Abstract
Staphylococcal superantigen-like proteins (SSLs) constitute a family of exoproteins exhibiting structural similarities to superantigens and enterotoxins but no superantigenic activity. In this article, we present evidence that SSL5 specifically binds to matrix metalloproteinase 9 (MMP-9) and inhibits its enzymatic activity. When human neutrophil cell lysate was applied to recombinant His-tagged SSL5 conjugated to Sepharose, the bound fraction gave a major band of approximately 100 kDa in SDS-polyacrylamide gel electrophoresis. This protein was identified as the proform of MMP-9 (proMMP-9) by peptide mass fingerprinting analysis. The recombinant SSL5-Sepharose also bound to proMMP-9 secreted by interleukin 8 (IL-8)-stimulated neutrophils and HT1080 fibrosarcoma cells. Surface plasmon resonance analysis revealed that recombinant SSL5 bound to proMMP-9 with rather high affinity (dissociation constant [KD] = 1.9 nM). Recombinant SSL5 was found to effectively inhibit MMP-9-catalyzed hydrolysis of gelatin and a synthetic fluorogenic peptide in a noncompetitive manner (Ki = 0.097 nM), as assessed by zymography and the fluorescence quenching method. Finally, the transmigration of neutrophils across Matrigel basement membranes in response to N-formyl-methionyl-leucyl-phenylalanine (FMLP) was suppressed by the presence of recombinant SSL5. We discuss possible roles that SSL5 may play in immune evasion of staphylococci by inhibiting MMP and interfering with leukocyte trafficking.
Staphylococcus aureus secretes various virulence factors and disturbs host defense systems. Exotoxins, such as alpha-toxin, hemolysins, and leukocidin, are thought to suppress host immunity via their cytotoxicity against leukocytes (9). On the other hand, superantigens, including toxic shock syndrome toxin 1 (TSST-1) and enterotoxins, induce unregulated activation of T cells by cross-linking major histocompatibility complex (MHC) class II molecules and T-cell antigen receptors, resulting in immunological perturbation (9). It was recently reported that exoproteins produced by staphylococci affect the functions of various molecules responsible for humoral and cell-mediated immunity (11), e.g., staphylococcus complement inhibitor (27), chemotaxis inhibitory protein of staphylococci (CHIPS) (26), and extracellular adherence protein (Eap) (7).
A family of exoproteins designated staphylococcal superantigen-like proteins (SSLs) has also been described, and these proteins possess structural similarity to staphylococcal TSST-1 and enterotoxins but exhibit no superantigenic activity. Eleven members of the SSL family of proteins have been identified to date, and the number of SSL members expressed in the cell varies from 7 to 11, depending on the strain of S. aureus (10). The amino acid sequence homology among these individual SSL proteins was found to be 36 to 67%, and their genes are located in so-called staphylococcal pathogenicity island 2 (SaPI2) in an order that is conserved among most strains. SSL proteins are characterized by the presence of an N-terminal β-barrel globular domain (known as the oligonucleotide/oligosaccharide-binding fold [OB fold]) linked to the C-terminal β-grasp domain, which is a structural feature common to TSST-1 and enterotoxins (33).
The secretion of several SSL proteins was upregulated when the bacteria were phagocytosed by lung epithelial cells (12), suggesting the relevance of SSLs to the protective mechanism of the bacteria against the host defense system. However, limited information on the functional aspects of SSLs has been available. Recently it was reported that the family member SSL7 bound to IgA and complement component C5, causing inhibition of IgA binding to its receptor on phagocytes and complement-dependent bactericidal activity (19). More recently, Bestebroer et al. reported that SSL5 bound to P-selectin glycoprotein ligand 1 (PSGL-1) expressed on leukocytes and inhibited the binding of PSGL-1 to P-selectin, an adhesion molecule expressed on activated endothelial cells and platelets (4). The P-selectin/PSGL-1 interaction plays a crucial role in recruitment of leukocytes to inflammatory and hemorrhagic sites (15, 22). The observation that SSL5 inhibited rolling of neutrophils on immobilized P-selectin suggested impairment of the initial step of neutrophil extravasation toward bacterial infection sites (4). Baker et al. analyzed the crystal structure of SSL5 complexed with a sialyl Lewis X tetrasaccharide (sLeX, sialic acid-α2-3-galactose-β1-4(fucose-α1-3)-N-acetylglucosamine), a key determinant of PSGL-1 binding to P-selectin, and demonstrated that sLeX bound to a specific site of the C-terminal domain of SSL5 (3). Because the sialyl-lactosamine unit (sialic acid-α2-3-galactose-β1-4-N-acetylglucosamine) is the critical motif for the SSL5/PSGL-1 interaction, SSL5 may recognize other glycoproteins carrying similar carbohydrate chains. In fact, human IgA receptor FcαRI (CD89) has been shown to be bound by SSL5 (3).
During a preliminary attempt to isolate PSGL-1 from human neutrophil cell lysate using immobilized recombinant SSL5 as an affinity ligand, we found that the proform of matrix metalloproteinase 9 (MMP-9) (gelatinase B, EC 3.4.24.35) was copurified with PSGL-1. MMP-9 is a member of the zinc-dependent endopeptidase family and degrades molecular components of basement membranes, including type IV collagen. This protease is stored in granules of neutrophils, extracellularly released as a latent proform upon stimulation, and then activated by other proteases, such as elastase (8, 25). It has been postulated that MMP-9 plays a major role in neutrophil transmigration to infection sites and inflammatory tissues through extracellular matrices (8, 17). Because MMP-9 from neutrophils is a glycoprotein consisting of 15% carbohydrates, it may interact with SSL5 through carbohydrate moieties. In this study, we characterized the interaction between SSL5 and MMP-9 and the effect of SSL5 on the enzymatic activity of MMP-9. The results indicated that recombinant SSL5 effectively inhibited MMP-9 activity and the formyl peptide-induced transmigration of neutrophils across Matrigel basement membranes, providing insight into the mechanism of escape of staphylococci from the host immune system.
MATERIALS AND METHODS
Reagents.
Dextran 200,000, bovine gelatin, dithiothreitol (DTT), iodoacetamide, and isopropyl-β-d-thiogalactopyranoside (IPTG) were purchased from Wako Pure Chemical Industries (Osaka, Japan). Restriction endonucleases and modifying enzymes were products of Gibco/BRL (Rockville, MD), TaKaRa (Osaka, Japan), and Toyobo (Osaka, Japan). Oligonucleotides were supplied by Hokkaido System Science Co., Ltd. (Sapporo, Japan). Trypsin, aprotinin, phenylmethylsulfonyl fluoride (PMSF), recombinant human IL-8, p-aminophenylmercuric acetate (APMA), N-formyl-methionyl-leucyl-phenylalanine (FMLP), 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), Brij 35, Nonidet P-40, and Triton X-100 were products of Sigma (St. Louis, MO). Ficoll-Paque, Ni Sepharose 6 Fast Flow, N-hydroxysuccinimide (NHS)-activated Sepharose 4FF, HiTrap desalting, and gelatin Sepharose 4B were purchased from GE Healthcare (Piscataway, NJ). Coomassie brilliant blue (CBB) R250 was purchased from Merck (Darmstadt, Germany). MMP-9 inhibitor I (a derivative of anthranilic acid) (20) and MMP-2/MMP-9 inhibitor I {(2R)-[(4-biphenylylsulfonyl)amino]-3-phenylpropionic acid (BiPS)} (29) were purchased from Calbiochem (San Diego, CA). Neuraminidase from Arthrobacter ureafaciens was a product of Nacalai (Kyoto, Japan).
Preparation of recombinant SSLs.
Genes for SSL5, SSL7, and SSL9 were amplified by PCR using genomic DNA of S. aureus (ATCC 27733) as a template and cloned into the pGEM-Teasy plasmid (Promega, Madison, WI). The primers used for PCR were 5′-GGG GAT CCA GAG CGA ACA TGA ATC AAA ATA TG-3′ (BamHI site underlined) and 5′-GGG GGT CGA CTT ATC TAA TAT TGG CTT CTA TTT TCT C-3′ (SalI site underlined) for SSL5, 5′-GGG ATC CAA AAA GAA AAG CAA GAG AGA G-3′ (BamHI site underlined) and 5′-GAA GCT TAA ATT TGT TTC AAA GTC AC-3′ (HindIII site underlined) for SSL7, and 5′-GGG GAT CCA GAA AGT AAA GTT GGA TGA AAC AC-3′ (BamHI site underlined) and 5′-GGG CTG CAG TTA ATT CAA ATT CAC TTC AAT ATT TTT A-3′ (PstI site underlined) for SSL9 (2). The sequences of the amplified and cloned DNA of SSLs were confirmed by a DNA sequencer (model 377A; Applied Biosystems, Foster City, CA). The insert DNA was then recovered and ligated with the expression vector pQE-32 (Qiagen, Chatsworth, CA) using the BamHI/SalI recognition sites for SSL5, the BamHI/HindIII recognition sites for SSL7, and the BamHI/PstI recognition sites for SSL9, respectively. The Escherichia coli strains JM109 (SSL5 and SSL7) and BL21 (SSL9) were transformed with the resultant constructs (pQE-32/SSL5, pQE-32/SSL7, and pQE32/SSL9), and the expression of recombinant SSLs was induced by culturing at 37°C for 4 h in the presence of 1 mM IPTG. The recombinant SSL proteins have an additional MHHHHHHGIQ sequence at their N termini, and the His-tagged SSLs (His6-SSL5, His6-SSL7, and His6-SSL9) were purified under denaturing conditions on a Ni Sepharose 6 Fast Flow column (GE Healthcare) according to the manufacturer's protocol. The proteins bound by the column were renatured by gradual replacement of the denaturing buffer (20 mM Tris-HCl, 0.5 M NaCl, 8 M urea, pH 7.5) with a native buffer (20 mM Tris-HCl, 0.5 M NaCl, pH 7.5) and eluted with 0.5 M imidazole. The His-tagged SSL proteins eluted from the column were desalted using HiTrap desalting (GE Healthcare). In some experiments, the SSL5 DNA was cloned into pGEX-5X-1 (GE Healthcare) to express glutathione S-transferase (GST)-tagged SSL5. The GST tag was cleaved off by digestion with Factor Xa protease (Qiagen) to obtain tag-free SSL5. The protein concentration was determined with a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL). Purified SSLs were stored in phosphate-buffered saline (PBS) containing 0.5% CHAPS at 4°C.
Coupling of SSLs to Sepharose.
Purified His-tagged SSLs were conjugated to NHS-activated Sepharose 4FF (GE Healthcare) according to the manufacturer's protocol. Briefly, recombinant SSL protein (1 mg/ml in 0.2 M NaHCO3) was applied to a column of NHS-activated Sepharose 4FF (1 ml of wet gel) that was pretreated with ice-cold 1 mM HCl, and the column was allowed to react for 1 h at room temperature. The column was then washed successively with 6 ml of high-pH buffer (0.5 M ethanolamine, 0.5 M NaCl, pH 8.3), 6 ml of low-pH buffer (0.1 M sodium acetate, 0.5 M NaCl, pH 4.0), and 6 ml of high-pH buffer again. The amount of protein coupled to Sepharose was approximately 1 mg protein/ml gel for each SSL, as estimated by the measurement of protein remaining in the supernatant after the coupling reaction. The SSL-conjugated Sepharose was stored in PBS containing 0.02% NaN3 until use.
Preparation of neutrophils.
Neutrophils were isolated from human peripheral blood by a combination of dextran sedimentation and Ficoll-Paque density gradient centrifugation as described previously (14). The purity of neutrophils was greater than 95% as assessed by Grünwald-Giemsa staining.
Isolation of SSL5-binding proteins.
Neutrophils (2 × 107 cells) were lysed with 1 ml of Lysis buffer (20 mM Tris-HCl, 0.5 M NaCl, 1% Nonidet P-40, pH 7.5) containing 1 mM PMSF and 1 μg/ml aprotinin. After the mixture was incubated at 4°C for 5 min, a clear cell lysate was obtained by centrifugation at 16,000 × g for 5 min. The cell lysate was mixed with His6-SSL5-Sepharose (25 μl of a 50% suspension) and incubated at 4°C for 1 h, and the Sepharose beads were washed five times with lysis buffer. Proteins bound to His6-SSL5-Sepharose were recovered by treatment with 40 μl of 1.5× Laemmli's sample buffer (75 mM Tris-HCl, pH 6.8, 1.5% SDS, 15% glycerol, 0.015% bromophenol blue) (18) for 10 min at room temperature and separated by electrophoresis on an SDS-polyacrylamide gel (7.5%), followed by staining with CBB.
By a similar procedure, proteins bound by His6-SSL5, His6-SSL7, and His6-SSL9 conjugated to Sepharose were isolated from the culture supernatants of IL-8-stimulated neutrophils and HT1080 human fibrosarcoma cells. Human neutrophils (2 × 107 cells) suspended in PBS were stimulated with IL-8 (50 ng/ml) at 37°C for 30 min, and the culture supernatant was collected by centrifugation at 2,500 × g for 5 min. HT1080 cells (1 × 107 cells) were cultured in RPMI medium at 37°C for 48 h, and the conditioned medium obtained was concentrated approximately 75-fold with a Microcon YM-30 centrifugal filter unit (Millipore Corp., Billerica, MA).
Peptide mass fingerprinting analysis.
Pieces of CBB-stained gel were washed three times with 25 mM NH4HCO3-50% (vol/vol) acetonitrile for 10 min each, dehydrated by the addition of acetonitrile, and dried in vacuo. The dried gel was treated with 25 mM NH4HCO3-10 mM dithiothreitol for 1 h at 56°C, washed with 25 mM NH4HCO3, and incubated with 25 mM NH4HCO3-10 mg/ml iodoacetamide for 45 min at room temperature in the dark. After the gel was successively washed once with 25 mM NH4HCO3 and twice with 25 mM NH4HCO3-50% (vol/vol) acetonitrile for 10 min and dried again, the reduced/alkylated proteins were digested by treatment of the gel with 10 μg/ml trypsin in 50 mM NH4HCO3 at 37°C for 16 h. The digested peptides were extracted with 5% (vol/vol) trifluoroacetic acid-50% (vol/vol) acetonitrile and subjected to peptide mass fingerprinting using a matrix-assisted laser desorption/ionization (MALDI) time-of-flight mass spectrometer (TOF MS) (AXIMA-CFRPLUS; Shimadzu/Kratos, Kyoto, Japan) equipped with a nitrogen laser (337 nm, 3-ns pulse width) using 2,5-dihydroxybenzoic acid (DHBA) as a matrix. MALDI-TOF MS analysis was conducted in the reflectron mode under the following conditions: accelerating voltage, 20 kV; measurement range, 500 to 4,000 m/z. Bradykinin fragments 1 to 7 ([M+H]+ = 757.3997) and synthetic peptide P14R ([M+H]+ = 1533.8582)) were used for external calibration. Peptide mass fingerprints were searched with the MASCOT search engines (Matrix Science, Boston, MA).
Purification of MMP-9 from human neutrophils.
MMP-9 was purified from FMLP-stimulated human neutrophil culture supernatants by the method of Masure et al. (21) with slight modification. Human neutrophils suspended in PBS (5 × 107 cells/ml, 1 ml) were stimulated with 1 μM FMLP at 37°C for 1 h. After centrifugation at 2,500 × g for 5 min, the supernatant was mixed with gelatin-Sepharose 4B (GE Healthcare; 0.2 ml of a 50% suspension). The mixture was incubated at 4°C for 1 h with gentle agitation, and gelatin-Sepharose 4B was washed three times with 1% Nonidet P-40 and once with PBS. MMP-9 was then eluted with 5% dimethyl sulfoxide (DMSO) and freed from DMSO by ultrafiltration with a Microcon YM-30 centrifugal filter unit (Millipore Corp.). The purity of MMP-9 was estimated to be >90%, as analyzed by SDS-polyacrylamide gel electrophoresis followed by silver staining. The concentration of MMP-9 was determined by enzyme-linked immunosorbent assay (ELISA) with a Biotrak MMP-9 activity assay system (GE Healthcare). Purified MMP-9 was stocked at −20°C in the presence of 10% glycerol.
SPR analysis.
The kinetic analysis of the SSL5-MMP-9 interaction was carried out by surface plasmon resonance (SPR) with a Biacore 2000 protein analyzer (GE Healthcare). His6-SSL5 was immobilized on a sensor chip CM5 (GE Healthcare) to a level of ∼5,000 resonance units (RU) using an amine coupling kit (GE Healthcare) according to the manufacturer's instructions. Serial dilutions of the purified proform of MMP-9 (proMMP-9) (6.8 to 109 nM) were injected over the chip surface at a flow rate of 20 μl/min. The association and dissociation kinetics were analyzed by using BIAevaluation software (version 3.0; GE Healthcare).
Gelatin zymography and reverse zymography.
MMP activity was detected by gelatin zymography essentially as described previously (30). Specimens were electrophoresed on a polyacrylamide gel (6.5%) containing gelatin (1.5 mg/ml) under nonreducing conditions. The gel was washed three times with washing buffer (50 mM Tris-HCl, 150 mM NaCl, 10 mM CaCl2, 0.02% of NaN3, pH 7.5) containing 2.5% Triton X-100 for 30 min for each step and incubated in washing buffer without Triton X-100 for 16 h. The gel was then stained with CBB and destained with 7.5% acetic acid-5% methanol.
Reverse zymography was performed according to the method described by Oliver et al. (24). Serial dilutions of His6-SSLs were separated on polyacrylamide gels (12.5%) containing gelatin (1.5 mg/ml) and proMMP-9 purified from human neutrophils (10.9 ng/ml). After electrophoresis, the gel was processed for staining and destaining as described above.
Assay for MMP activity.
MMP activity was assayed by two methods, using gelatin and a fluorogenic peptide as substrates, respectively. (i) ProMMP-9 (3.7 ng) was incubated with 15 μg of gelatin in the presence or absence of various amounts of His6-SSL5 (5.6 to 50 ng) in 30 μl of reaction buffer 1 (50 mM Tris-HCl, 0.33 mM APMA, 150 mM NaCl, 10 mM CaCl2, pH 7.5) at 37°C for 16 h. The reaction mixture was analyzed by SDS-polyacrylamide gel (10%) electrophoresis and CBB staining. (ii) ProMMP-9 (0.3 nM) was activated by treatment with 1 mM APMA at 37°C for 16 h and incubated with or without various concentrations of His6-SSL5 at room temperature for 2 h. The mixture was then incubated with a fluorescence-quenching substrate for matrix metalloproteinase, (7-methoxycoumarin-4-yl)acetyl-l-prolyl-leucylglycyl-l-leucyl-[Nb-(2,4-dinitrophenyl)-l-2,3-diaminopropionyl]-l-alanyl-l-arginine amide [MOCAc-PLGly-LA2pr(Dnp)-AR-NH2] (Peptide Institute, Inc., Osaka, Japan) (16), at a concentration of 0.625, 1.25, 2.5, 5.0, or 10.0 μM in 0.2 ml of reaction buffer 2 (50 mM Tris-HCl, 100 mM NaCl, 10 mM CaCl2, 0.1% Brij 35, pH 7.5) at 25°C, and the increase in fluorescence was monitored (excitation = 328 nm; emission = 393 nm) using a fluorescence spectrophotometer (F-4010; Hitachi, Tokyo, Japan). Fluorescence was measured every 30 s with a 6-s integration time. The reaction was allowed to proceed for 10 min, and the initial velocity of each reaction was determined using the data collected over 1 to 9 min.
Transmigration of neutrophils through reconstituted membranes.
The in vitro transmigration assay was performed according to the method described by Delclaux et al. (8) using a Boyden chamber system (cell culture inserts with 8-μm pores; BD Biosciences, San Jose, CA). The membranes between the upper and lower chambers were coated with Matrigel (BD Biosciences). Human neutrophil suspension in serum-free RPMI 1640 medium (1 × 105 cells/0.2 ml) with or without His6-SSL5 (10 μg/ml) was added to the upper chamber, and serum-free RPMI1640 medium containing 1 μM FMLP was added to the lower chamber. The chambers were incubated at 37°C for 2 h under a 5%-CO2 atmosphere. After the neutrophils remaining on the upper surface of the membrane were wiped off with cotton wool, the cells that had migrated to the bottom surface of the membrane were stained with crystal violet (5 mg/ml)-20% methanol and counted under a microscope.
RESULTS
Binding of proMMP-9 to recombinant His-tagged SSL5.
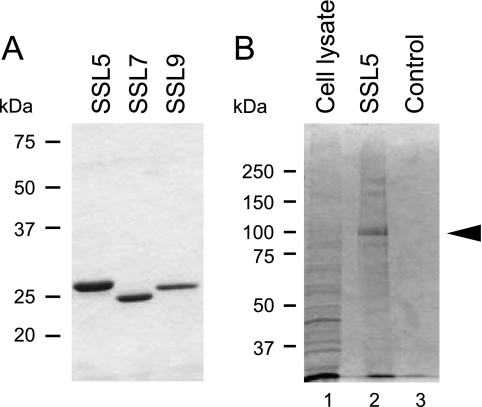
The recombinant His-tagged SSL proteins (His6-SSL5, His6-SSL7, and His6-SSL9) were expressed in E. coli and purified on a Ni-Sepharose column. The purified proteins were examined by SDS-polyacrylamide gel electrophoresis, and each preparation gave an apparently single band after staining with Coomassie brilliant blue (CBB) (Fig. 1 A). We then attempted to isolate PSGL-1 from human neutrophil lysate by using His6-SSL5-Sepharose as an affinity ligand. When the bound materials were analyzed by SDS-polyacrylamide gel electrophoresis under a reducing condition followed by CBB staining, a major protein band of ∼100 kDa was observed, as well as faint bands of ∼170 kDa and ∼250 kDa (Fig. 1B). Immunoblotting analysis indicated that PSGL-1 was also present in the bound fraction (data not shown), but there was little staining of PSGL-1 by CBB, probably due to the high carbohydrate content of PSGL-1. The peptide mass fingerprinting analysis of the tryptic fragments of the ∼100-kDa protein with MALDI-TOF MS and the MASCOT search engines resulted in proMMP-9 (proform of gelatinase B) as the best hit, exceeding the significance threshold for the database search. Major mass peaks detected by the MS analysis and the corresponding amino acid sequences of human proMMP-9 were as follows: m/z 671.07 for 678FYWR681, m/z 873.26 for 99CGVPDLGR106, m/z 1,001.37 for 578KLFFFSGR585, m/z 1,532.7 for 586QVWVYTGASVLGPR599, m/z 1,680.78 for 144AFALWSAVTPLTFTR158, and m/z 1,084.35 for 107FQTFEGDLK115 (GenBank accession number AAM97934: amino acid sequence for human MMP-9). The Mowse score and the sequence coverage of this analysis were 266 and 53%, respectively. Based on these data, the ∼100-kDa protein bound to His6-SSL5-Sepharose was identified as human proMMP-9.
FIG. 1.
Isolation of SSL5-binding proteins from human neutrophils. (A) Electrophoretic analysis of recombinant His-tagged SSL5, SSL7, and SSL9. These recombinant proteins were separated by SDS-polyacrylamide gel (12.5%) electrophoresis and stained with CBB. (B) The proteins bound to His6-SSL5-Sepharose were separated by electrophoresis on an SDS-polyacrylamide gel (10%) and stained with CBB. The major band (indicated by an arrowhead in lane 2) was subjected to peptide mass fingerprinting analysis. The whole neutrophil cell lysate (lane 1) and the fraction bound to control Sepharose (lane 3) were also applied to the gel.
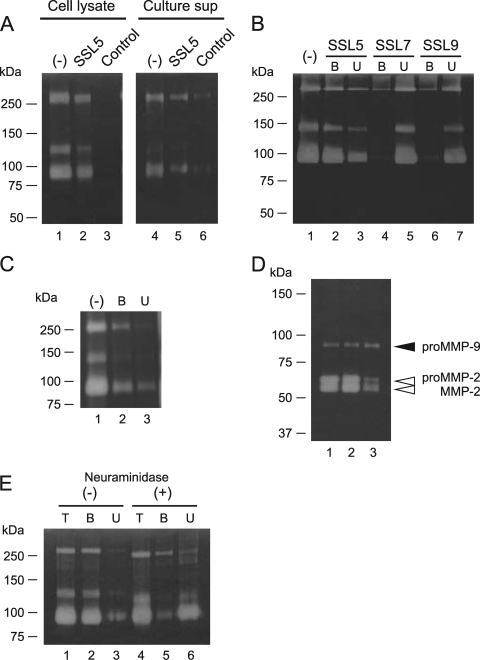
The interaction between His6-SSL5 and proMMP-9 was further examined by pull-down assay in combination with gelatin zymography. The neutrophil cell lysate and culture supernatant of IL-8-treated neutrophils were separately incubated with His6-SSL5-Sepharose, and the materials bound to His6-SSL5-Sepharose were analyzed by gelatin zymography. As shown in Fig. 2 A, both the bound fraction derived from the cell lysate and that derived from the culture supernatant gave three bands, which were assumed to be three forms of neutrophil proMMP-9—i.e., proMMP-9 monomer (∼92 kDa), a complex of proMMP-9 and neutrophil gelatinase-associated lipocalin (NGAL) (∼120 kDa), and proMMP-9 dimer (∼250 kDa) (25). The interaction between His6-SSL5 and proMMP-9 appears to be specific, because proMMP-9 from the culture supernatant of IL-8-treated neutrophils was not bound by His6-SSL7- or His6-SSL9-Sepharose (Fig. 2B). When we used tag-free SSL5-Sepharose prepared from the GST-SSL fusion protein, the bound fraction gave a similar electrophoretic profile (Fig. 2C). To examine whether His6-SSL5 binds to proMMP-9 from sources other than human neutrophils, we incubated conditioned medium of HT1080 human fibrosarcoma cells with His6-SSL5-Sepharose and analyzed the bound fraction by gelatin zymography. The band corresponding to proMMP-9 was detected with an intensity comparable to that obtained from the fraction bound to gelatin-Sepharose, which was used as a positive control (Fig. 2D, lanes 2 and 3). In contrast, the recovery of the band corresponding to proMMP-2 (gelatinase A) in the His6-SSL5-Sepharose-bound fraction was significantly lower than that in the gelatin-Sepharose-bound fraction.
FIG. 2.
Zymographic analysis of SSL5-binding proteins from human neutrophils. (A) Proteins recovered from the neutrophil cell lysate (lanes 1 to 3) and the culture supernatant of IL-8-treated neutrophils (lanes 4 to 6) by His6-SSL5-Sepharose were subjected to gelatin zymography. Lanes 1 and 4, unfractionated proteins; lanes 2 and 5, fractions bound to His6-SSL5-Sepharose; lanes 3 and 6, fractions bound to control Sepharose. (B) Culture supernatants of IL-8-stimulated neutrophils were incubated with His6-SSL5-Sepharose (lanes 2 and 3), His6-SSL7-Sepharose (lanes 4 and 5), or His6-SSL9-Sepharose (lanes 6 and 7) at 4°C for 1 h, and the bound (lanes 2, 4, and 6) and unbound (lanes 3, 5, and 7) fractions were subjected to gelatin zymography. The unfractionated culture supernatant was also subjected to the gel (lane 1). (C) The fractions bound (lane 2) and unbound (lane 3) to tag-free SSL5-Sepharose (prepared from the GST-SSL5 fusion protein) were analyzed by gelatin zymography. The unfractionated culture supernatant was also subjected to the gel (lane 1). (D) Serum-free conditioned media of HT1080 human fibrosarcoma cells were concentrated by ultrafiltration and fractionated with gelatin-Sepharose or His6-SSL5-Sepharose. Lane 1, unfractionated conditioned medium; lane 2, fraction bound to gelatin-Sepharose; lane 3, fraction bound to His6-SSL5-Sepharose. (E) ProMMP-9 purified from human neutrophils was treated with (lanes 4 to 6) or without (lanes 1 to 3) neuraminidasae (5 mU/ml) at 37°C for 1 h and incubated with His6-SSL5-Sepharose at 4°C for 1 h. Lanes 1 and 4, unfractionated sample; lanes 2 and 5, bound fractions; lanes 3 and 6, unbound fractions.
Since SSL5 has been reported to recognize sialic acid-containing oligosaccharide chains (3), we examined the binding of SSL5 to proMMP-9 purified from human neutrophils after neuraminidase treatment. As shown in Fig. 2E, desialylated proMMP-9 was recovered almost exclusively in the unbound fraction. Thus, removal of sialic acid residues from proMMP-9 reduced its binding affinity to His6-SSL5, suggesting that sialic acid-containing oligosaccharides are involved in the SSL5-MMP-9 interaction.
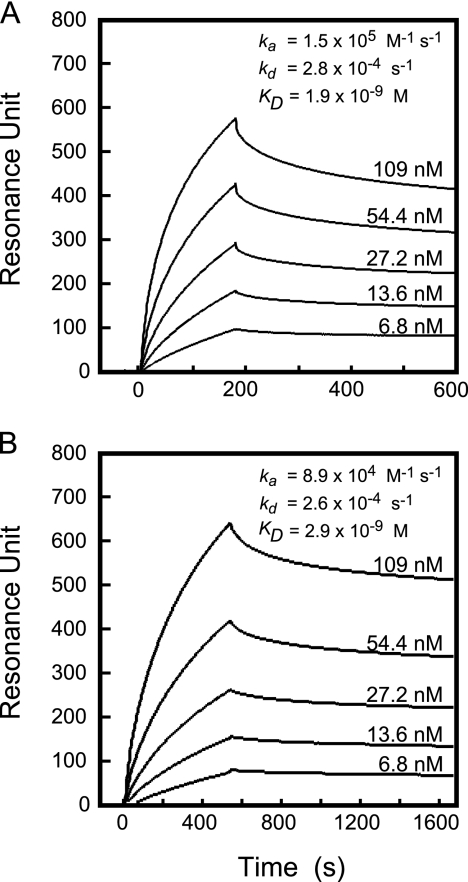
We next conducted a kinetic study of the interaction between His6-SSL5 and proMMP-9 by surface plasmon resonance (SPR) analysis with a Biacore system (Fig. 3 A). ProMMP-9 (6.8 to 109 nM) purified from neutrophils was analyzed on a His6-SSL5-immobilized sensor chip. Based on the sensorgrams, kinetic parameters were calculated using BIAevaluation software (GE Healthcare); the association rate constant (ka), dissociation rate constant (kd), and dissociation equilibrium constant (KD), values were estimated to be as follows: ka = 1.5 × 105 (1/Ms), kd = 2.8 × 10−4 (1/s), and KD = 1.9 nM, respectively. These data indicate that His6-SSL5 binds to proMMP-9 with rather high affinity. We subsequently analyzed the kinetics of the His6-SSL5 binding to an active form of MMP-9. After proMMP-9 was chemically activated by treatment with a sulfhydryl reagent, APMA (1 mM), at 37°C for 16 h, the activated MMP-9 thus obtained was subjected to SPR analysis in a manner similar to that described above. As shown in Fig. 3B, the KD value for activated MMP-9 was 2.9 nM, which was roughly comparable to the value for proMMP-9.
FIG. 3.
Sensorgram of binding of proMMP-9 to immobilized SSL5. ProMMP-9 from human neutrophils at various concentrations (6.8 to 109 nM) was allowed to bind to His6-SSL5 that had been immobilized on a CM5 sensor chip for 3 min at a flow rate of 0.02 ml/min and examined by surface plasmon resonance with a Biacore 2000 system (GE Healthcare) as described in Materials and Methods. (A) Unactivated proMMP-9; (B) APMA-activated MMP-9.
Inhibition of enzymatic activity of MMP-9 by His6-SSL5.
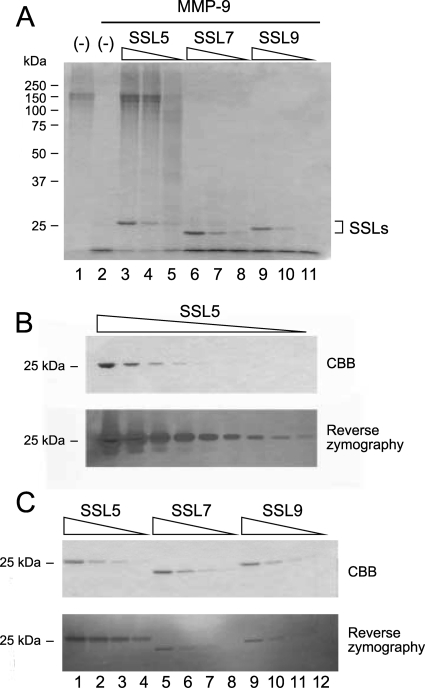
We examined the effects of His6-SSL5 on the enzymatic activity of MMP-9 using gelatin as a substrate. Neutrophil MMP-9 pretreated with APMA was incubated with gelatin (0.5 mg/ml) for 16 h, and the reaction mixture was analyzed by SDS-polyacrylamide gel electrophoresis. As shown in Fig. 4 A, APMA-activated MMP-9 completely degraded gelatin (lane 2), and the gelatinolysis catalyzed by MMP-9 was inhibited by the addition of His6-SSL5 (lanes 3 to 5) in a dose-dependent manner. However, neither His6-SSL7 (lanes 6 to 8) nor His6-SSL9 (lanes 9 to 11) inhibited the gelatinolytic activity of MMP-9 under these conditions. The inhibitory activity of His6-SSL5 was confirmed by reverse zymography using a polyacrylamide gel containing gelatin and purified MMP-9. When a series of His6-SSL5 dilutions was subjected to reverse zymography, the areas corresponding to His6-SSL5 remained more-intensely stained bands after CBB staining and destaining compared to bands for His6-SSL5 in regular SDS gel electrophoresis in the absence of gelatin and MMP-9 (Fig. 4B). His6-SSL7 and His6-SSL9, however, did not inhibit gelatinolysis catalyzed by MMP-9 (Fig. 4C).
FIG. 4.
Inhibition of the gelatinolytic activity of MMP-9 by SSLs. (A) Gelatin (0.5 mg/ml; 0.03 ml) was incubated with MMP-9 purified from human neutrophils (3.7 ng; final concentration, 1.3 nM) in the presence of various concentrations (5.6 to 50 ng/0.03 ml [7.3 to 65 nM], 3-fold serial dilutions) of His6-SSL5 (lanes 3 to 5), His6-SSL7 (lanes 6 to 8), or His6-SSL9 (lanes 9 to 11) at 37°C for 16 h as described in Materials and Methods. The reaction mixtures were analyzed by SDS-polyacrylamide gel (10%) electrophoresis followed by staining with CBB. Gelatin was also incubated with (lane 2) or without (lane 1) MMP-9 in the absence of SSLs as controls. (B) The inhibitory activity of His6-SSL5 was examined by reverse zymography. His6-SSL5 (0.023 to 150 μg/lane, 3-fold serial dilution) was loaded on a polyacrylamide gel (12.5%) containing gelatin (1.5 mg/ml) and MMP-9 (10.9 ng/ml). After electrophoresis, the gel was processed as described in Materials and Methods. (C) His6-SSL5, His6-SSL7, and His6-SSL9 (1.9 to 50 ng/lane, 3-fold serial dilution) were subjected to reverse zymography using a polyacrylamide gel (12.5%) containing gelatin and MMP-9 as described above.
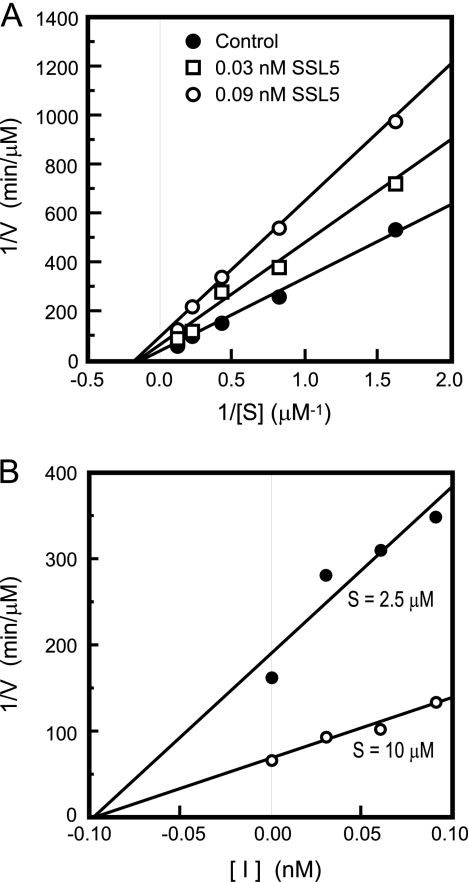
We next conducted kinetic analysis of the inhibition of MMP-9 activity by His6-SSL5 using a fluorescence-quenching substrate, MOCAc-PLGly-LA2pr(Dnp)-AR-NH2. The Lineweaver-Burk plot revealed that the slope and the y intercept of the plots obtained in the presence of distinct concentrations of His6-SSL5 varied significantly (Fig. 5 A) but that the x intercept was unchanged, indicating that His6-SSL5 is a noncompetitive inhibitor. The Ki value was also determined by Dixon plot analysis to be 0.097 nM (Fig. 5B).
FIG. 5.
Kinetic analysis of the inhibition of MMP-9 by His6-SSL5. (A) The Lineweaver-Burk plots of the hydrolysis of a fluorogenic peptide substrate (0.625, 1.25, 2.5, 5.0, and 10.0 μM) by APMA-activated MMP-9 in the absence (closed circles) or presence of His6-SSL5 at 0.03 nM (open squares) or 0.09 nM (open circles). (B) Dixon plots with a fluorogenic peptide substrate at 2.5 μM (closed circles) or 10 μM (open circles) in the presence of His6-SSL5 (0 to 0.09 nM).
Inhibition of neutrophil migration through reconstituted membranes by His6-SSL5.
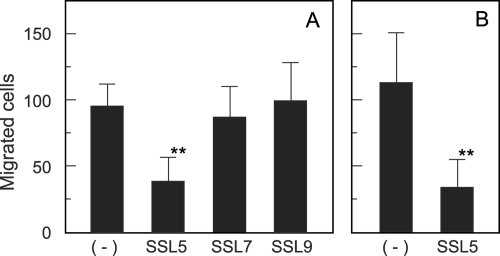
MMP-9 has been suggested to play a crucial role in the recruitment of neutrophils to inflammatory tissues (8, 17). We therefore assessed how His6-SSL5 affects the migration of neutrophils through extracellular matrix using the Boyden chamber system. The membrane between the upper and the lower chambers was coated with Matrigel or gelatin, and the formyl peptide FMLP (1 μM) was added to the lower chamber as a chemoattractant. Human neutrophils were then placed in the upper chamber, and the chambers were incubated at 37°C for 2 h to allow the cells to migrate through the membranes. Neutrophil transmigration induced by FMLP was effectively suppressed in the presence of MMP-9 inhibitor I or MMP-2/MMP-9 inhibitor I for either Matrigel- or gelatin-coated membranes (data not shown), confirming the previous observation that MMP-9 plays a crucial role in neutrophil invasion into the Matrigel basement membrane (8). Next, we examined the effect of His6-SSL5 on neutrophil transmigration across Matrigel-coated membranes. The addition of His6-SSL5 (10 μg/ml) to the upper chamber resulted in significant inhibition of the migration of neutrophils into the lower chamber through Matrigel-coated membranes (Fig. 6 A), whereas almost no inhibition was observed in the presence of His6-SSL7 or His6-SSL9. The transmigration across gelatin-coated membranes was similarly decreased by the addition of His6-SSL5 to approximately 30% of the control level (Fig. 6B).
FIG. 6.
Suppression by His6-SSL5 of FMLP-induced neutrophil transmigration across Boyden chamber membranes. A human neutrophil suspension in serum-free RPMI 1640 medium (1 × 105 cells/0.2 ml) was placed in the upper chamber, and RPMI 1640 medium containing 1 μM FMLP was added to the lower chamber. The chambers were then incubated at 37°C for 2 h, and cells that had migrated to the bottom surface of the membrane were counted under a microscope after being stained with crystal violet. The membranes between the upper and lower chambers had been coated with Matrigel (A) or gelatin (B). The assay was conducted in the presence or absence of His6-SSL5, His6-SSL7, or His6-SSL9 (10 μg/ml). The data are shown as the means with standard deviations of results for 9 fields selected at random. **, P < 0.01.
DISCUSSION
In this study, we provide lines of evidence that SSL5 specifically binds to human neutrophil MMP-9 and inhibits its enzymatic activity. MMP-9 has been thought to play crucial roles in leukocyte functions because inhibition of gelatinase activity has been shown to impair neutrophil infiltration of inflammatory tissues (5, 8, 23). The present study also showed that the transmigration of neutrophils across Matrigel basement membranes or gelatin-coated membranes was suppressed in the presence of recombinant SSL5, probably due to its inhibitory effect on MMP-9. Because neutrophils produce MMP-9 as a sole gelatinase but almost no MMP-2 (25), it is likely that neutrophil migration through collagenous connective tissues is seriously influenced by SSL5. Neutrophils function as the first line of host defense against bacterial infection, and thus, impairment of neutrophil recruitment surely results in an increased severity of infection. Calander et al. (6) evaluated the pathophysiological roles of MMP-9 in bacterially induced septic arthritis by using MMP-9-deficient mice and found that the deficient mice displayed a significantly high frequency and severity of arthritis and increased persistence of S. aureus compared to the wild-type mice. The aggravation of pathology in the deficient mice was thought to be caused by interference with extravasation and recruitment of leukocytes, which led to decreased bacterial clearance, indicating that MMP-9 plays an essential role in the defense against S. aureus infection.
SSL5 was previously reported to bind to leukocyte PSGL-1 and inhibit the interaction of PSGL-1 with P-selectin, an adhesion molecule expressed on activated endothelial cells (4). Because the P-selectin/PSGL-1 interaction plays a key role in leukocyte adhesion to activated endothelium, it is likely that SSL5 impairs tethering and rolling of leukocytes on activated endothelium and subsequent leukocyte extravasation. Taken together, these results suggest that S. aureus utilizes SSL5 to escape from host immunity through synergic inhibition of matrix-degrading enzymes, including MMP-9, and of leukocyte-endothelium adhesion. We also observed the interaction between SSL5 and PSGL-1 in the present study. PSGL-1 was recovered in the His6-SSL5-Sepharose-bound fraction when human neutrophil lysate was incubated with immobilized His6-SSL5. The KD value for the interaction between PSGL-1 and MMP-9 was reported to be 820 nM (4), whereas the KD value for proMMP-9/His6-SSL5 binding was estimated to be 1.9 nM (Fig. 3), indicating that SSL5 has a higher affinity for MMP-9 than for PSGL-1. The crystallographic analysis demonstrated that the binding of SSL5 to PSGL-1 was mediated mainly by carbohydrate chains of PSGL-1, especially those containing a sialyl lactosamine motif (3). Human neutrophil MMP-9 is a glycoprotein with a carbohydrate content of approximately 15% (wt/wt), and most carbohydrate chains are of the O-linked type (28). Thus, neutrophil MMP-9 shares a common type of carbohydrate unit with PSGL-1. When MMP-9 that was desialylated after neuraminidase treatment was examined for its ability to bind His6-SSL5 by SPR analysis, the KD value was increased about 5-fold, indicating that the removal of sialic acid residues from MMP-9 reduces the affinity to His6-SSL5 (S. Itoh, E. Hamada, and T. Tsuji, unpublished observation). This result suggested that sialic acid-containing carbohydrate chains of MMP-9 are involved in the SSL5/MMP-9 interaction. Van den Steen et al. similarly observed that the sensitivity of MMP-9 to its endogenous inhibitor TIMP-1 was attenuated after desialylation of MMP-9 (31). The carbohydrate moiety of MMP-9 may play a significant role in the regulation of its enzymatic activity through interaction with inhibitors.
The expression of SSLs has frequently been observed in virulent strains of methicillin-resistant S. aureus (MRSA) isolated from individuals with community-acquired infections and is increased upon phagocytosis by neutrophils (13, 32). Al-Shangiti et al. suggested that SSL7 and SSL9 affected the functions of antigen-presenting cells after their incorporation into monocytes and dendritic cells (1). A recent report also showed that SSL7 bound to IgA and complement component C5, causing inhibition of IgA binding to its receptor on phagocytes and complement-dependent bactericidal activity (19). These observations have suggested that SSLs are implicated in the pathogenicity of bacterial infections, presumably through their immunosuppressive activities.
The results provided in the present and preceding studies support the notion that the production of SSL5 by S. aureus facilitates the ability of bacteria to evade the host immune system via suppression of leukocyte trafficking caused by synergic inhibition of the P-selectin/PSGL-1 interaction and MMP-9 activity. To our knowledge, this is the first reported example of a bacterial exoprotein inhibiting host MMP activity. However, many other members of the SSL protein family remain functionally uncharacterized, and future studies should focus on elucidation of the functional roles of exoproteins, including SSLs, and their relevance to the pathogenesis of S. aureus infection.
Acknowledgments
We are grateful to Masaaki Kurihara (Kurihara Clinic, Tokyo, Japan) for his helpful discussion. We also thank Satoko Sakai and Makiko Masuyama (Hoshi University School of Pharmacy and Pharmaceutical Sciences) for their technical assistance.
This work was supported in part by the Ministry of Education, Culture, Sports, Science and Technology of Japan and by the Open Research Center Project.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 17 May 2010.
REFERENCES
- 1.Al-Shangiti, A. M., C. E. Naylor, S. P. Nair, D. C. Briggs, B. Henderson, and B. M. Chain. 2004. Structural relationships and cellular tropism of staphylococcal superantigen-like proteins. Infect. Immun. 72:4261-4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arcus, V., R. Langley, T. Proft, J. Fraser, and E. Baker. 2002. The Three-dimensional structure of a superantigen-like protein, SET3, from a pathogenicity island of the Staphylococcus aureus genome. J. Biol. Chem. 277:32274-32281. [DOI] [PubMed] [Google Scholar]
- 3.Baker, H. M., I. Basu, M. C. Chung, T. Caradoc-Davies, J. D. Fraser, and E. N. Baker. 2007. Crystal structures of the staphylococcal toxin SSL5 in complex with sialyl Lewis X reveal a conserved binding site that shares common features with viral and bacterial sialic acid binding proteins. J. Mol. Biol. 374:1298-1308. [DOI] [PubMed] [Google Scholar]
- 4.Bestebroer, J., M. J. Poppelier, L. H. Ulfman, P. J. Lenting, C. V. Denis, K. P. van Kessel, J. A. van Strijp, and C. J. de Haas. 2007. Staphylococcal superantigen-like 5 binds PSGL-1 and inhibits P-selectin-mediated neutrophil rolling. Blood 109:2936-2943. [DOI] [PubMed] [Google Scholar]
- 5.Betsuyaku, T., J. M. Shipley, Z. Liu, and R. M. Senior. 1999. Neutrophil emigration in the lungs, peritoneum, and skin does not require gelatinase B. Am. J. Respir. Cell Mol. Biol. 20:1303-1309. [DOI] [PubMed] [Google Scholar]
- 6.Calander, A. M., S. Starckx, G. Opdenakker, P. Bergin, M. Quiding-Jarbrink, and A. Tarkowski. 2006. Matrix metalloproteinase-9 (gelatinase B) deficiency leads to increased severity of Staphylococcus aureus-triggered septic arthritis. Microbes Infect. 8:1434-1439. [DOI] [PubMed] [Google Scholar]
- 7.Chavakis, T., M. Hussain, S. M. Kanse, G. Peters, R. G. Bretzel, J. I. Flock, M. Herrmann, and K. T. Preissner. 2002. Staphylococcus aureus extracellular adherence protein serves as anti-inflammatory factor by inhibiting the recruitment of host leukocytes. Nat. Med. 8:687-693. [DOI] [PubMed] [Google Scholar]
- 8.Delclaux, C., C. Delacourt, M. P. D'Ortho, V. Boyer, C. Lafuma, and A. Harf. 1996. Role of gelatinase B and elastase in human polymorphonuclear neutrophil migration across basement membrane. Am. J. Respir. Cell Mol. Biol. 14:288-295. [DOI] [PubMed] [Google Scholar]
- 9.Dinges, M. M., P. M. Orwin, and P. M. Schlievert. 2000. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 13:16-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzgerald, J. R., S. D. Reid, E. Ruotsalainen, T. J. Tripp, M. Liu, R. Cole, P. Kuusela, P. M. Schlievert, A. Jarvinen, and J. M. Musser. 2003. Genome diversification in Staphylococcus aureus: molecular evolution of a highly variable chromosomal region encoding the Staphylococcal exotoxin-like family of proteins. Infect. Immun. 71:2827-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster, T. J. 2005. Immune evasion by staphylococci. Nat. Rev. Microbiol. 3:948-958. [DOI] [PubMed] [Google Scholar]
- 12.Garzoni, C., P. Francois, A. Huyghe, S. Couzinet, C. Tapparel, Y. Charbonnier, A. Renzoni, S. Lucchini, D. P. Lew, P. Vaudaux, W. L. Kelley, and J. Schrenzel. 2007. A global view of Staphylococcus aureus whole genome expression upon internalization in human epithelial cells. BMC Genomics 8:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holtfreter, S., K. Bauer, D. Thomas, C. Feig, V. Lorenz, K. Roschack, E. Friebe, K. Selleng, S. Lovenich, T. Greve, A. Greinacher, B. Panzig, S. Engelmann, G. Lina, and B. M. Broker. 2004. egc-encoded superantigens from Staphylococcus aureus are neutralized by human sera much less efficiently than are classical staphylococcal enterotoxins or toxic shock syndrome toxin. Infect. Immun. 72:4061-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itoh, S., C. Susuki, K. Takeshita, K. Nagata, and T. Tsuji. 2007. Redistribution of P-selectin glycoprotein ligand-1 (PSGL-1) in chemokine-treated neutrophils: a role of lipid microdomains. J. Leukoc. Biol. 81:1414-1421. [DOI] [PubMed] [Google Scholar]
- 15.Johnston, G. I., R. G. Cook, and R. P. McEver. 1989. Cloning of GMP-140, a granule membrane protein of platelets and endothelium: sequence similarity to proteins involved in cell adhesion and inflammation. Cell 56:1033-1044. [DOI] [PubMed] [Google Scholar]
- 16.Knight, C. G., F. Willenbrock, and G. Murphy. 1992. A novel coumarin-labelled peptide for sensitive continuous assays of the matrix metalloproteinases. FEBS Lett. 296:263-266. [DOI] [PubMed] [Google Scholar]
- 17.Kolaczkowska, E., M. Chadzinska, A. Scislowska-Czarnecka, B. Plytycz, G. Opdenakker, and B. Arnold. 2006. Gelatinase B/matrix metalloproteinase-9 contributes to cellular infiltration in a murine model of zymosan peritonitis. Immunobiology 211:137-148. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli, U. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.Langley, R., B. Wines, N. Willoughby, I. Basu, T. Proft, and J. D. Fraser. 2005. The staphylococcal superantigen-like protein 7 binds IgA and complement C5 and inhibits IgA-Fc alpha RI binding and serum killing of bacteria. J. Immunol. 174:2926-2933. [DOI] [PubMed] [Google Scholar]
- 20.Levin, J. I., J. M. Chen, M. T. Du, F. C. Nelson, T. Wehr, J. F. DiJoseph, L. M. Killar, S. Skala, A. Sung, M. A. Sharr, C. E. Roth, G. Jin, R. Cowling, L. Di, M. Sherman, Z. B. Xu, C. J. March, K. M. Mohler, R. A. Black, and J. S. Skotnicki. 2001. The discovery of anthranilic acid-based MMP inhibitors. Part 3: incorporation of basic amines. Bioorg. Med. Chem. Lett. 11:2975-2978. [DOI] [PubMed] [Google Scholar]
- 21.Masure, S., P. Proost, J. Van Damme, and G. Opdenakker. 1991. Purification and identification of 91-kDa neutrophil gelatinase. Release by the activating peptide interleukin-8. Eur. J. Biochem. 198:391-398. [DOI] [PubMed] [Google Scholar]
- 22.McEver, R. P., and R. D. Cummings. 1997. Role of PSGL-1 binding to selectins in leukocyte recruitment. J. Clin. Invest. 100:S97-S103. [PubMed] [Google Scholar]
- 23.Nagaoka, I., and S. Hirota. 2000. Increased expression of matrix metalloproteinase-9 in neutrophils in glycogen-induced peritoneal inflammation of guinea pigs. Inflamm. Res. 49:55-62. [DOI] [PubMed] [Google Scholar]
- 24.Oliver, G. W., J. D. Leferson, W. G. Stetler-Stevenson, and D. E. Kleiner. 1997. Quantitative reverse zymography: analysis of picogram amounts of metalloproteinase inhibitors using gelatinase A and B reverse zymograms. Anal. Biochem. 244:161-166. [DOI] [PubMed] [Google Scholar]
- 25.Opdenakker, G., P. E. Van den Steen, B. Dubois, I. Nelissen, E. Van Coillie, S. Masure, P. Proost, and J. Van Damme. 2001. Gelatinase B functions as regulator and effector in leukocyte biology. J. Leukoc. Biol. 69:851-859. [PubMed] [Google Scholar]
- 26.Postma, B., M. J. Poppelier, J. C. van Galen, E. R. Prossnitz, J. A. van Strijp, C. J. de Haas, and K. P. van Kessel. 2004. Chemotaxis inhibitory protein of Staphylococcus aureus binds specifically to the C5a and formylated peptide receptor. J. Immunol. 172:6994-7001. [DOI] [PubMed] [Google Scholar]
- 27.Rooijakkers, S. H., M. Ruyken, A. Roos, M. R. Daha, J. S. Presanis, R. B. Sim, W. J. van Wamel, K. P. van Kessel, and J. A. van Strijp. 2005. Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat. Immunol. 6:920-927. [DOI] [PubMed] [Google Scholar]
- 28.Rudd, P. M., T. S. Mattu, S. Masure, T. Bratt, P. E. Van den Steen, M. R. Wormald, B. Kuster, D. J. Harvey, N. Borregaard, J. Van Damme, R. A. Dwek, and G. Opdenakker. 1999. Glycosylation of natural human neutrophil gelatinase B and neutrophil gelatinase B-associated lipocalin. Biochemistry 38:13937-13950. [DOI] [PubMed] [Google Scholar]
- 29.Tamura, Y., F. Watanabe, T. Nakatani, K. Yasui, M. Fuji, T. Komurasaki, H. Tsuzuki, R. Maekawa, T. Yoshioka, K. Kawada, K. Sugita, and M. Ohtani. 1998. Highly selective and orally active inhibitors of type IV collagenase (MMP-9 and MMP-2): N-sulfonylamino acid derivatives. J. Med. Chem. 41:640-649. [DOI] [PubMed] [Google Scholar]
- 30.Tsuji, T., Y. Kawada, M. Kai-Murozono, S. Komatsu, S. Han, K. Takeuchi, H. Mizushima, K. Miyazaki, and T. Irimura. 2002. Regulation of melanoma cell migration and invasion by laminin-5 and α3β1 integrin (VLA-3). Clin. Exp. Metastasis 19:127-134. [DOI] [PubMed] [Google Scholar]
- 31.Van den Steen, P. E., G. Opdenakker, M. R. Wormald, R. A. Dwek, and P. M. Rudd. 2001. Matrix remodelling enzymes, the protease cascade and glycosylation. Biochim. Biophys. Acta 1528:61-73. [DOI] [PubMed] [Google Scholar]
- 32.Voyich, J. M., K. R. Braughton, D. E. Sturdevant, A. R. Whitney, B. Said-Salim, S. F. Porcella, R. D. Long, D. W. Dorward, D. J. Gardner, B. N. Kreiswirth, J. M. Musser, and F. R. DeLeo. 2005. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 175:3907-3919. [DOI] [PubMed] [Google Scholar]
- 33.Williams, R. J., J. M. Ward, B. Henderson, S. Poole, B. P. O'Hara, M. Wilson, and S. P. Nair. 2000. Identification of a novel gene cluster encoding staphylococcal exotoxin-like proteins: characterization of the prototypic gene and its protein product, SET1. Infect. Immun. 68:4407-4415. [DOI] [PMC free article] [PubMed] [Google Scholar]