Abstract
Infection models are essential tools for studying microbial pathogenesis. Murine models are considered the “gold standard” for studying in vivo infections caused by Aspergillus species, such as A. fumigatus. Recently developed molecular protocols allow rapid construction of high numbers of fungal deletion mutants, and alternative infection models based on cell culture or invertebrates are widely used for screening such mutants to reduce the number of rodents in animal experiments. To bridge the gap between invertebrate models and mice, we have developed an alternative, low-cost, and easy-to-use infection model for Aspergillus species based on embryonated eggs. The outcome of infections in the egg model is dose and age dependent and highly reproducible. We show that the age of the embryos affects the susceptibility to A. fumigatus and that increased resistance coincides with altered chemokine production after infection. The progress of disease in the model can be monitored by using egg survival and histology. Based on pathological analyses, we hypothesize that invasion of embryonic membranes and blood vessels leads to embryonic death. Defined deletion mutant strains previously shown to be fully virulent or partially or strongly attenuated in a mouse model of bronchopulmonary aspergillosis showed comparable degrees of attenuation in the egg model. Addition of nutrients restored the reduced virulence of a mutant lacking a biosynthetic gene, and variations of the infectious route can be used to further analyze the role of distinct genes in our model. Our results suggest that embryonated eggs can be a very useful alternative infection model to study A. fumigatus virulence and pathogenicity.
Aspergillus fumigatus is a ubiquitous mold with the ability to cause life-threatening disease in immunocompromised human patients (19). Despite the development of novel antimycotics, the lethality of invasive aspergillosis in human patients is still high and can exceed 50% (14, 57). Thus, a better understanding of the infectious process and the fungal factors involved in pathogenesis is essential for development of more effective treatments. One approach to identify virulence factors is to study fungal gene deletion mutants in appropriate infection models. The most commonly used infection models for A. fumigatus are laboratory rodents, especially mice. These models are well characterized and have been critical for understanding host-pathogen interactions, as well as for developing better therapeutic approaches (11). However, ethical considerations and legal restrictions limit the use of mammals for infection studies. Additionally, animal experiments are costly and require specialized facilities and specially trained personal, which further limits the availability of mammalian models to researchers. Therefore, several alternative infection models for fungal pathogens have been developed in recent years, ranging from cell lines and tissue cultures to invertebrate hosts. Insect models, like the fruit fly (Drosophila melanogaster) (28, 29) and larvae of the greater wax moth (Galleria mellonella) (21, 30, 37, 42, 43), have been successfully used to investigate virulence traits of A. fumigatus and the efficacy of antimycotic treatment. However, these models are limited compared to mammalian hosts; the limitations include, for example, the low temperature required for experiments, the route of infection, and certain aspects of the immune response. One possible way to bridge the gap between insect and mammalian hosts is to use embryonated eggs as an alternative infection model.
Birds of all ages are susceptible to aspergillosis, and this disease has been described for a variety of avian species, both birds in captivity and wild birds (54). Aspergillosis outbreaks can cause significant economic losses in the poultry industry (41) and have prompted the development of vaccination strategies to prevent Aspergillus infections (46). The avian immune response to Aspergillus infections is similar to the mammalian response with respect to the beneficial effect of a type 1 response, the role of macrophages and heterophils (avian immune cells similar to mammalian neutrophils), and the production of specific antibodies (10, 27, 33). Although these features suggest that birds could be an interesting and potential animal model for studying aspergillosis (11, 47), the same ethical, legal, financial, and logistical restrictions that apply to experiments with mammals apply to experiments with birds. However, embryonated bird eggs could provide an alternative model, which would fulfill the demand for refinement of animal models. Fertilized eggs are readily available from commercial breeders at low cost, are easy to handle, and require little specialized equipment and no specialized facilities or personal. Since embryonated eggs have been successfully used to study virulence features of various bacteria (2, 55) and the fungal pathogen Candida albicans (16, 17), we investigated the potential of embryonated eggs as an alternative model for studying the virulence of A. fumigatus.
MATERIALS AND METHODS
A. fumigatus strains and preparation of conidia for infection.
A. fumigatus wild-type strains CBS 144.89 (Centralbureau voor Schimmelcultures, Utrecht, Netherlands), ATCC 46645 (American Type Culture Collection, Manassas, VA), and Af293 (Fungal Research Trust) (www.aspergillus.man.ac.uk), as well as laboratory strains CEA17ΔakuB (13) and AfS35 (24), which are deficient for nonhomologous recombination, were used in this study. ΔacuD, ΔmcsA, ΔhcsA, and ΔgliP deletion mutants and corresponding complemented strains have been described previously (20, 25, 48, 49). Malt agar slants or plates were inoculated with conidia and incubated at room temperature for 7 days. Conidia were suspended in sterile phosphate-buffered saline (PBS) containing 0.1% Tween 20 (AppliChem GmbH, Darmstadt, Germany) and filtered through a 40-μm cell strainer (BD Bioscience, Heidelberg, Germany). After the conidia were counted, the suspension was diluted with PBS to obtain the desired concentration and used within a few hours for egg infection. PBS alone was used as a negative control for infection experiments.
Preparation of eggs and infection of the CAM.
Fertilized “weiße Leghorn” chicken eggs were obtained from a local producer and stored at 8°C for a maximum of 7 days prior to incubation. Incubation was performed at 37.6°C and 50 to 60% relative humidity in a specialized incubator (BSS 300; Grumbach, Germany). From the fourth day of incubation onward, the eggs were turned four times a day until they were infected. Vitality was assessed daily by candling. Infection of the chorioallantoic membrane (CAM) was performed as described by Härtl et al. (17). Briefly, after the shell was wiped with a disinfectant (Braunol; Braun, Melsungen, Germany), it was perforated at the blunt end and on the side using a dentist's drill. An artificial air chamber on the side was then formed by applying negative pressure at the hole in the blunt end. After perforation of the shell membrane, a 0.1-ml inoculum was applied via the artificial air chamber to the CAM using a sterile 1-ml syringe. The holes were then sealed with paraffin. Survival was monitored for up to 8 days by candling. Unless indicated otherwise, eggs were infected on day 10 of incubation (“developmental day 10”). Twenty eggs were infected for each group, and experiments were repeated at least two times. Survival data were plotted as Kaplan-Meyer curves and were analyzed statistically by a log rank test using Graph Pad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA).
Lysine supplementation.
For lysine supplementation experiments, harvested conidia were diluted to obtain the desired concentration in PBS containing either 2.5 mM or 5 mM lysine. Additional lysine doses on days 1 to 4 postinfection (p.i.) were applied using 100 μl 5 mM lysine in PBS via the hole used for infection with a sterile needle and syringe.
i.p. infection of chicken embryos.
For intraperitoneal (i.p.) infection, an artificial air chamber was constructed as described above. A small circular saw blade on a dentist's drill was then used to carefully cut a 1- by 1.5-cm window into the shell on the side of the egg without damaging the CAM. The cut shell fragment was removed with sterile forceps. The embryo was located through the intact CAM and carefully grabbed with atraumatic sterile forceps to bring it into a position which allowed intraperitoneal injection through the intact CAM. The window was covered with UV-sterilized Parafilm, which was fixed to the shell with adhesive tape. Twenty eggs per group were infected, and the experiments were repeated two times. Survival data were plotted as Kaplan-Meyer curves and were analyzed statistically by a log rank test using Graph Pad Prism version 5.00 for Windows.
Reisolation.
Dead eggs that were identified by candling or eggs that were humanely killed by chilling on ice for 1 h followed by decapitation and exsanguination were randomly selected from various experiments. Following disinfection of the surface of an egg with 70% ethanol, the shell was opened with sterile scissors. The CAM surrounding the shell perforation site was removed with sterile forceps and swabbed onto malt agar plates. The embryo was then carefully separated from its surrounding membranes and placed in 70% ethanol for 2 to 3 min. Using sterile scissors and forceps, the abdominal cavity was opened. The liver was removed and swapped onto malt agar plates. The plates were incubated at room temperature for 54 h prior to macroscopic evaluation of fungal growth.
Histological examination of CAM and liver.
For sample preparation, embryos were killed by chilling the eggs on ice for 1 h. The egg surface was disinfected with 70% ethanol. Using sharp scissors, the upper half of each shell was cut and removed, leaving the CAM attached to the removed shell fragment. The CAM was then cut into samples that were suitable sizes and fixed in 10% formalin. The liver was removed during postmortem examination and fixed in 10% formalin. Samples were embedded in paraffin and cut into 5-μm sections. Mounted sections were stained with either hematoxylin and eosin (H&E) or periodic acid-Schiff (PAS) stain using standard protocols and were examined using bright-field microscopy at the magnification indicated below.
RNA isolation and transcription analysis using quantitative real-time RT-PCR.
Embryos were infected on the CAM as described above with 103 conidia of strain CEA17ΔakuB on either developmental day 10 or developmental day 12. At different time points, the embryos were killed by chilling the eggs on ice for 1 h, and the eggs were opened as described above. For PBS controls and infected eggs up to 24 h p.i., a round piece of the CAM (2 to 2.5 cm in diameter) surrounding the drilled hole was removed. At later time points, when fungal mycelia were visible, an 8-mm biopsy punch was used to excise and remove CAM adjacent to mycelia. Spleens were removed at postmortem examination. All samples were placed into RNAlater RNA stabilization reagent (Qiagen, Hilden, Germany) immediately after removal and incubated at 4°C overnight on a tube rotator prior to storage at −20°C. For total RNA isolation, 20 to 30 mg (CAM) or 5 mg (spleen) of tissue was used. Due to the small size of the spleens, two or three spleens were pooled for one RNA preparation. Sample lysis, removal of genomic DNA, and RNA isolation were performed with QIAshredder, RNase-free DNase Set, and an RNeasy minikit (all obtained from Qiagen) used according to the manufacturer's recommendations. The quality and quantity of eluted RNA were determined by spectral analysis using a NanoDrop ND-100 spectrophotometer (peqlab, Erlangen, Germany). Only samples with A260/A280 and A260/A230 ratios of about 2 were used in subsequent analyses. The concentration of RNA was adjusted to 100 ng/μl immediately before reverse transcription-PCR (RT-PCR) was performed. Previously described primers and temperature protocols for K60, inducible nitric oxide synthase (iNOS), gamma interferon (IFN-γ), and the internal control glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (5, 7, 40) were used. For macrophage inflammatory protein 1β (MIP-1β) the following primers were used with an annealing temperature of 59°C: forward primer 5′-TCCTGCTGCTTCACCTACATCT-3′ and reverse primer 5′-ATGAACACAACACCAGCATGAG-3′. Interleukin-1β (IL-1β) was analyzed using an annealing temperature of 57°C with the following primers: forward primer 5′-TTTTTGAGCCCGTCACCTTC-3′ and reverse primer 5′-TACAGCGCAATGTTGAGCCTC-3′. Quantitative real-time RT-PCRs were performed using a one-step quantitative RT-PCR MasterMix Plus for SYBR green I kit (Eurogentec, Seraing, Belgium) according to the manufacturer's instructions. Amplification and detection of specific products were performed with a 7300 real-time PCR system (Applied Biosystems, Foster City, CA) using the dissociation curve mode to check the specificity of amplification products. Specific mRNA levels were quantified using the threshold method (38) and were normalized using the changes in the cycle threshold (ΔCT values) for GAPDH. The results were expressed as fold changes compared to the PBS control determined by calculating 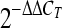 . Two groups at a given time point were compared by the unpaired, two-tailed t test using Graph Pad Prism version 5.00 for Windows.
. Two groups at a given time point were compared by the unpaired, two-tailed t test using Graph Pad Prism version 5.00 for Windows.
RESULTS
Dose-dependent mortality and comparison of different wild-type and laboratory A. fumigatus strains.
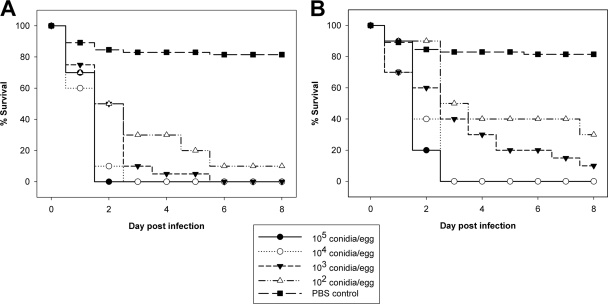
In order to determine the appropriate infectious dose and to determine whether A. fumigatus kills embryos in a dose-dependent manner, eggs were infected on developmental day 10 with 107 to 102 conidia of wild-type and laboratory strains (CBS 144.89, ATCC 46645, Af293, CEA17ΔakuB, and AfS35). Developmental day 10 was chosen as the time of infection since at this age viable embryos can be identified reliably and because initial experiments showed that embryos that are this age tolerate manipulation of the shell and the natural air space (data not shown). Infectious doses of 104 conidia per egg or higher resulted in 100% lethality within 2 to 4 days in all individual experiments, irrespective of the strain used. Infection with 103 conidia per egg resulted in a delay in killing, but there was still 90 to 100% lethality. Reducing the infectious dose to 102 conidia per egg further delayed and reduced lethality. This dose dependence was observed for all strains, was statistically significant (P < 0.0001, log rank test for trend), and is shown for CEA17ΔakuB and ATCC 46645 in Fig. 1A and B, respectively.
FIG. 1.
Dose-dependent mortality of embryonated eggs infected with A. fumigatus CEA17ΔakuB (A) and A. fumigatus ATCC 46645 (B). Eggs were infected with the doses indicated on developmental day 10 (n = 20 for 105 and 104 conidia/egg; n = 40 for 103 and 102 conidia/egg). Experiments were performed twice. Survival was monitored daily for 8 days, and the results are expressed as Kaplan-Meyer curves. The log rank test for trend was significant for both strains (P < 0.0001) (the results for the PBS control were not included in the statistical analysis).
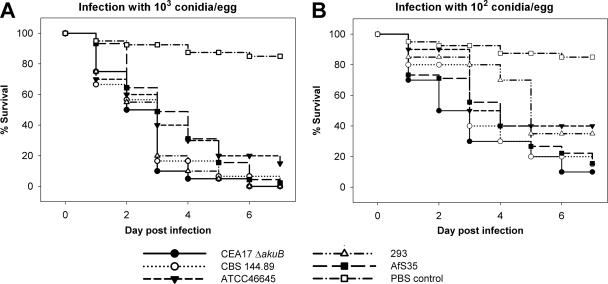
Treatment with all wild-type and laboratory strains tested (CEA17ΔakuB, CBS 144.89, ATCC 46645, Af293, and AfS35) resulted in indistinguishable lethality rates if eggs were infected with 104 or more conidia (data not shown) and similar mortality rates after infection with 103 and 102 conidia (Fig. 2A and B); 80 to 95% of PBS mock-infected embryos survived until the end of the observation period. Embryonic death in the control group could be attributed to manipulation since the embryos died mainly 1 to 2 days after infection. It could also have been caused by genetic or developmental defects independent of the experimental procedure. The variability with an infectious dose of 103 conidia per egg was found to be very low, and 85 to 100% mortality was observed consistently on day 3 or 4 p.i. These variations were within the viability range for PBS control eggs.
FIG. 2.
Virulence of wild-type and laboratory A. fumigatus strains in embryonated eggs. Embryonated eggs were inoculated with 103 (A) or 102 (B) conidia per egg on developmental day 10 (20 eggs per group per experiment; three independent experiments per strain per infectious dose). Survival was monitored daily for 8 days, and the results are expressed as Kaplan-Meyer curves. The log rank test did not indicate that there were significant differences between strains (P > 0.05).
Macroscopic and histological alterations during infection.
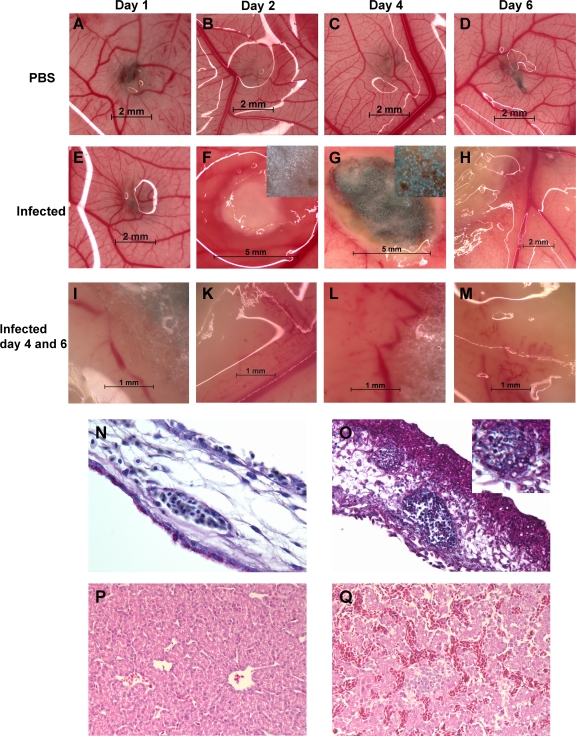
In order to analyze morphological modifications of the CAM and to determine fungal growth, eggs containing viable embryos were selected randomly on days 1, 2, 4, and 6 p.i. Ten eggs per day per group (PBS control eggs and eggs infected with 102 conidia of A. fumigatus CEA17ΔakuB) were analyzed. In the PBS control eggs, the entire CAM was uniformly nerved with well-defined branched blood vessels. No signs of trauma from the application process were observed at the site of inoculation (Fig. 3A to D). In infected eggs, mycelia were macroscopically visible in most eggs from day 2 p.i. onward (Fig. 3F to H). The size of the area covered by mycelium increased over time to a maximum diameter of ca. 3 cm. Sporulation was frequently observed on days 4 and 6 in eggs in which the artificial air chamber formed at the time of infection did not recede; in contrast, sporulation was rarely seen in eggs in which the artificial air chamber receded. To determine whether a visible mycelium and sporulation were correlated with death and virulence, we simultaneously investigated dead eggs and eggs which had survived 7 days of infection in survival experiments with different wild-type and mutant strains. However, irrespective of the strain investigated and the viability of the embryo, ∼70 to 80% of the eggs contained mycelium, and in 50 to 70% of these eggs there was sporulation. Thus, the presence of macroscopically visible mycelium and sporulation did not correlate with viability.
FIG. 3.
Macroscopic and microscopic changes in infected embryonated eggs. Eggs were infected on developmental day 10. (A to M) Macroscopic changes on the CAM surrounding the hole drilled for infection (darker area). (A to D) PBS control. The days after infection are indicated at the top. (E to H) Infection with 102 A. fumigatus CEA17ΔakuB conidia/egg. The insets in panels F and G show higher magnifications of mycelia. (I to M) Higher magnifications of eggs infected with A. fumigatus on day 4 after infection (I and K) and on day 6 after infection (L and M). (N to Q) Histological analysis of the CAM (N and O) and livers (P and Q) of infected embryos. (N and O) CAM stained with PAS stain. Magnification, ×63. (N) PBS control. (O) CAM infected with 102 A. fumigatus CEA17ΔakuB conidia/egg (inset, higher magnification of left blood vessel). (P and Q) Liver stained with H&E. Magnification, ×20. (P) PBS control. (Q) Liver after infection with 102 A. fumigatus CEA17ΔakuB conidia/egg.
To elucidate whether A. fumigatus was present on CAM with no macroscopically visible mycelia, culturing of CAM was performed for a total of 154 infected eggs and 20 PBS control eggs. No microorganisms were isolated from any of the PBS control eggs. In contrast, A. fumigatus was readily reisolated from the CAM of 134 infected eggs, demonstrating that fungal cells were present in the CAM in the absence of visible mycelium. The 20 remaining infected eggs, for which fungal growth was not detected on agar plates, generally were eggs that were infected with low numbers of conidia (102 conidia). Since only a small part of the CAM was excised, the infected regions might have been missed in these eggs. Alternatively, the immune system might have been able to clear the infection when very low doses of conidia were used.
A typical feature observed in most (>90%) infected eggs and all eggs containing macroscopically visible mycelium was areas where there were altered blood vessel patterns in the CAM. In the vicinity of hyphae, blood vessels either were completely absent (Fig. 3G, K, and M) or appeared to be very thin (Fig. 3H) or blotchy (Fig. 3I, L, and M). Alterations were observed mainly for smaller vessels, while larger vessels appeared to be unchanged (Fig. 3H and K). Invasion of A. fumigatus hyphae into blood vessels was confirmed by histological analysis of infected CAM. Hyphae grew through the outer epithelium of the CAM, formed a tight mycelium in the inner layer of connective tissue, and penetrated blood vessels (Fig. 3O).
The invasion of blood vessels by hyphae prompted us to investigate whether A. fumigatus could disseminate via the CAM into internal organs of the embryo, as A. fumigatus is commonly found in the livers of infected birds (22, 32, 54). We performed necropsies of 40 dead embryos infected with 102 to 105 conidia of CEA17ΔakuB and cultured liver tissue from these embryos. A. fumigatus was reisolated from 7 of the 40 livers; all culture-positive livers originated from embryos infected with 104 or 105 conidia. In general, no gross alterations of internal organs were observed upon necropsy; however, some livers of A. fumigatus-infected embryos appeared to be darker. Thus, we analyzed embryonic livers from embryos that were alive on days 3 and 6 after infection with 102 conidia histologically. The livers of infected embryos had enlarged blood vessels with an abundance of erythrocytes, indicating that there was liver congestion (Fig. 3Q).
As nutrient acquisition by a fungus during growth on the CAM might impair nutrient availability for the embryo, we weighed surviving uninfected and infected embryos on days 1, 2, 4, 6, and 8 p.i. (10 embryos for each day and group). No significant differences in weight were observed.
Influence of developmental age on the outcome of infection and the immune response.
Since the embryonic immune system matures during development, we investigated whether the age of embryos at the time of infection influences the outcome of A. fumigatus infection. In this analysis eggs were infected on developmental days 10, 12, and 15. Compared to the results for embryos infected on developmental day 10, the onset of mortality was clearly delayed in older embryos, and survival was significantly greater for eggs infected on developmental days 12 and 15 (P < 0.05 for every individual experiment, P < 0.0001 for summarized data shown in Fig. 4). As the experiments had to be terminated on developmental day 20 to avoid full development and hatching of chicks (day 21), eggs infected on developmental day 15 could be monitored for only 5 days p.i. The mortality rate during these 5 days resembled that of eggs infected on developmental day 12. Whether differences in mortality rates could occur later in the time course of infection could not be determined with the experimental design used.
FIG. 4.
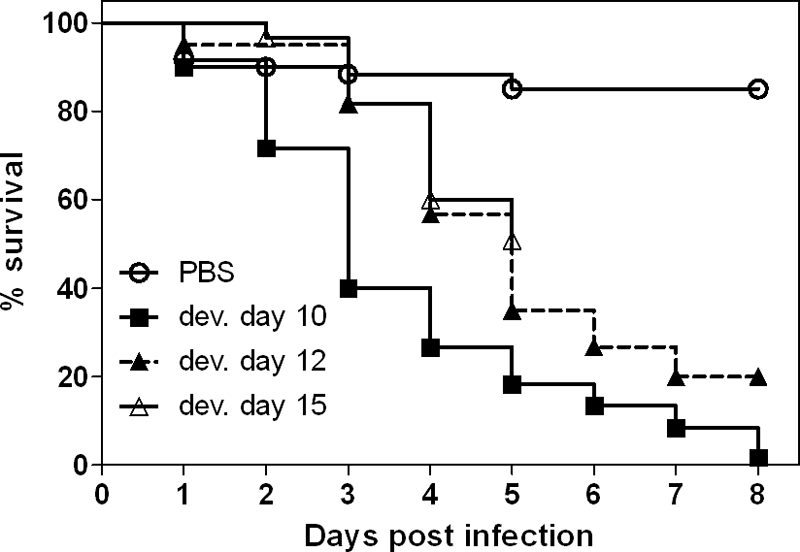
Influence of embryonic age at the time of infection on mortality. Eggs were infected on developmental (dev.) day 10, 12, or 15 with 102 A. fumigatus CEA17ΔakuB conidia/egg (20 eggs per age per experiment), and three independent experiments were performed. Survival was monitored daily for 8 days, and the results are expressed as Kaplan-Meyer curves. Embryos infected on developmental days 12 and 15 survived significantly longer than less-developed embryos (P < 0.001, log rank test).
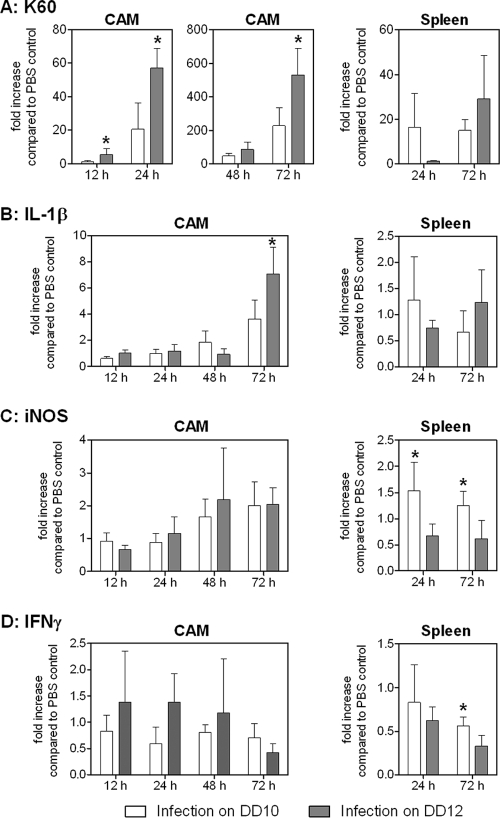
To analyze whether immune maturation during embryogenesis influences the immune response, we determined the levels of transcription of genes encoding the proinflammatory immune mediators K60 (CXCLi1), iNOS, IFN-γ, MIP-1β, and IL-1β locally in the CAM of PBS controls and infected eggs on either developmental day 10 or developmental day 12. The basal levels of transcription of these factors in PBS control eggs did not vary significantly over time (data not shown). The greatest increase in transcription level after infection with A. fumigatus was observed for the K60 chemokine gene. The levels of K60 gene transcription in infected embryos steadily increased over time and were significantly higher 12 h, 24 h, and 72 h p.i. in embryos infected on developmental day 12 than in embryos infected on developmental day 10 (Fig. 5A). Transcription of the proinflammatory cytokine IL-1β gene was moderately increased at 72 h p.i., and the increase was significantly greater for embryos infected when they were older (Fig. 5B). The levels of transcription of the iNOS gene increased little over time, and there was no difference between the groups (Fig. 5C). The levels of transcription of the genes coding for IFN-γ (Fig. 5D) and MIP-1β (data not shown) were not influenced by infection.
FIG. 5.
Levels of transcription of chemokines, cytokines, and iNOS in the CAM and spleen after infection with A. fumigatus CEA17ΔakuB. Eggs were infected on the CAM with 103 conidia on either developmental day 10 (DD10) or developmental day 12 (DD12). Transcripts were quantified by real-time quantitative RT-PCR, and the results were normalized using GAPDH and are expressed as fold increases compared to age- and time-matched PBS controls (5 eggs per group per time point). The two groups for each time point were compared by using an unpaired, two-tailed t test. Asterisks indicate significantly (P < 0.05) higher levels of transcription.
To assess the systemic immune response, levels of transcription in the spleen were analyzed 24 h and 72 h p.i. In the spleen, no upregulation was observed for the genes encoding IL-1β (Fig. 5B) and MIP-1β (data not shown). The K60 gene showed earlier upregulation in the spleens of embryos infected on developmental day 10, but the transcript levels at 72 h p.i. were higher in older embryos (Fig. 5A); however, none of the differences were statistically significant. The iNOS transcript levels in the spleens of embryos infected on developmental day 10 were significantly higher than the iNOS transcript levels in the spleens of embryos infected on developmental day 12 (Fig. 5C), but they were only ≤1.5-fold higher than the iNOS transcript levels in the spleens of PBS control embryos. Similarly, the IFN-γ transcript levels in the spleens were significantly higher in embryos infected on developmental day 10 than in embryos infected on developmental day 12 at 72 h p.i., but they were less than the transcript levels for the PBS controls (Fig. 5D).
Thus, an increased local immune response of embryos at later developmental stages coincided with reduced mortality after A. fumigatus infection.
Infection with A. fumigatus deletion mutants.
To determine whether embryonated eggs are a suitable alternative model for detecting differences in the virulence of A. fumigatus deletion mutants, mutants with defined virulence potential in cortisone acetate-treated mice were used to infect embryonated eggs on developmental day 10. Based on the results of the previous experiments, 103 conidia per egg were used to induce 90 to 100% lethality. Since partial virulence defects might not be detectable at a high infectious dose which rapidly causes 100% lethality, we also performed infection experiments with 102 conidia per egg. The results for the virulence of mutants were highly reproducible in these experiments, and the total mortality rates differed 10 to 15% between experiments.
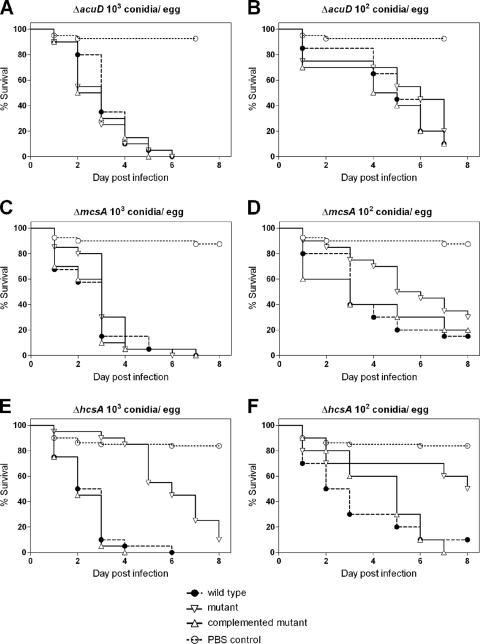
An A. fumigatus mutant with the acuD isocitrate lyase gene deleted (ΔacuD mutant) has previously been shown to be fully virulent in a mouse model (48). Likewise, this mutant was fully virulent in the egg model (Fig. 6A and B). Deletion of the mcsA methylcitrate synthase gene results in attenuation of virulence in mice which is more pronounced with a lower infectious dose (20). Analogous to the situation in the murine model, a ΔmcsA mutant displayed dose-dependent attenuation in the egg model. At a higher dose the mutant was fully virulent (Fig. 6C), but it showed significant attenuation of virulence at a lower dose (Fig. 6D) (P < 0.05). Finally, we found that a homocitrate synthase mutant (ΔhcsA mutant), which is strongly attenuated in mice infected intranasally (49), was significantly and strongly attenuated in eggs infected via the CAM on developmental day 10, irrespective of the infectious dose (Fig. 6E and F) (P < 0.05).
FIG. 6.
Virulence of defined A. fumigatus deletion mutants in embryonated eggs. Eggs (20 eggs per group per experiment; three independent experiments per strain and infectious dose) were infected on developmental day 10 with 103 conidia/egg (A, C, and E) or 102 conidia/egg (B, D, and F). Survival was monitored daily for 8 days, and the results are expressed as Kaplan-Meyer curves. (A and B) Isocitrate lyase (acuD) mutant. (C and D) Methylcitrate synthase (mcsA) mutant. (E and F) Homocitrate synthase (hcsA) mutant.
Our results show that there was a significant decrease in mortality in older embryos infected with A. fumigatus CEA17ΔakuB (Fig. 4), which was accompanied by changes in the chemokine and cytokine expression pattern (Fig. 5). These findings might have been related to progression of immune maturation and increased host defense. Therefore, we hypothesized that the partial virulence defect of the A. fumigatus ΔmcsA mutant might be more pronounced in embryos infected at a later developmental stage. Thus, the ΔmcsA mutant was also tested in eggs infected on developmental day 12. In accordance with our hypothesis, this mutant showed significantly reduced virulence (P < 0.01) at a higher dose (103 conidia per egg) in embryos infected on developmental day 12 (Fig. 7A). The rates of mortality caused by 103 mutant conidia in older embryos were comparable to the rates of mortality caused by the wild-type and complemented mutant when a 1-log-lower infection dose was used (Fig. 7A); thus, this outcome closely resembled the outcome of infection with this strain in cortisone acetate-treated mice (20).
FIG. 7.
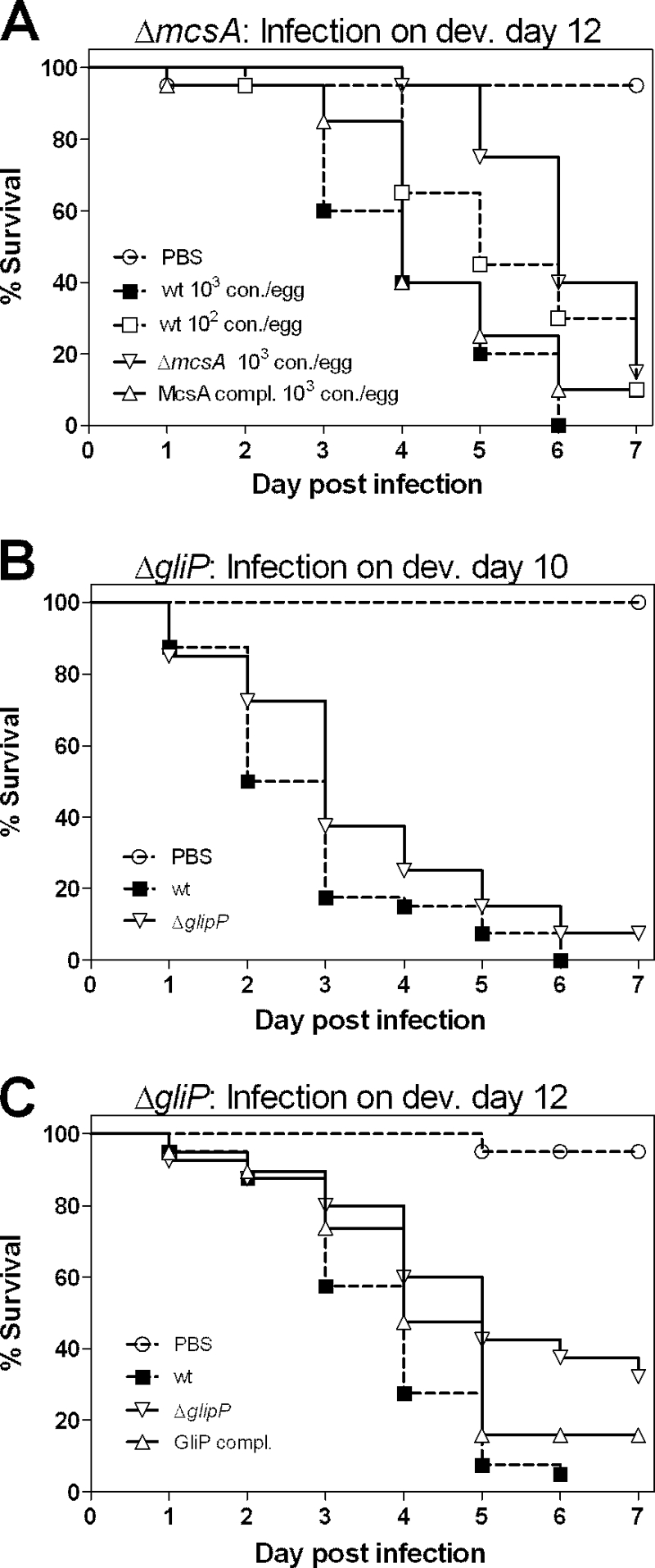
Virulence of the A. fumigatus methylcitrate synthase (ΔmcsA) mutant and the gliotoxin-deficient (ΔgliP) mutant in older embryonated eggs. (A) Methylcitrate synthase mutant. Eggs (20 eggs per group per experiment; two independent experiments) were infected on developmental day 12 (dev. day 12) with 103 conidia/egg of the mutant and the complemented strain. Infection with the wild-type control was performed for two groups using 103 and 102 conidia/egg. wt, wild type; con., conidia; McsA compl., complemented methylcitrate synthase mutant. (B and C) Gliotoxin-deficient mutant. Eggs (20 eggs per group per experiment; two independent experiments) were infected on developmental day 10 (B) or developmental day 12 (C) with 103 conidia/egg of the wild-type control strain, the mutant, and the complemented strain (data are shown only for developmental day 12). Survival was monitored daily for 7 days, and results are expressed as Kaplan-Meyer curves. GliP compl., complemented gliotoxin mutant. Analysis using the log rank test revealed significant attenuation of the ΔmcsA mutant (P < 0.01) and the ΔgliP mutant (P < 0.001) in embryos infected on day 12.
To further test the hypothesis that the age of embryos is related to immune maturation and host defense, we tested a gliotoxin-deficient strain (ΔgliP mutant) in the egg model. The role of gliotoxin in virulence has been shown to depend on the immune status of the host, as gliotoxin-deficient A. fumigatus strains are attenuated in cortisone acetate-treated mice but fully virulent in leucopenic mice (51). We found that the ΔgliP mutant exhibited full virulence in eggs infected on developmental day 10 with either 103 conidia (Fig. 7B) or a lower dose (102 conidia) (data not shown). In contrast, the ΔgliP mutant was significantly attenuated (P < 0.01) in eggs infected with 103 conidia on developmental day 12 (Fig. 7C).
We have recently shown that in mice the reduced virulence potential of a ΔhcsA lysine auxotrophic mutant can be restored by supplementation of the drinking water with lysine if the water is supplemented continuously after infection (49). To test whether lysine supplementation likewise restores virulence in the egg model, we performed two sets of supplementation experiments with the egg model. Supplementation with 100 μl 2.5 mM lysine on the day of infection did not influence the virulence of the ΔhcsA mutant (Fig. 8A). However, addition of 100 μl 5 mM lysine daily from the day of infection until day 4 p.i. restored the virulence of the ΔhcsA mutant (Fig. 8B). Thus, lysine supplementation had comparable results in the murine and egg models.
FIG. 8.
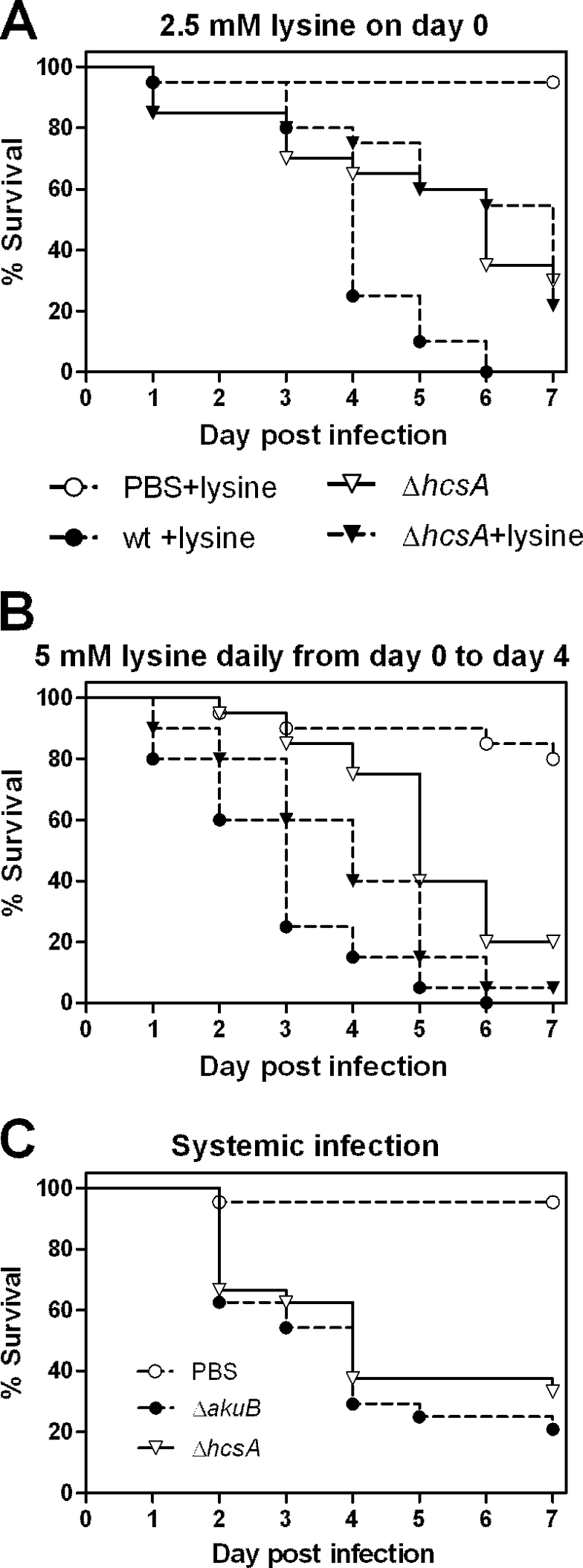
Effects of lysine supplementation and systemic application on the virulence of the ΔhcsA mutant. Eggs (20 eggs per group per experiment; two independent experiments) were infected on developmental day 10 (A and B) or developmental day 12 (C) with 103 conidia/egg of the mutant and the wild-type strain (wt). In the experiment whose results are shown in panel A 100 μl 2.5 mM lysine was added at the time of infection, and in the experiment whose results are shown in panel B 100 μl 5 mM lysine was added at the time of infection and daily during the first 4 days p.i. Survival was monitored daily for 7 days, and the results are expressed as Kaplan-Meyer curves. (B) The survival of embryos infected with the ΔhcsA mutant and grown with 5 mM lysine was significantly different from the survival of embryos infected with the ΔhcsA mutant without lysine (P < 0.02, log rank test) and wild-type strain-infected embryos (P < 0.05, log rank test). (C) The log rank test did not indicate that there were significant differences between strains after systemic infection (P = 0.35).
The route of infection can have a major effect on the outcome of infection experiments. While the A. fumigatus ΔhcsA mutant shows strong attenuation in a murine pulmonary model, this mutant is fully virulent if it is inoculated systemically into mice (49). To test if similar experiments can be performed in the egg model, we infected embryos on developmental day 12 intraperitoneally with 103 conidia of the wild type and the ΔhcsA mutant. Developmental day 12 was chosen because preliminary experiments revealed that embryos at this stage are more resistant to the necessary manipulations. We still observed approximately 30% mortality in all groups, including the PBS mock-infected embryos, in the first 24 h p.i. This early mortality was probably due to the more extensive manipulations necessary to perform the systemic infection procedure. However, >95% of the PBS-treated embryos that were alive on day 1 p.i. survived until the end of the experiment. Therefore, a survival analysis was performed only for embryos that survived the first 24 h p.i. We reproducibly observed no difference between the mortality after systemic infection with the wild type and the mortality after systemic infection with the ΔhcsA mutant (Fig. 8C); these results are comparable to the results obtained for systemic murine infection, in contrast to the results obtained for infection of the CAM (Fig. 6E and F).
DISCUSSION
Embryonated eggs have been used for decades as a convenient alternative infection model for studying viruses and for determination of bacterial virulence determinants (2, 18, 23, 55). However, to our knowledge, they have never been used as a model to study the pathogenesis of filamentous fungi, such as A. fumigatus.
Embryonated eggs can be infected via the CAM, the albumen, the yolk sack, or the allantoic cavity or by direct injection into the embryo or major blood vessels. We decided to infect eggs via the CAM for the following reasons. (i) The CAM is a thin, translucent membrane consisting of two epithelial layers separated by loose connective tissue (44). Pathogens applied to the outer layer of the CAM have to invade the outer epithelial layer to access nutrients, and invasion is an important virulence determinant for A. fumigatus in the lung. (ii) The CAM is highly vascularized in order to mediate both nutrient transport from the albumen and yolk sack to the embryo and, importantly, gas exchange during the latter two-thirds of embryonic development (44). High levels of vascularization and gas exchange are also characteristics of mammalian pulmonary tissue. (iii) Aseptic application of solutions onto the CAM is technically straightforward, and the required manipulation is well tolerated by the embryo (56).
Our results demonstrate that embryonated eggs are highly susceptible to A. fumigatus infection via the CAM in a dose-dependent manner. This fungus proliferates radially on the CAM, invading the tissue and blood vessels; these findings resemble histological findings for human patients (9, 31) and experimentally infected mice (4, 53). Similar to murine models of pulmonary invasive aspergillosis, in which dissemination to internal organs is rarely observed, dissemination from the CAM to the embryonic liver was rare and occurred only if high infectious doses (≥104 conidia per egg) were applied. We examined only dissemination to liver, and the fungus might infect other internal organs at a higher rate. However, we observed no gross pathological alterations of internal organs. Additionally, in disseminated aspergillosis in human patients and during natural infections in birds, A. fumigatus is found in the liver, demonstrating the general ability of A. fumigatus to thrive in the liver (15, 22, 32, 50, 54, 59). Consequently, direct destruction of internal organs of the embryo by invading fungi is unlikely to be the cause of mortality in this model. Since we observed rapid growth of A. fumigatus on the CAM, we considered the possibility that nutrient competition might contribute to pathogenesis. Nutrient deprivation should have led to reduced growth rates of infected embryos and reduced weight compared to the results for control embryos. However, the growth rates of infected and control embryos were indistinguishable during the first 4 days of infection, which is the time period when most embryos died. Given that fungal growth on the CAM was associated with macroscopically visible destruction of blood vessels, we hypothesize that impairment of blood flow is the most likely cause of embryonic death during A. fumigatus infection. This hypothesis is supported by the histological evidence of liver congestion in infected embryos, indicating abnormal blood flow or pressure.
Sporulation is generally not observed in pulmonary aspergillosis of humans and in infection models with mice and other rodents. In contrast, sporulation is regularly observed in aspergillosis in birds (6, 45, 52). Most likely, sporulation inside the host is favored if the fungus grows on a mucosa-air interface, like that found in the avian lung air sacks (54). The natural and artificial air chamber in fertilized eggs also is an interface between mucosa (CAM) and air and thus promotes sporulation. Therefore, it was not surprising that sporulation was regularly observed in infected eggs. However, the frequency and quantity of sporulation did not correlate with mortality, as we did not observe differences in sporulation between strains with different virulence properties or upon postmortem analysis of dead and surviving embryos. Therefore, sporulation at the outer surface and blood vessel infiltration seem to be unrelated, and the latter process is the main cause of embryonic death.
Immunosuppression is essential for establishment of A. fumigatus infections in mammalian models of pulmonary aspergillosis. Due to incomplete maturation of the immune system, chicken embryos can be considered naturally immunocompromised. While the phagocytic system of the innate immunity develops early during ontogenesis and both functional macrophages and heterophils are present on developmental day 10, the spleen is colonized by lymphocytes in several waves which expand into the posthatching period (12). The expression of Toll-like receptors, cytokines, and effector molecules, such as defensins, increases with embryonic age (1, 34). We speculated that if the interaction of A. fumigatus with the host's immune system influences the outcome of infection of embryonated eggs, older embryos should display increased resistance to infection. We indeed observed delayed mortality in older embryos, suggesting that the host immune response plays a role in pathogenesis in the embryonated egg model. Additionally, age-dependent differences in the levels of transcription of proinflammatory immune mediators between embryos infected on either developmental day 10 or developmental day 12 were observed. The greatest increase in mRNA levels was observed for K60, a CXCL chemokine with functions orthologous to those of human CXCL8. K60 has been shown to be associated with avian early proinflammatory responses and influx of heterophils after bacterial infection (26, 58). We observed early upregulation of K60 in the CAM of infected embryos and a continuous increase in the transcript level over time. Notably, the transcript levels were significantly higher in older embryos. In mice, the level of neutrophil recruitment mediated by the chemokines KC and MIP-2 has been shown to influence survival. Low levels of KC and MIP-2 were associated with decreased neutrophil recruitment and increased mortality, while overexpression of KC increased resistance of mice to A. fumigatus infection (35). Thus, the greater local transcription of K60 in older embryos in the egg model may lead to an increased influx of neutrophils and might therefore explain increased resistance. This hypothesis is supported by our observation that the level of expression of the proinflammatory cytokine IL-1β is significantly higher in the CAM of older embryos.
In contrast to the findings for K60 and IL-1β, the levels of transcription of IFN-γ were not elevated in infected embryos compared to the PBS controls at any time, independent of the age at the time of infection. IFN-γ has been shown to be involved in murine resistance to A. fumigatus infections, as an impaired IFN-γ response is associated with mortality in murine models of invasive aspergillosis (3, 8). Upon A. fumigatus infection, IFN-γ is upregulated in immunocompetent mice but not in immunocompromised mice. Therefore, the absence of an IFN-γ response in infected chicken embryos suggests that embryos on developmental day 12 should be considered naturally immunocompromised. It might also partially explain the observed high susceptibility of chicken embryos to A. fumigatus infection. We analyzed iNOS transcription, because iNOS has been shown to be upregulated in chickens after infection with invasive Salmonella strains (5). However, infection with A. fumigatus resulted in only a minor increase in local levels of iNOS gene transcription. While this finding might indicate that there is a general immune deficiency during embryogenesis, the immune deficiency likely does not affect the outcome of infection, as killing of A. fumigatus by mammalian macrophages has been shown to be independent of iNOS (39). Finally, there were no differences in transcription of the macrophage chemokine MIP-1β between infected and noninfected embryos, suggesting that attraction of heterophils by K60 rather than macrophages is the predominant immune response in infected embryos. This resembles the important role of neutrophils in combating A. fumigatus infections in murine model systems (36).
Testing the virulence of genetically defined fungal mutants in animal models is a common approach for identifying fungal factors involved in pathogenesis (11). Thus, an important characteristic for testing the validity of alternative models for infection research is that attenuation of mutants detected in the “gold standard” infection model can be similarly detected in the alternative model. Therefore, we tested A. fumigatus mutants with defined virulence in mice in the egg model. The mutants were chosen based on their different degrees of attenuation (virulence indistinguishable from wild-type virulence, partially attenuated, and strongly attenuated) and normal growth in vitro on rich media. We observed a good correlation between the murine and egg models. Using 20 eggs per group in every independent experiment, we found that the mortality rates were highly reproducible in experiments. However, the limited observation period for infected eggs prior to hatching might hamper detection of subtle differences in virulence in attenuated mutants.
Partial attenuation of the ΔmcsA mutant was detected either by using a lower infectious dose or by infecting eggs at a later developmental stage (day 12). As we assume that immune maturation leads to delayed killing in older embryos, we hypothesize that infection of older embryos might correlate best with nonneutropenic murine models, such as treatment with cortisone acetate, while younger embryos seem to resemble neutropenic murine models using cyclophosphamide. Consistent with this hypothesis, we found that a gliotoxin-deficient strain (ΔgliP mutant) was fully virulent if embryos were infected on developmental day 10, irrespective of the infectious dose. In older embryos, however, the ΔgliP mutant was attenuated. Similarly, a ΔgliP mutant has been shown to be fully virulent in neutropenic mice but is attenuated in mice immunosuppressed with corticosteroids (51).
While reduced mortality rates with genetically defined fungal mutants indicate that the deleted or disrupted gene is involved in pathogenesis, mortality rates alone do not provide information about the affected pathogenicity mechanisms. One option to obtain additional information is to perform pathological and histological examinations, like those that we performed with embryos to characterize the egg model. Additionally, the virulence of mutants that show a defect in nutrient acquisition can be restored by supplementation or variation of the route of infection. We have recently used supplementation of the drinking water with lysine to demonstrate that the lysine auxotrophy of a ΔhcsA mutant is responsible for the observed attenuation in mice (49). Likewise, we restored virulence in the egg model by supplying additional lysine, thus demonstrating that supplementation experiments are feasible in the alternative model. Attenuation of a ΔhcsA mutant in mice was observed only after pulmonary infection and not after systemic infection (49). Similarly, the ΔhcsA mutant was fully virulent in the egg model if it was applied systemically. Thus, variation of the infectious route is possible in the alternative model, and the results closely resemble the results for mice.
In summary, our data show that embryonated eggs provide the basis for a highly reproducible alternative model for studying the pathogenicity of A. fumigatus. Since this model is inexpensive and easy to perform and it does not require specialized facilities (only a humidity-controlled incubator and an appropriate drill are needed), infection of embryonated eggs could be used widely. By using embryos at different developmental stages for infection, hosts with different levels of immunocompetence can be used, similar to the different established models of immunosuppression in mice. Additionally, the egg model allows investigation of fungal invasion processes by histological analysis of infected membranes, which is not possible when alternative systems, such as cell lines, are used. The role of factors required for nutrient acquisition can be studied using nutrient supplementation. Furthermore, by using different routes of infection it is possible to clarify whether the factors being studied are locally and/or systemically involved in virulence. Prescreening of selected strains in the egg model and analysis of virulence mechanisms that may be involved might aid in choosing the appropriate mouse model for further testing of mutants. Therefore, the embryonated egg model may help to significantly reduce the numbers of mammals needed for infection studies.
Acknowledgments
This work was partially supported by the “Netzwerk Grundlagenforschung” of the Leibniz Institute for Natural Product Research and Infection Biology—Hans-Knöll-Institut (HKI) and by the Federal Ministry of Education and Health (BMBF).
The ΔgliP strain was kindly provided by Axel Brakhage and Thorsten Heinekamp, Department for Molecular and Applied Microbiology, Leibniz Institute for Natural Product Research and Infection Biology, Jena, Germany. We thank Birgit Weber, Ursula Stöckel, and Gudrun Steinmetzer for excellent technical help and Duncan Wilson for critical reading of the manuscript.
Editor: G. S. Deepe, Jr.
Footnotes
Published ahead of print on 26 April 2010.
REFERENCES
- 1.Abdul-Careem, M. F., D. B. Hunter, M. D. Lambourne, J. Barta, and S. Sharif. 2007. Ontogeny of cytokine gene expression in the chicken spleen. Poult. Sci. 86:1351-1355. [DOI] [PubMed] [Google Scholar]
- 2.Adam, R., S. Mussa, D. Lindemann, T. A. Oelschlaeger, M. Deadman, D. J. Ferguson, R. Moxon, and H. Schroten. 2002. The avian chorioallantoic membrane in ovo—a useful model for bacterial invasion assays. Int. J. Med. Microbiol. 292:267-275. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong-James, D. P., S. A. Turnbull, I. Teo, J. Stark, N. J. Rogers, T. R. Rogers, E. Bignell, and K. Haynes. 2009. Impaired interferon-gamma responses, increased interleukin-17 expression, and a tumor necrosis factor-alpha transcriptional program in invasive aspergillosis. J. Infect. Dis. 200:1341-1351. [DOI] [PubMed] [Google Scholar]
- 4.Balloy, V., M. Huerre, J. P. Latge, and M. Chignard. 2005. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infect. Immun. 73:494-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berndt, A., A. Wilhelm, C. Jugert, J. Pieper, K. Sachse, and U. Methner. 2007. Chicken cecum immune response to Salmonella enterica serovars of different levels of invasiveness. Infect. Immun. 75:5993-6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cacciuttolo, E., G. Rossi, S. Nardoni, R. Legrottaglie, and P. Mani. 2009. Anatomopathological aspects of avian aspergillosis. Vet. Res. Commun. 33:521-527. [DOI] [PubMed] [Google Scholar]
- 7.Carvajal, B. G., U. Methner, J. Pieper, and A. Berndt. 2008. Effects of Salmonella enterica serovar Enteritidis on cellular recruitment and cytokine gene expression in caecum of vaccinated chickens. Vaccine 26:5423-5433. [DOI] [PubMed] [Google Scholar]
- 8.Centeno-Lima, S., H. Silveira, C. Casimiro, P. Aguiar, and V. E. do Rosario. 2002. Kinetics of cytokine expression in mice with invasive aspergillosis: lethal infection and protection. FEMS Immunol. Med. Microbiol. 32:167-173. [DOI] [PubMed] [Google Scholar]
- 9.Chiang, L. Y., D. C. Sheppard, F. N. Gravelat, T. F. Patterson, and S. G. Filler. 2008. Aspergillus fumigatus stimulates leukocyte adhesion molecules and cytokine production by endothelial cells in vitro and during invasive pulmonary disease. Infect. Immun. 76:3429-3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chute, H. L. 1975. Characteristics of immunity in fungal infections. Am. J. Vet. Res. 36:601-602. [PubMed] [Google Scholar]
- 11.Clemons, K. V., and D. A. Stevens. 2005. The contribution of animal models of aspergillosis to understanding pathogenesis, therapy and virulence. Med. Mycol. 43(Suppl. 1):S101-S110. [DOI] [PubMed] [Google Scholar]
- 12.Cooper, M. D., C. L. Chen, R. P. Bucy, and C. B. Thompson. 1991. Avian T cell ontogeny. Adv. Immunol. 50:87-117. [DOI] [PubMed] [Google Scholar]
- 13.da Silva Ferreira, M. E., M. R. Kress, M. Savoldi, M. H. Goldman, A. Härtl, T. Heinekamp, A. A. Brakhage, and G. H. Goldman. 2006. The akuB(KU80) mutant deficient for nonhomologous end joining is a powerful tool for analyzing pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 5:207-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallien, S., S. Fournier, R. Porcher, J. Bottero, P. Ribaud, A. Sulahian, G. Socie, and J. M. Molina. 2008. Therapeutic outcome and prognostic factors of invasive aspergillosis in an infectious disease department: a review of 34 cases. Infection 36:533-538. [DOI] [PubMed] [Google Scholar]
- 15.Gottfredsson, M., and H. Steingrimsdottir. 2006. Disseminated invasive aspergillosis in a patient with acute leukaemia. Acta Biomed, 77(Suppl. 2):10-13. [PubMed] [Google Scholar]
- 16.Gow, N. A., Y. Knox, C. A. Munro, and W. D. Thompson. 2003. Infection of chick chorioallantoic membrane (CAM) as a model for invasive hyphal growth and pathogenesis of Candida albicans. Med. Mycol. 41:331-338. [DOI] [PubMed] [Google Scholar]
- 17.Härtl, A., H. G. Hillesheim, W. Kunkel, and E. J. Schrinner. 1995. The Candida infected hen's egg. An alternative test system for systemic anticandida activity. Arzneimittelforschung 45:926-928. (In German.) [PubMed] [Google Scholar]
- 18.Herren, C. D., A. Mitra, S. K. Palaniyandi, A. Coleman, S. Elankumaran, and S. Mukhopadhyay. 2006. The BarA-UvrY two-component system regulates virulence in avian pathogenic Escherichia coli O78:K80:H9. Infect. Immun. 74:4900-4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hohl, T. M., and M. Feldmesser. 2007. Aspergillus fumigatus: principles of pathogenesis and host defense. Eukaryot. Cell 6:1953-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibrahim-Granet, O., M. Dubourdeau, J. P. Latgé, P. Ave, M. Huerre, A. A. Brakhage, and M. Brock. 2008. Methylcitrate synthase from Aspergillus fumigatus is essential for manifestation of invasive aspergillosis. Cell. Microbiol. 10:134-148. [DOI] [PubMed] [Google Scholar]
- 21.Jackson, J. C., L. A. Higgins, and X. Lin. 2009. Conidiation color mutants of Aspergillus fumigatus are highly pathogenic to the heterologous insect host Galleria mellonella. PLoS One 4:e4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khosravi, A. R., H. Shokri, T. Ziglari, A. R. Naeini, Z. Mousavi, and H. Hashemi. 2008. Outbreak of severe disseminated aspergillosis in a flock of ostrich (Struthio camelus). Mycoses 51:557-559. [DOI] [PubMed] [Google Scholar]
- 23.King, V., A. Bavetsia, and N. Bumstead. 1993. Effect of host lineage on the virulence of Campylobacter jejuni/coli in the chick embryo model. FEMS Microbiol. Lett. 106:271-274. [DOI] [PubMed] [Google Scholar]
- 24.Krappmann, S., C. Sasse, and G. H. Braus. 2006. Gene targeting in Aspergillus fumigatus by homologous recombination is facilitated in a nonhomologous end-joining-deficient genetic background. Eukaryot. Cell 5:212-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kupfahl, C., T. Heinekamp, G. Geginat, T. Ruppert, A. Hartl, H. Hof, and A. A. Brakhage. 2006. Deletion of the gliP gene of Aspergillus fumigatus results in loss of gliotoxin production but has no effect on virulence of the fungus in a low-dose mouse infection model. Mol. Microbiol. 62:292-302. [DOI] [PubMed] [Google Scholar]
- 26.Laurent, F., R. Mancassola, S. Lacroix, R. Menezes, and M. Naciri. 2001. Analysis of chicken mucosal immune response to Eimeria tenella and Eimeria maxima infection by quantitative reverse transcription-PCR. Infect. Immun. 69:2527-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehmann, P. F. 1985. Immunology of fungal infections in animals. Vet. Immunol. Immunopathol. 10:33-69. [DOI] [PubMed] [Google Scholar]
- 28.Lionakis, M. S., and D. P. Kontoyiannis. 2005. Fruit flies as a minihost model for studying drug activity and virulence in Aspergillus. Med. Mycol. 43(Suppl. 1):S111-S114. [DOI] [PubMed] [Google Scholar]
- 29.Lionakis, M. S., R. E. Lewis, G. S. May, N. P. Wiederhold, N. D. Albert, G. Halder, and D. P. Kontoyiannis. 2005. Toll-deficient Drosophila flies as a fast, high-throughput model for the study of antifungal drug efficacy against invasive aspergillosis and Aspergillus virulence. J. Infect. Dis. 191:1188-1195. [DOI] [PubMed] [Google Scholar]
- 30.Maerker, C., M. Rohde, A. A. Brakhage, and M. Brock. 2005. Methylcitrate synthase from Aspergillus fumigatus. Propionyl-CoA affects polyketide synthesis, growth and morphology of conidia. FEBS J. 272:3615-3630. [DOI] [PubMed] [Google Scholar]
- 31.Marr, K. A., T. Patterson, and D. Denning. 2002. Aspergillosis. Pathogenesis, clinical manifestations, and therapy. Infect. Dis. Clin. North Am. 16:875-894, vi. [DOI] [PubMed] [Google Scholar]
- 32.Martin, M. P., K. P. Bouck, J. Helm, M. J. Dykstra, D. P. Wages, and H. J. Barnes. 2007. Disseminated Aspergillus flavus infection in broiler breeder pullets. Avian Dis. 51:626-631. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Quesada, J., A. Nieto-Cadenazzi, and J. M. Torres-Rodriguez. 1993. Humoral immunoresponse of pigeons to Aspergillus fumigatus antigens. Mycopathologia 124:131-137. [DOI] [PubMed] [Google Scholar]
- 34.Meade, K. G., R. Higgs, A. T. Lloyd, S. Giles, and C. O'Farrelly. 2009. Differential antimicrobial peptide gene expression patterns during early chicken embryological development. Dev. Comp. Immunol. 33:516-524. [DOI] [PubMed] [Google Scholar]
- 35.Mehrad, B., M. Wiekowski, B. E. Morrison, S. C. Chen, E. C. Coronel, D. J. Manfra, and S. A. Lira. 2002. Transient lung-specific expression of the chemokine KC improves outcome in invasive aspergillosis. Am. J. Respir. Crit. Care Med. 166:1263-1268. [DOI] [PubMed] [Google Scholar]
- 36.Mircescu, M. M., L. Lipuma, N. van Rooijen, E. G. Pamer, and T. M. Hohl. 2009. Essential role for neutrophils but not alveolar macrophages at early time points following Aspergillus fumigatus infection. J. Infect. Dis. 200:647-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mylonakis, E. 2008. Galleria mellonella and the study of fungal pathogenesis: making the case for another genetically tractable model host. Mycopathologia 165:1-3. [DOI] [PubMed] [Google Scholar]
- 38.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Philippe, B., O. Ibrahim-Granet, M. C. Prevost, M. A. Gougerot-Pocidalo, M. Sanchez Perez, A. Van der Meeren, and J. P. Latge. 2003. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect. Immun. 71:3034-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pieper, J., U. Methner, and A. Berndt. 2008. Heterogeneity of avian gammadelta T cells. Vet. Immunol. Immunopathol. 124:241-252. [DOI] [PubMed] [Google Scholar]
- 41.Pollock, C. 2003. Fungal diseases of columbiformes and anseriformes. Vet. Clin. North Am. Exot. Anim. Pract. 6:351-361. [DOI] [PubMed] [Google Scholar]
- 42.Reeves, E. P., C. G. Messina, S. Doyle, and K. Kavanagh. 2004. Correlation between gliotoxin production and virulence of Aspergillus fumigatus in Galleria mellonella. Mycopathologia 158:73-79. [DOI] [PubMed] [Google Scholar]
- 43.Renwick, J., P. Daly, E. P. Reeves, and K. Kavanagh. 2006. Susceptibility of larvae of Galleria mellonella to infection by Aspergillus fumigatus is dependent upon stage of conidial germination. Mycopathologia 161:377-384. [DOI] [PubMed] [Google Scholar]
- 44.Ribatti, D., B. Nico, A. Vacca, L. Roncali, P. H. Burri, and V. Djonov. 2001. Chorioallantoic membrane capillary bed: a useful target for studying angiogenesis and anti-angiogenesis in vivo. Anat. Rec. 264:317-324. [DOI] [PubMed] [Google Scholar]
- 45.Richard, J. L., R. C. Cutlip, J. R. Thurston, and J. Songer. 1981. Response of turkey poults to aerosolized spores of Aspergillus fumigatus and aflatoxigenic and nonaflatoxigenic strains of Aspergillus flavus. Avian Dis. 25:53-67. [PubMed] [Google Scholar]
- 46.Richard, J. L., J. R. Thurston, R. C. Cutlip, and A. C. Pier. 1982. Vaccination studies of aspergillosis in turkeys: subcutaneous inoculation with several vaccine preparations followed by aerosol challenge exposure. Am. J. Vet. Res. 43:488-492. [PubMed] [Google Scholar]
- 47.Schmidt, A. 2002. Animal models of aspergillosis—also useful for vaccination strategies? Mycoses 45:38-40. [DOI] [PubMed] [Google Scholar]
- 48.Schöbel, F., O. Ibrahim-Granet, P. Ave, J. P. Latge, A. A. Brakhage, and M. Brock. 2007. Aspergillus fumigatus does not require fatty acid metabolism via isocitrate lyase for development of invasive aspergillosis. Infect. Immun. 75:1237-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schöbel, F., I. D. Jacobsen, and M. Brock. 2 April 2010, posting date. Evaluation of lysine biosynthesis as antifungal drug target: biochemical characterization of Aspergillus fumigatus homocitrate synthase and virulence studies. Eukaryot. Cell. doi: 10.1128/EC.00020-10. [DOI] [PMC free article] [PubMed]
- 50.Shirkhoda, A., G. Lopez-Berestein, J. M. Holbert, and M. A. Luna. 1986. Hepatosplenic fungal infection: CT and pathologic evaluation after treatment with liposomal amphotericin B. Radiology 159:349-353. [DOI] [PubMed] [Google Scholar]
- 51.Spikes, S., R. Xu, C. K. Nguyen, G. Chamilos, D. P. Kontoyiannis, R. H. Jacobson, D. E. Ejzykowicz, L. Y. Chiang, S. G. Filler, and G. S. May. 2008. Gliotoxin production in Aspergillus fumigatus contributes to host-specific differences in virulence. J. Infect. Dis. 197:479-486. [DOI] [PubMed] [Google Scholar]
- 52.Stedham, M. A., T. J. Bucci, and R. R. Maronpot. 1968. Sexual and asexual phases of Aspergillus nidulans in an egret. Mycopathol Mycol. Appl. 36:289-292. [DOI] [PubMed] [Google Scholar]
- 53.Stephens-Romero, S. D., A. J. Mednick, and M. Feldmesser. 2005. The pathogenesis of fatal outcome in murine pulmonary aspergillosis depends on the neutrophil depletion strategy. Infect. Immun. 73:114-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tell, L. A. 2005. Aspergillosis in mammals and birds: impact on veterinary medicine. Med. Mycol. 43(Suppl. 1):S71-S73. [DOI] [PubMed] [Google Scholar]
- 55.Townsend, M. K., N. J. Carr, J. G. Iyer, S. M. Horne, P. S. Gibbs, and B. M. Pruss. 2008. Pleiotropic phenotypes of a Yersinia enterocolitica flhD mutant include reduced lethality in a chicken embryo model. BMC Microbiol. 8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vargas, A., M. Zeisser-Labouebe, N. Lange, R. Gurny, and F. Delie. 2007. The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv. Drug Deliv. Rev. 59:1162-1176. [DOI] [PubMed] [Google Scholar]
- 57.Vonberg, R. P., and P. Gastmeier. 2006. Nosocomial aspergillosis in outbreak settings. J. Hosp. Infect. 63:246-254. [DOI] [PubMed] [Google Scholar]
- 58.Withanage, G. S., P. Kaiser, P. Wigley, C. Powers, P. Mastroeni, H. Brooks, P. Barrow, A. Smith, D. Maskell, and I. McConnell. 2004. Rapid expression of chemokines and proinflammatory cytokines in newly hatched chickens infected with Salmonella enterica serovar Typhimurium. Infect. Immun. 72:2152-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yeom, S. K., H. J. Kim, J. H. Byun, A. Y. Kim, M. G. Lee, and H. K. Ha. 21 September 2009, posting date. Abdominal aspergillosis: CT findings. Eur. J. Radiol. 32 doi: 10.1016/j.ejrad.2009.08.016. [DOI] [PubMed]