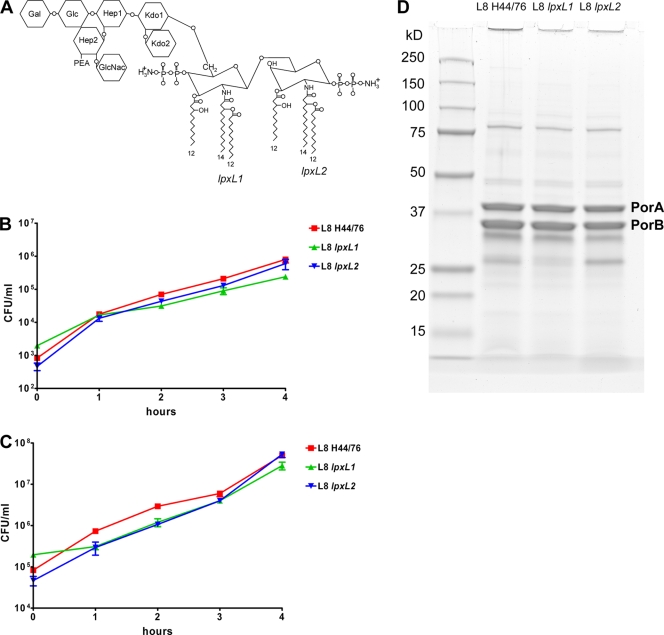
The lpxL1 and lpxL2 mutants were generated in strain H44/76 (immunotype L8). Their LPS structures are shown in Fig. 1 A. The lpxL1 mutant has penta-acylated lipid A missing the secondary acyl chain at the 2′ position. The lpxL2 mutant also has penta-acylated lipid A but lacks the secondary acyl chain at the 2 position. Lipid A was directly extracted from whole bacteria by the ammonium-isobutyrate method and analyzed by nanoelectrospray tandem mass spectrometry to verify the mutant structures (17). Both the L8 lpxL1 LPS and the L8 lpxL2 LPS were found to have >95% penta-acyl lipid A (results not shown). The growth of the lpxL1 and lpxL2 mutants in whole blood of C57BL/6 mice was compared to that of the parental strain. The blood was incubated with two doses of each strain, 1 × 103 CFU/ml and 1 × 105 CFU/ml, and the numbers of bacteria in the blood after 1, 2, 3, and 4 h were determined (Fig. 1B and C). For both doses, growth was very similar for all strains in whole blood. Finally, the outer membrane protein profiles of the different strains were compared (Fig. 1D). Minor differences in the expression of Opa proteins were visible; however, in our experience the presence or absence of particular Opa proteins does not affect virulence in the i.p. mouse model (G. P. J. M. van den Dobbelsteen, unpublished results). Overall, the outer membrane profiles did not show major differences, suggesting that the lpxL mutations did not have unanticipated effects on the expression of proteins possibly involved in the virulence of N. meningitidis.
FIG. 1.
Characterization of N. meningitidis strains L8 H44/76, L8 lpxL1, and L8 lpxL2. (A) Schematic structure of LPS from the wild-type strain H44/76 (immunotype L8). The acyl chains missing in the lpxL1 and lpxL2 mutants are indicated with green and blue, respectively. (B and C) Growth of strain H44/76 and the lpxL1 and lpxL2 mutants in whole blood from C57BL/6JIco mice. Results of one of two independent experiments are shown. Data are expressed as means of triplicates, and error bars indicate standard errors of the means (SEM). The outcome for an initial dose of approximately 1 × 103 CFU/ml is shown in panel B and that for an initial dose of approximately 1 × 105 CFU/ml in panel C. (D) Outer membrane complex proteins of strain H44/76 and the lpxL1 and lpxL2 mutants separated by SDS-PAGE and stained with Coomassie blue.
In vitro biological activity of lpxL mutants.
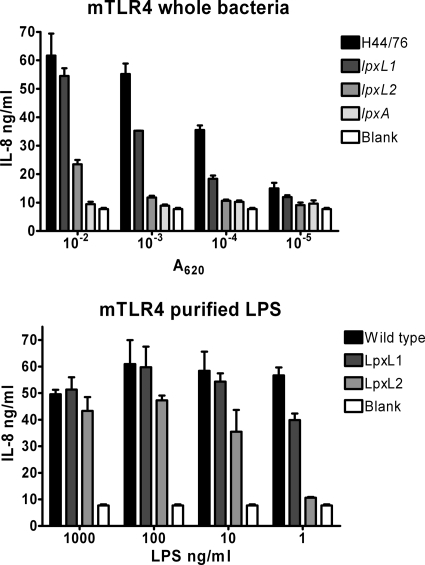
HEK293 cells stably transfected with murine TLR4, MD-2, and CD14 were stimulated with different doses of heat-inactivated whole bacterial strains: H44/76, the lpxL1 mutant, the lpxL2 mutant, and the lpxA mutant, which is completely LPS deficient (44). In addition, purified wild-type LPS, LpxL1 LPS, and LpxL2 LPS were tested. Activation of the LPS receptor complex was assessed by measuring IL-8 production by the HEK293 cells with ELISA (Fig. 2). As expected, wild-type bacteria strongly activated the murine LPS receptor complex, while lpxA mutant bacteria did not activate the receptor complex at all. Compared to the wild-type strain, lpxL1 mutant bacteria were about 10-fold less efficient in activating murine TLR4/MD-2, and lpxL2 mutant bacteria were about 100-fold less efficient. Similar results were obtained with purified LPS from these strains (Fig. 2).
FIG. 2.
Comparison of murine TLR4 activation by the lpxL mutants and the wild-type strain. HEK293 cells transfected with mouse TLR4, MD-2, and CD14 were stimulated with different concentrations of heat-inactivated whole H44/76 bacteria, lpxL1 bacteria, lpxL2 bacteria, lpxA bacteria, or purified LPS from these strains. After overnight incubation, the IL-8 concentration in the supernatant was measured by ELISA. Results of one representative experiment of three independent experiments are shown. Data are expressed as means of triplicates, and error bars indicate SEM.
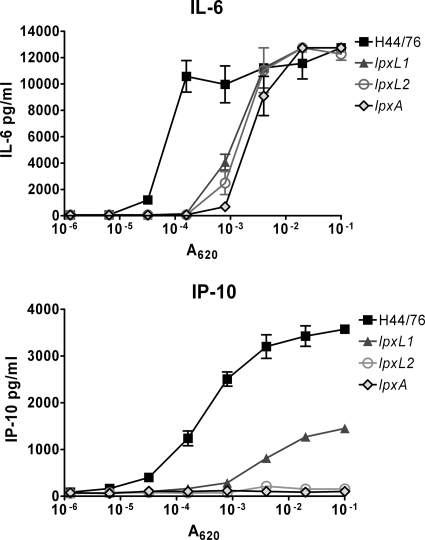
The bacteria also contain other components that can be recognized by pattern recognition receptors that contribute to the innate immune response (36). Therefore, we also investigated the influence of recognition of lpxL lipid A with immune cells that also express other pattern recognition receptors. The murine macrophage cell line J774A.1 was stimulated with titrations of the H44/76, lpxL1, lpxL2, or lpxA strain. We measured IL-6 and IP-10 induction (Fig. 3). IL-6 is induced by the MyD88-dependent pathway and can therefore also be induced by other TLRs, such as TLR2. In contrast, IP-10 is induced by the TRIF-dependent pathway. Therefore, LPS should be the only component of the bacteria capable of inducing IP-10. In agreement with this, differences between the lipid A mutants were most pronounced for IP-10, which was not induced at all by the lpxL2 and lpxA mutants and was induced about 25-fold less than the wild type by the lpxL1 mutant. For IL-6, all the lipid A mutants were less active than the wild type, but differences among them were small (Fig. 3). Taken together, these results demonstrate that the wild-type strain is the most potent activator of mouse immune cells and that the lpxL2 mutant is the weakest activator. As expected, these effects were TLR4 dependent.
FIG. 3.
Activation of J774A.1 cells with H44/76 and the lpxL1, lpxL2, and lpxA mutants. J774A.1 cells were stimulated overnight with titrations of heat-inactivated strains. Concentrations of IL-6 and IP-10 in the supernatant were determined by ELISA. Results of one representative experiment of three independent experiments are shown. Data are expressed as means of triplicates, and error bars indicate SEM.
Mouse model of meningococcal sepsis.
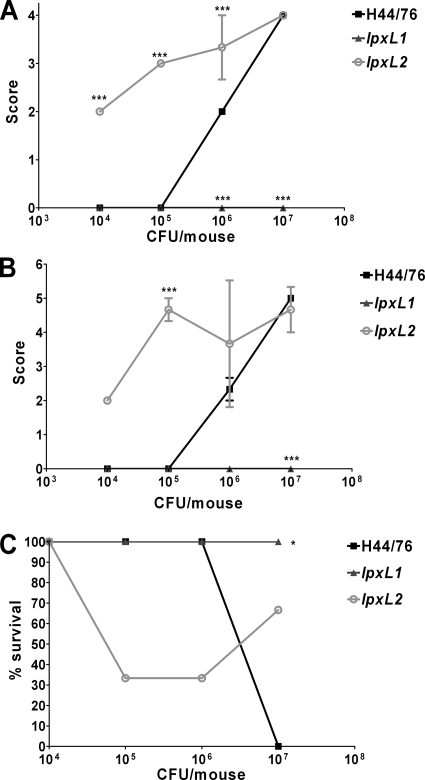
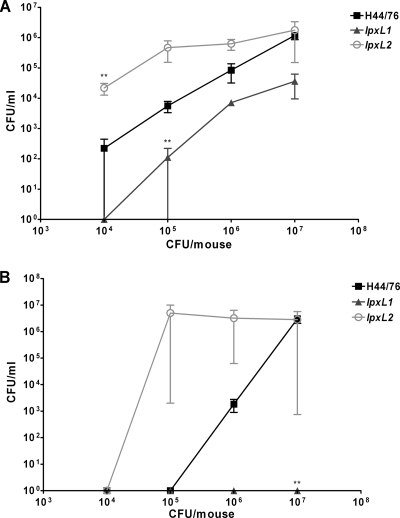
Next, we compared the virulence of the wild-type strain, the lpxL1 mutant, and the lpxL2 mutant in a mouse model of meningococcal sepsis. We anticipated that the results might be different for different dosages. Therefore, the mice were challenged i.p. with four different doses of each strain: 1 × 104, 1 × 105, 1 × 106, and 1 × 107 CFU/mouse. There were three female C57BL/6 mice per group. Since N. meningitidis needs iron for growth but cannot sequester iron from murine proteins (19), mice were injected i.p. with iron-dextran a few hours before injection with bacteria and again 24 h later. Animal health was monitored twice a day over a period of 48 h, and a health score was assigned to each mouse at each time point. A score of 0 means no visible symptoms, and a score of 6 means death. To limit suffering, mice that were severely ill (with a score of 5) were euthanized. In our experience, animals that reach that stage will not recover but will die later on. Animals that received the two lowest doses of the wild-type strain did not show any signs of illness, animals that received 1 × 106 CFU of H44/76 had moderate symptoms, and animals that received the highest dose of H44/76 were severely ill after 19 and 25 h (Fig. 4A and B). Interestingly, even the lowest dose of the lpxL2 mutant gave the mice moderate symptoms, and mice that received higher doses of the lpxL2 mutant had more severe symptoms. On the other hand, none of the mice that received the lpxL1 mutant showed any signs of illness, even with the highest dose. After 19 h, all animals were still alive and none had a score higher than 4. However, after 25 h, two mice were dead (one in the group that received 1 × 106 CFU of the lpxL2 mutant and one in the group that received 1 × 107 CFU of the lpxL2 mutant) and others had a score of 5, so they were euthanized (Fig. 4C). Only the highest dose of H44/76 was lethal, whereas lower doses of the lpxL2 mutant were lethal to some but not all mice in the group. In contrast, the highest dose of the lpxL2 mutant was less lethal than the highest dose of H44/76 or lower doses of the lpxL2 mutant. Obviously, all mice that received the lpxL1 mutant survived. Moreover, all mice that survived the first 25 h were practically symptom free after 43 h (data not shown).
FIG. 4.
Disease severity in mice infected with strain H44/76 or the lpxL1 or lpxL2 mutant. After infection, the health of the animals was monitored regularly, and a heath score was given at each time point. The score ranged from healthy (0) to death (6). There were three mice per group. (A and B) Health scores 19 h after infection (A) and 25 h after infection (B). (C) Percentage of survivors in each group after 25 h. Animals with a score of 5 that were killed at this time point were counted as nonsurvivors. Results of one representative experiment of two independent experiments are shown. Data are expressed as means for three mice, and error bars indicate SEM. Asterisks indicate that the group receiving the lpxL mutant was significantly different from the group receiving the same dose of wild-type strain H44/76. *, P < 0.05; **, P < 0.01; ***, indicates P < 0.001.
Meningococcal growth in vivo.
A sample of blood was taken from the animals after 2 and 19 h to determine the bacterial load in the circulation (Fig. 5). Already after 2 h the difference in fitness between the strains was visible. At the lower doses, more lpxL2 mutant bacteria were found in the blood than wild-type bacteria. However, there were fewer lpxL1 mutant bacteria than wild-type bacteria in the blood. After 19 h, none of the mice that received the lpxL1 mutant had any bacteria in their blood, consistent with no signs of sickness. Only the mice that received the two highest doses of H44/76 had bacteria in their blood after 19 h. However, mice that received an initial dose of 1 × 105 CFU or more of the lpxL2 mutant had a high bacterial load at that time point. Overall, the number of bacteria in the blood correlated strongly with illness severity. These results demonstrate that at lower doses, the lpxL2 mutant survived better in vivo than the wild-type strain. In contrast, the lpxL1 mutant was more easily cleared by the mice than the wild-type strain.
FIG. 5.
Number of bacteria in blood after infection. Samples of blood were taken from mice infected with strain H44/76 or the lpxL1 or lpxL2 mutant at 2 h (A) and 19 h (B) after administration. Serial dilutions of blood in PBS were plated to determine CFU. Results of one representative experiment of two independent experiments are shown. Data are expressed as means for three mice, and error bars indicate SEM. Asterisks indicate that the group that received an lpxL mutant was significantly different from the group that received the same dose of wild-type strain H44/76. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Murine cytokine response.
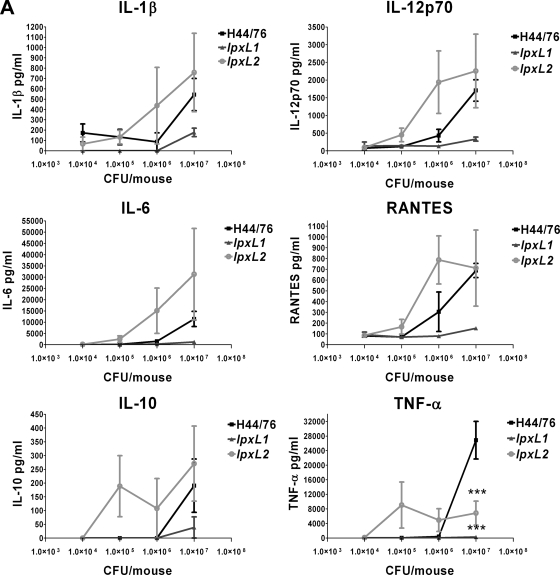
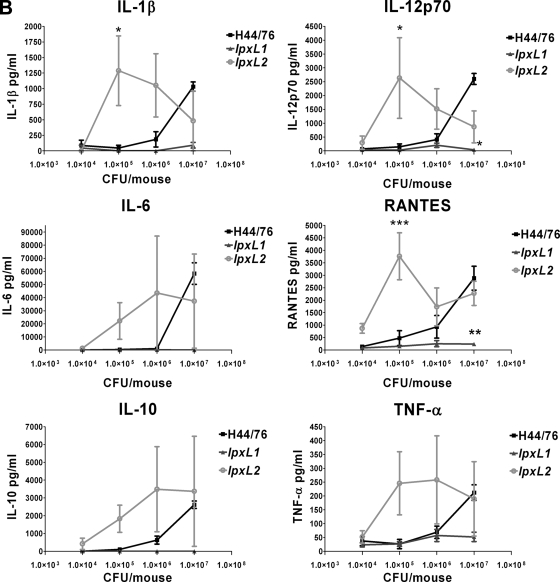
The concentrations of a number of cytokines important in the innate immune response were also measured in the serum after 2 and 19 h. The levels of the following cytokines were determined: IL-1β, IL-6, TNF-α, IL-12p70, IL-10, and RANTES. The levels of all cytokines at 2 h after challenge correlated very well with the number of bacteria in the blood (Fig. 6A). A high number of bacteria gave high levels of cytokines and vice versa. At lower doses the lpxL2 mutant induced more cytokine production than the wild-type strain. The lpxL1 mutant induced less cytokine production than the wild-type strain. Surprisingly, there was not much difference in the cytokine-inducing capabilities of the different strains when the bacterial load in the blood was approximately equal, although the wild-type strain was clearly more biologically active than the lpxL mutants in vitro (Fig. 3). For example, the initial dose of 1 × 107 CFU gave approximately the same number of wild-type and lpxL2 bacteria in the blood after 2 h (Fig. 5A), yet wild-type bacteria did not induce higher levels of cytokines than lpxL2 mutant bacteria after 2 h. One important exception, however, was TNF-α, which was much higher in mice that received wild-type bacteria (Fig. 6A). The levels of cytokines in the blood at 19 h after challenge also correlated strongly with bacterial load (Fig. 6B). Again, lpxL2 bacteria induced higher levels of cytokines than wild-type bacteria at the lower doses, whereas cytokines were practically undetectable in blood of animals infected with lpxL1 bacteria.
FIG. 6.
Cytokine levels in plasma of mice after infection with H44/76 or the lpxL1 or lpxL2 mutant. At 2 h (A) or 19 h (B) after infection with H44/76 or the lpxL1 or lpxL2 mutant, blood samples were taken from the mice. Cytokine levels in the plasma were determined with a six-plex Bio-Plex assay (Bio-Rad) containing beads for mouse IL-1β, IL-6, IL-10, IL-12p70, RANTES, and TNF-α. Data are expressed as means for three mice, and error bars indicate SEM. Asterisks indicate that the group that received an lpxL mutant was significantly different from the group that received the same dose of wild-type strain H44/76. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
Our study demonstrates that the lpxL2 mutant was much more virulent in mice than the wild-type strain, in contrast to the lpxL1 mutant, which was completely avirulent. The overall differences between the three strains shown in Fig. 4 to 66 follow the same trend in mice at all doses (with the exception of the highest), i.e., lpxL2 mutant > wild type > lpxL1 mutant. Therefore, the conclusions about their relative effects are not based on results from a single group of three mice but were repeatedly seen for different groups of mice. Although the outer membrane profiles and growth in whole mouse blood were very similar, it cannot be excluded that differences between these strains other than lipid A structure alone contributed to the great variation in virulence. However, in our past experiences with other strains in the same model, we never observed such large differences in virulence. The higher virulence was reflected by a higher bacterial load, higher cytokine levels, and more severe symptoms. Overall, there was a very strong correlation between these three parameters. In humans it was also found that meningococcal disease patients who died had higher bacterial loads than patients who survived (11). Other parameters indicative of disease severity were also associated with bacterial load in that study. Presumably, a higher bacterial load leads to higher cytokine levels. In humans, disease severity and mortality are also associated with higher cytokine levels (21).
Why is the lpxL2 mutant more virulent than the wild-type strain in our mouse model? We demonstrated that lpxL2 mutant bacteria activate murine TLR4/MD-2 much less efficiently than wild-type bacteria. Moreover, lpxL2 mutant bacteria were less active on murine macrophages than wild-type bacteria. The importance of TLR4 in protection against Gram-negative bacterial pathogens has been widely demonstrated in mice (3, 5, 40, 47, 54). Similarly, others have demonstrated that the virulence of certain Gram-negative bacteria in mice can be related to the low activity of their LPS (26, 30, 55). For example, the plague bacillus Yersinia pestis normally produces tetra-acyl LPS at mammalian body temperature, which is poorly recognized by TLR4. The wild-type strain was virulent in mice, in contrast to a modified strain that produced hexa-acyl LPS at 37°C. However, the modified strain was fully virulent in TLR4-deficient mice, demonstrating the importance of evasion of TLR4 activation for this bacterium (30). It is possible that lpxL2 mutant bacteria also evade recognition by the innate immune system, which promotes their survival in mice. TLR4-deficient mice have indeed been demonstrated to be more susceptible to experimental meningococcal infection (56). In humans also, the importance of LPS detection by the host is illustrated by the finding that individuals with rare mutations in TLR4 are more prone to meningococcal disease (15, 16, 42). In addition, the lpxL2 mutant could benefit indirectly from impaired TLR4 recognition. Besides TLR4, N. meningitidis activates TLR2 and TLR9 (29). It has been demonstrated that LPS upregulates TLR9 in mouse macrophages (2). Therefore, infection with the lpxL2 mutant might also reduce recognition by TLR9, which is important in the host defense against meningococcal disease (41).
Why, then, is the lpxL1 mutant less virulent than the wild-type strain in mice, although it is less well recognized by TLR4? Other host defense mechanisms are also important in the protection against N. meningitidis. The complement system plays a major role, as demonstrated by the fact that individuals with complement deficiencies are very susceptible to infection with Neisseria species (32). Another important defense mechanism includes antimicrobial peptides (4). Having lipid A with six acyl chains or more can protect bacteria from antimicrobial peptides (28, 31). Indeed, it has been demonstrated that N. meningitidis lipid A mutants are less resistant to such peptides (48). LPS structure is also known to influence the complement sensitivity of N. meningitidis (37). However, whether the acylation pattern of lipid A has any effect on complement sensitivity has not been studied to our knowledge. Thus, the lpxL1 mutant may be less virulent than the wild-type strain because of increased sensitivity to host defense mechanisms other than TLR4. It is likely that the lpxL2 mutant is also more sensitive to these other defense mechanisms, but that is widely compensated for by the evasion of TLR4 recognition. On other hand, the lpxL1 mutant might still be recognized by TLR4 well enough to clear the bacteria.
We show that in vitro the induction of IL-6 is not much different in the lpxL1 and lpxL2 whole bacteria but that IP-10 induction is much lower after lpxL2 mutant stimulation than after lpxL1 mutant stimulation. Presumably, activation of other pattern recognition receptors such as TLR2 contributes to IL-6 production via the MyD88 pathway, which compensates for the difference in the biological activities of lpxL1 and lpxL2 LPS. However, the lpxL1 and lpxL2 mutants clearly differ in their ability to induce IP-10, likely because IP-10 is induced via the TRIF pathway, which is activated only by LPS. Activation of the TRIF pathway leads to the production of type I IFN (33). Interestingly, it has been demonstrated that type I IFN treatment of mice infected with Salmonella enterica serovar Typhimurium leads to reduced lethality (9).
Somewhat in contrast to our findings, Plant et al. reported that a serogroup C wild-type strain and its isogenic lpxA mutant induced similar amounts of cytokines and caused equivalent disease severity in mice (34). Moreover, TLR4−/− mice were protected from disease, rather than being more susceptible. Differences in experimental design might be an explanation. Plant et al. used 1 × 108 and 5 × 108 CFU per mouse and no exogenous iron source. The advantage of not using an iron source is that possible effects of iron on the host immune response are ruled out, but a disadvantage is that higher doses of bacteria are needed to infect the mice. Thus, they used doses higher than our highest dose. We also found little difference between the wild-type strain and the lpxL2 mutant at the highest dose. Possibly the lpxL2 mutation has an advantage at lower bacterial numbers, because TLR4 recognition can be evaded. However, at high bacterial numbers, other pattern recognition receptors are sufficiently activated. At high bacterial loads, having TLR4 is probably only a disadvantage for the host, because it contributes significantly to the excessive production of proinflammatory cytokines, which can be lethal.
We show that the lpxL2 mutant induces smaller amounts of cytokines in vitro than the wild-type strain. In contrast, at the lower doses the lpxL2 mutant induced higher levels of cytokines in vivo. This apparent discrepancy can be explained by the fact that stimulation of cells in vitro was performed with inactivated bacteria but mice were infected with live bacteria. If unchecked by effective defense mechanisms, live bacteria can quickly reach much higher densities, while in vitro the densities used are always known. Presumably, the lpxL2 mutant induced more cytokines and chemokines in vivo because much higher levels of bacteria were present in the blood due to less efficient clearing by the immune system. However, it should be noted that with the highest dose of wild-type and lpxL2 bacteria, which led to similar levels of bacteria in the blood, both strains induced similar amounts of cytokines. It is possible that with such high numbers of bacteria in the blood, cytokine production reaches a plateau and the other pattern recognition receptor ligands compensate for the difference in LPS activity. One exception was TNF-α after 2 h, which was much higher in the blood of mice challenged with the wild-type strain, suggesting that specifically TNF-α production is more dependent on LPS. Interestingly, it has been demonstrated previously that the levels of LPS in the cerebrospinal fluid of patients with meningococcal disease correlated with levels of TNF-α but not with levels of IL-6 or IL-1 (52).
We recently reported that a surprisingly large fraction of N. meningitidis disease isolates have mutations in lpxL1 (17). The lpxL1 mutants activate human TLR4 much less efficiently than wild-type bacteria. Therefore, an explanation for the high frequency of lpxL1 mutation could be that it creates an advantage for the bacteria, because they can evade TLR4 recognition and subsequent clearing by the innate immune system. In the present study we wanted to test the influence of lipid A structure on virulence in a mouse model of meningococcal sepsis. However, a complicating factor is that lpxL1 mutant bacteria activate murine TLR4 much better than human TLR4. Here we show that an lpxL2 mutant activates murine TLR4 less efficiently than the lpxL1 mutant. Moreover, we demonstrate that the lpxL2 mutant is more virulent in mice than the wild-type strain, in contrast to the lpxL1 mutant, which is much less virulent. How the lpxL2 mutant behaves in mice might be a good model of how the lpxL1 mutant behaves in humans. There are also differences, however. We previously showed that meningitis patients infected with an lpxL1 mutant were less severely ill than patients infected with a wild-type strain (17). However, in the present study, mice infected with lpxL2 mutant bacteria had higher morbidity and mortality than mice infected with equivalent amounts of wild-type bacteria, at least at the lower bacterial doses used. These differences might relate to the fact that humans are much more sensitive to the effects of endotoxin than mice (10). It has been shown previously that the dose of endotoxin needed to induce a certain amount of IL-6 in mice was 250 times greater than the dose needed for humans. Moreover, endotoxin induced a rapid physiological response in humans but not in mice. Of course, the mouse model differs in several crucial respects from a human infection, including (i) the different route of infection; (ii) the much higher infective dose used, which means that the direct contribution of LPS to pathogenicity may be higher; (iii) the absence of any preexisting specific immunity; and (iv) the differential activity of many host-specific factors such as Opa adhesins, iron-sequestering proteins, and complement regulators (19). However, in spite of these differences, our results clearly demonstrate the crucial role of N. meningitidis lipid A structure in virulence, possibly through its effect on the degree of TLR4 activation. Moreover, our results suggest that the lipid A structure is important in the bacteria's defense against other host immune mechanisms as well.
Acknowledgments
We thank Maarten Schipper for advice on statistics.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 3 May 2010.
REFERENCES
- 1.Akashi, S., Y. Nagai, H. Ogata, M. Oikawa, K. Fukase, S. Kusumoto, K. Kawasaki, M. Nishijima, S. Hayashi, M. Kimoto, and K. Miyake. 2001. Human MD-2 confers on mouse Toll-like receptor 4 species-specific lipopolysaccharide recognition. Int. Immunol. 13:1595-1599. [DOI] [PubMed] [Google Scholar]
- 2.An, H., H. Xu, Y. Yu, M. Zhang, R. Qi, X. Yan, S. Liu, W. Wang, Z. Guo, Z. Qin, and X. Cao. 2002. Up-regulation of TLR9 gene expression by LPS in mouse macrophages via activation of NF-kappaB, ERK and p38 MAPK signal pathways. Immunol. Lett. 81:165-169. [DOI] [PubMed] [Google Scholar]
- 3.Banus, H. A., R. J. Vandebriel, R. H. de, J. A. Dormans, N. J. Nagelkerke, F. R. Mooi, B. Hoebee, H. J. van Kranen, and T. G. Kimman. 2006. Host genetics of Bordetella pertussis infection in mice: significance of Toll-like receptor 4 in genetic susceptibility and pathobiology. Infect. Immun. 74:2596-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergman, P., L. Johansson, H. Wan, A. Jones, R. L. Gallo, G. H. Gudmundsson, T. Hokfelt, A. B. Jonsson, and B. Agerberth. 2006. Induction of the antimicrobial peptide CRAMP in the blood-brain barrier and meninges after meningococcal infection. Infect. Immun. 74:6982-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernheiden, M., J. M. Heinrich, G. Minigo, C. Schutt, F. Stelter, M. Freeman, D. Golenbock, and R. S. Jack. 2001. LBP, CD14, TLR4 and the murine innate immune response to a peritoneal Salmonella infection. J. Endotoxin Res. 7:447-450. [PubMed] [Google Scholar]
- 6.Beutler, B., and E. T. Rietschel. 2003. Innate immune sensing and its roots: the story of endotoxin. Nat. Rev. Immunol. 3:169-176. [DOI] [PubMed] [Google Scholar]
- 7.Brandtzaeg, P., K. Bryn, P. Kierulf, R. Ovstebo, E. Namork, B. Aase, and E. Jantzen. 1992. Meningococcal endotoxin in lethal septic shock plasma studied by gas chromatography, mass-spectrometry, ultracentrifugation, and electron microscopy. J. Clin. Invest. 89:816-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandtzaeg, P., P. Kierulf, P. Gaustad, A. Skulberg, J. N. Bruun, S. Halvorsen, and E. Sorensen. 1989. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J. Infect. Dis. 159:195-204. [DOI] [PubMed] [Google Scholar]
- 9.Bukholm, G., B. P. Berdal, C. Haug, and M. Degre. 1984. Mouse fibroblast interferon modifies Salmonella typhimurium infection in infant mice. Infect. Immun. 45:62-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Copeland, S., H. S. Warren, S. F. Lowry, S. E. Calvano, and D. Remick. 2005. Acute inflammatory response to endotoxin in mice and humans. Clin. Diagn. Lab. Immunol. 12:60-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darton, T., M. Guiver, S. Naylor, D. L. Jack, E. B. Kaczmarski, R. Borrow, and R. C. Read. 2009. Severity of meningococcal disease associated with genomic bacterial load. Clin. Infect. Dis. 48:587-594. [DOI] [PubMed] [Google Scholar]
- 12.Dixon, D. R., and R. P. Darveau. 2005. Lipopolysaccharide heterogeneity: innate host responses to bacterial modification of lipid A structure. J. Dent. Res. 84:584-595. [DOI] [PubMed] [Google Scholar]
- 13.Ernst, R. K., K. N. Adams, S. M. Moskowitz, G. M. Kraig, K. Kawasaki, C. M. Stead, M. S. Trent, and S. I. Miller. 2006. The Pseudomonas aeruginosa lipid A deacylase: selection for expression and loss within the cystic fibrosis airway. J. Bacteriol. 188:191-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ernst, R. K., E. C. Yi, L. Guo, K. B. Lim, J. L. Burns, M. Hackett, and S. I. Miller. 1999. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science 286:1561-1565. [DOI] [PubMed] [Google Scholar]
- 15.Faber, J., N. Henninger, A. Finn, W. Zenz, F. Zepp, and M. Knuf. 2009. A Toll-like receptor 4 variant is associated with fatal outcome in children with invasive meningococcal disease. Acta Paediatr. 98:548-552. [DOI] [PubMed] [Google Scholar]
- 16.Faber, J., C. U. Meyer, C. Gemmer, A. Russo, A. Finn, C. Murdoch, W. Zenz, C. Mannhalter, B. U. Zabel, H. J. Schmitt, P. Habermehl, F. Zepp, and M. Knuf. 2006. Human toll-like receptor 4 mutations are associated with susceptibility to invasive meningococcal disease in infancy. Pediatr. Infect. Dis. J. 25:80-81. [DOI] [PubMed] [Google Scholar]
- 17.Fransen, F., S. G. Heckenberg, H. J. Hamstra, M. Feller, C. J. Boog, J. P. van Putten, D. van de Beek, A. van der Ende, and P. van der Ley. 2009. Naturally occurring lipid A mutants in Neisseria meningitidis from patients with invasive meningococcal disease are associated with reduced coagulopathy. PLoS. Pathog. 5:e1000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gioannini, T. L., and J. P. Weiss. 2007. Regulation of interactions of Gram-negative bacterial endotoxins with mammalian cells. Immunol. Res. 39:249-260. [DOI] [PubMed] [Google Scholar]
- 19.Gorringe, A. R., K. M. Reddin, S. G. Funnell, L. Johansson, A. Rytkonen, and A. B. Jonsson. 2005. Experimental disease models for the assessment of meningococcal vaccines. Vaccine 23:2214-2217. [DOI] [PubMed] [Google Scholar]
- 20.Guo, L., K. B. Lim, J. S. Gunn, B. Bainbridge, R. P. Darveau, M. Hackett, and S. I. Miller. 1997. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science 276:250-253. [DOI] [PubMed] [Google Scholar]
- 21.Hackett, S. J., A. P. Thomson, and C. A. Hart. 2001. Cytokines, chemokines and other effector molecules involved in meningococcal disease. J. Med. Microbiol. 50:847-859. [DOI] [PubMed] [Google Scholar]
- 22.Hajjar, A. M., R. K. Ernst, J. H. Tsai, C. B. Wilson, and S. I. Miller. 2002. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat. Immunol. 3:354-359. [DOI] [PubMed] [Google Scholar]
- 23.Kahler, C. M., and D. S. Stephens. 1998. Genetic basis for biosynthesis, structure, and function of meningococcal lipooligosaccharide (endotoxin). Crit. Rev. Microbiol. 24:281-334. [DOI] [PubMed] [Google Scholar]
- 24.Kawasaki, K., R. K. Ernst, and S. I. Miller. 2004. 3-O-deacylation of lipid A by PagL, a PhoP/PhoQ-regulated deacylase of Salmonella typhimurium, modulates signaling through Toll-like receptor 4. J. Biol. Chem. 279:20044-20048. [DOI] [PubMed] [Google Scholar]
- 25.Kawasaki, K., R. K. Ernst, and S. I. Miller. 2004. Deacylation and palmitoylation of lipid A by Salmonellae outer membrane enzymes modulate host signaling through Toll-like receptor 4. J. Endotoxin Res. 10:439-444. [DOI] [PubMed] [Google Scholar]
- 26.Khan, S. A., P. Everest, S. Servos, N. Foxwell, U. Zahringer, H. Brade, E. T. Rietschel, G. Dougan, I. G. Charles, and D. J. Maskell. 1998. A lethal role for lipid A in Salmonella infections. Mol. Microbiol. 29:571-579. [DOI] [PubMed] [Google Scholar]
- 27.Lien, E., T. K. Means, H. Heine, A. Yoshimura, S. Kusumoto, K. Fukase, M. J. Fenton, M. Oikawa, N. Qureshi, B. Monks, R. W. Finberg, R. R. Ingalls, and D. T. Golenbock. 2000. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J. Clin. Invest. 105:497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller, S. I., R. K. Ernst, and M. W. Bader. 2005. LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol. 3:36-46. [DOI] [PubMed] [Google Scholar]
- 29.Mogensen, T. H., S. R. Paludan, M. Kilian, and L. Ostergaard. 2006. Live Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis activate the inflammatory response through Toll-like receptors 2, 4, and 9 in species-specific patterns. J. Leukoc. Biol. 80:267-277. [DOI] [PubMed] [Google Scholar]
- 30.Montminy, S. W., N. Khan, S. McGrath, M. J. Walkowicz, F. Sharp, J. E. Conlon, K. Fukase, S. Kusumoto, C. Sweet, K. Miyake, S. Akira, R. J. Cotter, J. D. Goguen, and E. Lien. 2006. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat. Immunol. 7:1066-1073. [DOI] [PubMed] [Google Scholar]
- 31.Munford, R. S. 2008. Sensing gram-negative bacterial lipopolysaccharides: a human disease determinant? Infect. Immun. 76:454-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicholson, A., and I. H. Lepow. 1979. Host defense against Neisseria meningitidis requires a complement-dependent bactericidal activity. Science 205:298-299. [DOI] [PubMed] [Google Scholar]
- 33.Palsson-McDermott, E. M., and L. A. O'Neill. 2004. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 113:153-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plant, L., H. Wan, and A. B. Jonsson. 2007. Non-lipooligosaccharide-mediated signalling via Toll-like receptor 4 causes fatal meningococcal sepsis in a mouse model. Cell. Microbiol. 9:657-669. [DOI] [PubMed] [Google Scholar]
- 35.Poltorak, A., P. Ricciardi-Castagnoli, S. Citterio, and B. Beutler. 2000. Physical contact between lipopolysaccharide and Toll-like receptor 4 revealed by genetic complementation. Proc. Natl. Acad. Sci. U. S. A. 97:2163-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitt, C., A. Villwock, and O. Kurzai. 2009. Recognition of meningococcal molecular patterns by innate immune receptors. Int. J. Med. Microbiol. 299:9-20. [DOI] [PubMed] [Google Scholar]
- 37.Schneider, M. C., R. M. Exley, S. Ram, R. B. Sim, and C. M. Tang. 2007. Interactions between Neisseria meningitidis and the complement system. Trends Microbiol. 15:233-240. [DOI] [PubMed] [Google Scholar]
- 38.Schromm, A. B., K. Brandenburg, H. Loppnow, A. P. Moran, M. H. Koch, E. T. Rietschel, and U. Seydel. 2000. Biological activities of lipopolysaccharides are determined by the shape of their lipid A portion. Eur. J. Biochem. 267:2008-2013. [DOI] [PubMed] [Google Scholar]
- 39.Schromm, A. B., K. Brandenburg, H. Loppnow, U. Zahringer, E. T. Rietschel, S. F. Carroll, M. H. Koch, S. Kusumoto, and U. Seydel. 1998. The charge of endotoxin molecules influences their conformation and IL-6-inducing capacity. J. Immunol. 161:5464-5471. [PubMed] [Google Scholar]
- 40.Schurr, J. R., E. Young, P. Byrne, C. Steele, J. E. Shellito, and J. K. Kolls. 2005. Central role of Toll-like receptor 4 signaling and host defense in experimental pneumonia caused by gram-negative bacteria. Infect. Immun. 73:532-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sjolinder, H., T. H. Mogensen, M. Kilian, A. B. Jonsson, and S. R. Paludan. 2008. Important role for Toll-like receptor 9 in host defense against meningococcal sepsis. Infect. Immun. 76:5421-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smirnova, I., N. Mann, A. Dols, H. H. Derkx, M. L. Hibberd, M. Levin, and B. Beutler. 2003. Assay of locus-specific genetic load implicates rare Toll-like receptor 4 mutations in meningococcal susceptibility. Proc. Natl. Acad. Sci. U. S. A. 100:6075-6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Somerville, J. E., Jr., L. Cassiano, and R. P. Darveau. 1999. Escherichia coli msbB gene as a virulence factor and a therapeutic target. Infect. Immun. 67:6583-6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steeghs, L., R. den Hartog, A. den Boer, B. Zomer, P. Roholl, and P. van der Ley. 1998. Meningitis bacterium is viable without endotoxin. Nature 392:449-450. [DOI] [PubMed] [Google Scholar]
- 45.Steeghs, L., A. M. Keestra, A. van Mourik, H. Uronen-Hansson, P. van der Ley, R. Callard, N. Klein, and J. P. van Putten. 2008. Differential activation of human and mouse Toll-like receptor 4 by the adjuvant candidate LpxL1 of Neisseria meningitidis. Infect. Immun. 76:3801-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stephens, D. S., B. Greenwood, and P. Brandtzaeg. 2007. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet 369:2196-2210. [DOI] [PubMed] [Google Scholar]
- 47.Supajatura, V., H. Ushio, A. Nakao, K. Okumura, C. Ra, and H. Ogawa. 2001. Protective roles of mast cells against enterobacterial infection are mediated by Toll-like receptor 4. J. Immunol. 167:2250-2256. [DOI] [PubMed] [Google Scholar]
- 48.Tzeng, Y. L., K. D. Ambrose, S. Zughaier, X. Zhou, Y. K. Miller, W. M. Shafer, and D. S. Stephens. 2005. Cationic antimicrobial peptide resistance in Neisseria meningitidis. J. Bacteriol. 187:5387-5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Ley, P., J. E. Heckels, M. Virji, P. Hoogerhout, and J. T. Poolman. 1991. Topology of outer membrane porins in pathogenic Neisseria spp. Infect. Immun. 59:2963-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Ley, P., L. Steeghs, H. J. Hamstra, J. ten Hove, B. Zomer, and L. van Alphen. 2001. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect. Immun. 69:5981-5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Deuren, M., P. Brandtzaeg, and J. W. van der Meer. 2000. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin. Microbiol. Rev. 13:144-166, table. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waage, A., A. Halstensen, R. Shalaby, P. Brandtzaeg, P. Kierulf, and T. Espevik. 1989. Local production of tumor necrosis factor alpha, interleukin 1, and interleukin 6 in meningococcal meningitis. Relation to the inflammatory response. J. Exp. Med. 170:1859-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Westphal, O., and J. K. Jann. 1965. Bacterial lipopolysaccharides extraction with phenol-water and further application of the procedure. Methods Carbohydr. Chem. 5:83-91. [Google Scholar]
- 54.Wieland, C. W., S. Florquin, N. A. Maris, K. Hoebe, B. Beutler, K. Takeda, S. Akira, and T. van der Poll. 2005. The MyD88-dependent, but not the MyD88-independent, pathway of TLR4 signaling is important in clearing nontypeable Haemophilus influenzae from the mouse lung. J. Immunol. 175:6042-6049. [DOI] [PubMed] [Google Scholar]
- 55.Wolfe, D. N., A. M. Buboltz, and E. T. Harvill. 2009. Inefficient Toll-like receptor-4 stimulation enables Bordetella parapertussis to avoid host immunity. PLoS One 4:e4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woods, J. P., J. A. Frelinger, G. Warrack, and J. G. Cannon. 1988. Mouse genetic locus Lps influences susceptibility to Neisseria meningitidis infection. Infect. Immun. 56:1950-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]