Abstract
The acquisition of iron during the infection process is essential for the growth of pathogenic microorganisms (S. C. Andrews, Adv. Microb. Physiol. 40:281-351, 1998; H. M. Baker, B. F. Anderson, and E. N. Baker, Proc. Natl. Acad. Sci. U. S. A. 100:3579-3583, 2003). Since the solubility of iron is low and it is toxic at low concentrations, following uptake, iron is stored in subcellular microenvironments in the iron storage protein ferritin (C. Cheers and M. Ho, J. Reticuloendothel. Soc. 34:299-309, 1983). Here, we show that ferritin-like proteins (Frl) are highly conserved in the genus Listeria and demonstrate that these proteins are present in both the cytoplasm and cell wall fractions of these bacteria. Even though Frl is expressed under different growth conditions, transcriptional mapping revealed that its regulation is complex. When bacteria are grown in brain heart infusion medium, extracellular expression involves both sigma A (SigA)- and sigma B (SigB)-dependent promoters; however, during intracellular growth, initiation of transcription is additionally SigB dependent. The expression of Frl is greatly enhanced in bacteria grown in the presence of blood, and a mutant strain lacking the frl gene was defective for growth in this medium. Using the monoclonal antibody (MAb) specific for Frl, we demonstrate that administration of anti-Frl MAb prior to infection confers antilisterial resistance in vivo, evidenced in reduced bacterial load and increased survival rates, thereby demonstrating the in vivo significance of upregulated cell surface-associated Frl expression. In vitro studies revealed that the antilisterial resistance is due to increased listerial phagocytosis.
Listeria monocytogenes, the causative agent of listeriosis, is a well-described food-borne pathogen known to infect immunocompromised individuals, leading to severe clinical symptoms, such as meningitis and septicemia (16, 29, 38). L. monocytogenes is widely considered to be one of the leading causes of food-borne infections with high case fatalities (40). Also, infection with L. monocytogenes during pregnancy can lead to choriomeningitis and miscarriage (29, 37). Immunity to systemic infections in listeriosis has been linked to cellular immunity and more recently to components of the innate immune response (4). The role of the humoral response is less studied, and only a few listerial antigens have been identified as targets of antibodies following listerial infections (23). Nevertheless, taking the severity of clinical manifestations associated with L. monocytogenes infection into consideration, an understanding of the pathogenesis and role of bacterial antigens targeted for recognition by different arms of the immune response is required to design effective strategies for inducing resistance against L. monocytogenes infection.
Recently, we identified the listerial ferritin-like protein as a target of the humoral response following infection of mice with pathogenic L. monocytogenes. The ferritin-like protein of L. monocytogenes (Frl) (9, 30, 32) is a member of the family of bacterial Dps proteins (DNA binding proteins from starved cells). These proteins bear resemblance to ferritins and heme-containing ferritins (bacterioferritins) in both structure and function (2, 3, 25) and are iron storage proteins that remove intracellular ferrous iron and maintain the metal in a nontoxic metal form. These proteins assemble as dodecameric structures that are endowed not only with high thermal stability but also with an unusual resistance at low pH (15). Previous studies have demonstrated that the expression of Frl is both growth phase and temperature dependent and is required for resisting oxidative stress and overcoming low iron availability (32). The expression of Frl is also induced under conditions of iron limitation and is regulated by Fur, the ferric uptake regulator, which binds within the promoter region of the frl gene (19). Our own work showed that Frl contributes to pathogenesis in mice, in particular at an early time point following infection (30).
The ferritin-like protein of L. monocytogenes has variously been designated Flp (ferritin-like protein), Frm (for L. monocytogenes), or Fri (for Listeria innocua). We have sequenced the ferritin-like protein genes from all of the known species of the genus Listeria. To avoid confusion and make it evident that the ferritin-like protein of L. monocytogenes is strongly homologous to the ferritin-like protein of L. innocua, we suggest Frl (ferritin-like protein in Listeria species) as a common name for all the related proteins in this species.
Conflicting data on the localization of Frl exist in the literature, where it has been described as a cytoplasmic protein but also has been found in the secreted protein fraction of L. monocytogenes (13, 34). To determine the cellular localization of Frl, we purified the protein and raised a monoclonal antibody (MAb) capable of recognizing both Frl of L. monocytogenes and Frl of L. innocua. Using the MAb, we examined the presence of the protein in cytoplasmic, cell wall, and supernatant fractions of L. monocytogenes. Since Frl is required early in infection, we checked for its expression on bacteria grown in the presence of blood. Furthermore, we evaluated the effects of selective Frl inhibition by using the MAb in in vivo L. monocytogenes infection.
MATERIALS AND METHODS
Mice.
Six- to 8-week-old female BALB/c mice, purchased from Harlan Winkelmann (Borchen, Germany) and kept at the Giessen University mouse facility, were used in all experiments.
Bacteria.
Wild-type L. monocytogenes strain EGD-e (21), its isogenic EGD-eΔsigB (13) and EGD-eΔfrl deletion mutants as well as its complemented EGD-eΔfrl::pPL-frl derivative (30) were used in this study. Bacteria were grown in brain heart infusion (BHI) broth and agar at the indicated temperatures. In all experiments, fresh cultures of bacteria, prepared from an overnight culture, were used. Briefly, bacteria were grown in BHI broth and agar, harvested in the exponential growth phase, and washed twice with phosphate-buffered saline (PBS). The pellet was resuspended in PBS, and the bacterial concentration was calibrated by optical absorption. Further dilutions were prepared in PBS to obtain required numbers of bacteria for infection.
Antibodies.
The major monoclonal antibodies used in this study were the murine anti-Frl IgG2b MAb (referred to as anti-Frl MAb) and the control low-endotoxin, azide-free (LEAF) murine anti-human CD129 IgG2b MAb (referred to as control; Biolegend, San Diego, CA). In addition, the rat anti-mouse CD32/16 IgG2b (LEAF) MAb (Biolegend, San Diego, CA) was utilized for blocking Fc-γRII (CD32) and Fc-γRIII (CD16) binding of IgG. The anti-Frl MAb was produced against purified listerial ferritin-like protein as previously described (31), isotyped using a mouse monoclonal isotyping kit (Calbiochem, La Jolla, CA), and purified using protein A-Sepharose (Amersham Pharmacia, Sweden). The endotoxin content in this antibody was estimated and showed less than 10 endotoxin units per milligram protein using a Limulus amebocyte lysate QCL-1000 bacterial endotoxin quantitation kit (BioWhittaker Inc., Walkersville, MD). The concentration of anti-Frl MAb was determined by an optical density at 280 nm reading and Bradford analysis (8). Anti-Frl MAb has shown efficient binding to purified Frl in immunoblots and in enzyme-linked immunosorbent assays. For studies involving the localization of protein to cell wall or cytoplasmic fractions, we used the N81 MAb raised against L. monocytogenes ActA and a polyclonal rabbit antibody raised against purified L. monocytogenes PrfA protein.
Mouse infection.
The in vivo effect of anti-Frl MAb on bacterial survival was demonstrated by measuring bacterial growth kinetics in the organs of mice infected with L. monocytogenes following treatment with the respective monoclonal antibodies. Groups of 12 mice were injected intraperitoneally (i.p.) with 500 μg of anti-Frl MAb or control MAb 1 day before i.p. infection with 3 × 103 CFU of wild-type L. monocytogenes. At the indicated time points following infection, spleens and livers were aseptically isolated from 3 mice per group and bacterial growth was determined by plating 10-fold serial dilutions of the organ homogenates on BHI plates. The detection limit of this procedure was 102 CFU per organ. Colonies were counted after 24 h of incubation at 37°C. In a protection study, groups of 15 mice were treated with the antibodies as described above or left untreated 1 day before i.p. infection with a lethal dose (105 CFU) of L. monocytogenes. Survival of mice was monitored over 12 days postinfection and expressed as a percentage of survival following a lethal infection with L. monocytogenes.
Intracellular growth and immunofluorescence studies.
The effects of anti-Frl MAb on the intracellular growth of L. monocytogenes were determined by infection of the mouse macrophage-like cell line P388D1 or mouse peritoneum-derived macrophages. P388D1 cells were kept in RPMI 1640 (PAN Biotech, Aidenbach, Germany) supplemented with 10% (vol/vol) fetal calf serum (FCS) and 2 mM l-glutamine. Peritoneal macrophages were aseptically isolated from 4- to 6-week-old BALB/c female mice, propagated, and maintained in Dulbecco modified Eagle medium (PAN Biotech, Aidenbach, Germany) supplemented with 1 g/liter d-glucose, 2 mM l-glutamine, 25 mM HEPES, sodium pyruvate, 20% (vol/vol) macrophage colony-stimulating factor, 5% (vol/vol) nonessential amino acids, and 10% (vol/vol) FCS.
The eukaryotic cells were incubated with 5 μg of anti-Frl MAb, control MAb, and/or 5% fresh-frozen or heat-inactivated (80°C for 1 h) naive mouse serum for 30 min at 37°C. Thereafter, cells were infected with wild-type L. monocytogenes or its isogenic Δfrl mutant at a multiplicity of infection (MOI) of 10. At 1 h postinfection, cells were washed and kept in 20 μg/ml gentamicin-containing medium to kill extracellular bacteria. At the indicated time points, cells were lysed with 0.2% (vol/vol) NP-40 and plated on BHI plates in serial dilutions. In order to functionally block the Fc-γ receptors on P388D1 macrophages, cells were incubated with 2 μg of rat anti-mouse CD32/CD16 for 1 h or left untreated. Then, cells were washed twice and further incubated with or without anti-Frl MAb for 30 min, followed by infection with wild-type L. monocytogenes as described above.
For immunofluorescence studies, P388D1 cells were infected with L. monocytogenes at an MOI of 10. Cells were not treated with gentamicin in order to maintain the extracellular bacteria. At 2 h postinfection, macrophages were washed, fixed with 4% formaldehyde, and permeabilized with 0.2% (vol/vol) Triton X-100 in PBS. Bacteria were visualized by labeling with Cy3-conjugated anti-Frl MAb (red) and staining intracellular F-actin by using Oregon Green 488-conjugated phalloidin under a fluorescence microscope (Zeiss, Germany). Images were captured and processed using KS300 software (Zeiss, Germany).
Extraction of bacterial proteins and immunoblotting.
Fifty-milliliter exponentially grown bacterial cultures were harvested by centrifugation at 15,000 rpm for 20 min and washed with PBS. Pelleted cells were resuspended in 1% SDS and incubated for 45 min at 37°C with gentle shaking. Cells were centrifuged for 20 min at 15,000 rpm and 4°C. Supernatants containing solubilized cell wall-associated proteins were removed, aliquoted, and kept at −80°C (27, 36). Cytoplasmic proteins under the same bacterial growth conditions were isolated as previously described (10).
Soluble antigens were separated by 12.5% SDS-PAGE and then transferred onto an Immobilon FL membrane (Millipore) by using a semidry electroblotting transfer cell system (Bio-Rad, Germany). Immunoblot analysis was performed using anti-mouse Frl MAb. Fluorescent dye-labeled IRDye 800CW goat anti-mouse IgG (Li-Cor Biosciences) was used as the secondary antibody. Protein bands were viewed using the Odyssey infrared imaging system (Li-Cor Biosciences).
Growth in blood and flow cytometry analysis.
The ability of L. monocytogenes and its isogenic Δfrl deletion mutant to survive in blood was examined by incubating either L. monocytogenes or the Δfrl mutant (2 × 104 CFU) in fresh heparinized mouse blood at 37°C for 3 h. Tenfold serial dilutions were plated on BHI plates. The detection limit was 102 CFU. Colonies were counted after 24 h of incubation at 37°C.
For flow cytometry studies, exponentially grown wild-type L. monocytogenes (2 × 104 CFU) bacteria were incubated in either 1 ml fresh heparinized mouse blood or 1 ml BHI medium at 37°C for 3 h. Erythrocytes were lysed with sterile distilled water, and bacterial pellets were incubated on ice with anti-Frl MAb for 1 h. Bacteria were washed 3 times with cold PBS, followed by incubation with fluorescein isothiocyanate (FITC)-labeled anti-mouse IgG MAb (BioLegend, San Diego, CA) for 30 min to detect the Frl expression on the bacterial cell surface. Flow cytometry was performed using a FACSCalibur flow cytometer and further analyzed with CellQuest software (Becton Dickinson, Mountain View, CA).
Listerial RNA isolation and promoter mapping of Frl transcripts.
Promoter start sites of mRNA transcripts were determined by 454 sequencing. Thus, total RNA was extracted from L. monocytogenes grown either extracellularly in BHI medium or intracellularly in P388D1 cells as previously described (13). Transcriptome sequencing was carried out by Eurofins MWG (Germany) as recommended by the manufacturer. Briefly, first-strand cDNA synthesis was primed with an N6 randomized primer. Then 454 adapters A (5′-CCATCTCATCCCTGCGTGTCTCCGACTCAG-3′) and B (5′-CTGAGACTGCCAAGGCACACAGGGGATAGG-3′) were ligated to the 5′ and 3′ ends of the cDNAs. The cDNAs were finally amplified with PCR using a proofreading enzyme. Normalization was carried out by one cycle of denaturation and reassociation of the cDNAs. Reassociated double-stranded cDNAs were separated from the remaining single-stranded cDNAs (sscDNAs) (normalized cDNAs) by passing the mixture over a hydroxylapatite column. After hydroxylapatite chromatography, the sscDNAs were amplified with 15 PCR cycles. For titanium sequencing, the cDNAs in the size range of 500 to 700 bp were eluted from preparative agarose gels. An aliquot of the size-fractionated cDNAs was analyzed on a 1.5% agarose gel. Clipped sequencing reads were aligned against the L. monocytogenes EGD-e genome sequence (21) by using software based on the BLAST algorithm. Matching criteria for correct alignments were 80% identity and 80% coverage. Reads shorter than 20 bases were not taken into account. Predicted transcript start sites were visualized using the Integrative Genomics Viewer (IGV; Broad Institute, Cambridge, MA).
Quantitative PCR analysis.
Quantitative real-time PCR was carried out using the 7900HT fast real-time PCR system (Applied Biosystems) as previously described (13). Isolated RNA was reverse transcribed into cDNA by SuperScript II reverse transcriptase (Invitrogen) and subjected to quantitative real-time PCR in a final volume of 25 μl by using a QuantiTect SYBR green PCR kit (Qiagen) according to the manufacturer's instructions. Forward (5′-GATGGTTTCCACACTGGA-3′) and reverse (5′-TAATAAGTATAATCTATTTCCACATCA-3′) primers (purchased from Eurofins MWG Operon) were selected to produce an amplicon length of about 100 bp.
A standard curve was generated for this primer pair by using different copy numbers of genomic DNA from EGD-e. For each primer pair, a negative control (water), an RNA sample without reverse transcriptase (to determine genomic DNA contamination), and a sample with a known amount of copy numbers (to test the efficiency of the reaction) were included as controls during cDNA quantification. After real-time PCR, all samples were run on a 2.0% agarose gel to verify that only a single band was produced.
Statistical analysis.
Data are representative of at least three independent experiments. Significance of the represented data was calculated using a paired Student t test and analysis of variance. Data are expressed as means ± standard errors.
RESULTS
Ferritin-like protein is conserved among the genus Listeria.
Comparison of the ferritin-like protein sequences from L. monocytogenes, L. innocua, and L. welshimeri showed strong conservation of this protein family in these species (21, 24). We sequenced the ferritin-like proteins from representative strains of the three remaining Listeria species, namely, L. ivanovii, L. seeligeri, and L. grayi, and compared them to sequences reported previously. Genomic analysis of the ferritin-like protein locus showed that this protein is highly conserved across the species L. monocytogenes, L. innocua, L. welshimeri, L. ivanovii, and L. seeligeri but that the predicted protein in L. grayi shows significant divergence in keeping with its taxonomic distance to the other species (see Fig. S1 in the supplemental material). Interestingly, the Frl proteins encoded by the pathogenic species L. monocytogenes and L. ivanovii are identical. The remaining proteins, except that from L. grayi, have substitutions at either one or two sites, viz., Gln114 and/or Asn126.
Cell wall-associated expression of Frl.
Frl has previously been detected in cytoplasmic extracts (20); however, Frl has also been detected in the culture supernatants of growing bacterial cultures (39). To localize Frl in the bacteria, we used a monoclonal antibody recognizing common epitopes in the Frl protein of L. monocytogenes and the Frl protein of L. innocua (anti-Frl MAb). We analyzed cell wall and cytoplasmic fractions for the presence of Frl and, as expected, detected Frl in the cytoplasmic fraction of L. monocytogenes. However, Frl was also detected as a component of cell wall fractions isolated from these bacteria (Fig. 1 A). Control experiments using antibodies to ActA, a cell wall-associated protein, or antibodies to PrfA, a cytoplasmically expressed protein, confirmed that Frl is indeed also a component of the listerial cell wall. An analysis of cell wall fractions isolated from representative strains of L. ivanovii, L. seeligeri, L. innocua, L. welshimeri, and L. grayi also revealed that these proteins were localized to the cell wall fraction of these bacteria (Fig. 1B).
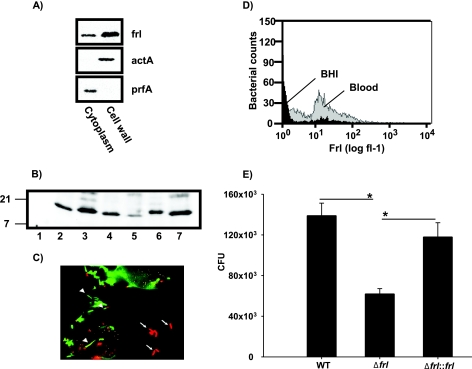
FIG. 1.
Frl is a surface-accessible protein in L. monocytogenes. (A) Soluble cell wall and cytoplasmic fractions (5 μg) from L. monocytogenes were transferred onto an Immobilon FL membrane (Pall) and incubated with anti-Frl MAb, anti-ActA MAb, or anti-PrfA polyclonal antibody, followed by incubation with IRDye 800CW goat anti-mouse or IRDye 680CW goat anti-rabbit IgG. Blots were visualized using the Odyssey infrared imaging system (Li-Cor). (B) Soluble cell wall fractions from the L. monocytogenes Δfrl mutant (lane 1), wild-type L. monocytogenes (2), L. innocua (3), L. ivanovii (4), L. welshimeri (5), L. grayi (6), and L. seeligeri (7) were blotted and incubated with anti-Frl MAb as described for panel A. Numbers on the left refer to the apparent molecular weights in thousands. (C) The macrophage-like P388D1 cell line was infected with L. monocytogenes. At 2 h postinfection, cells were fixed with 4% formaldehyde. Extracellular (arrows) and intracellular (arrowheads) Listeria bacteria were visualized by Cy3-conjugated anti-Frl MAb (red) under a fluorescence microscope. Oregon Green 488-conjugated phalloidin was used to stain actin tails of intracellular Listeria bacteria. (D) Expression levels of Frl were measured on the surface of bacteria after growth in blood or BHI medium by flow cytometry using a FACSCalibur flow cytometer. Bacteria were stained with FITC-labeled anti-Frl MAb. The histogram was created and analyzed with CellQuest software. The x axis represents the logarithmic number of cells exposing Frl on their surfaces. (E) Wild-type L. monocytogenes (2 × 104 CFU), its isogenic Δfrl mutant, and the complemented Δfrl::frl mutant strain were grown in mouse blood for 3 h. Bacteria were plated out and quantified. Bars represent geometric means ± standard errors of the means (SEM) (*, P < 0.05 for the wild type versus the Δfrl mutant and P < 0.01 for the Δfrl::frl mutant versus the Δfrl mutant).
We used confocal laser scanning microscopy to reveal that Frl is exposed on the bacterial cell surface of both extracellularly and intracellularly growing bacteria following infection of the macrophage-like cell line P388D1 (Fig. 1C). Frl was detected on the surface of bacteria growing in BHI medium. In infected cells, staining with the anti-Frl MAb indicated the presence of large aggregates of Frl that were not associated with cytoplasmically located bacteria; this was not observed in uninfected cells.
Sigma B factor stimulates intracellular Frl expression.
Previous studies have indicated that the listerial frl gene is carried by a monocistronic transcript with a putative binding sequence for the ferric uptake regulator (Fur) upstream of its putative start site (19, 32). However, transcription of frl is complex and involves three promoters, corresponding to two sigma A- and one sigma B-dependent transcriptional start site (32). Since Frl is also expressed during intracellular growth, we wondered whether there were qualitative differences in the use of SigA- and SigB-dependent promoters during extra- and intracellular growth of bacteria. We used quantitative direct sequencing of transcripts isolated from bacteria grown in BHI medium or extracted from intracellular bacteria of L. monocytogenes-infected P388D1 cells to examine for qualitative differences in promoter use under these different growth conditions. The expression of the frl gene grown in BHI medium was dependent on transcription originating from all three promoter start sites (P1, 6 transcripts; P2, 1 transcript; and P3, 65 transcripts). However, during intracellular growth, transcription initiation from the SigB-dependent promoter (P2) was greatly enhanced (P1, 7 transcripts; P2, 37 transcripts; and P3, 58 transcripts) (see Fig. S2A and B in the supplemental material). To confirm that the transcription data really reflect the intracellular and extracellular conditions, the expression of the PrfA-dependent gene uhpT (lmo0838), encoding the hexose phosphate transporter, was analyzed. Under conditions of extracellular growth, where uhpT is poorly expressed, we detected a single transcript, whereas during intracellular growth induction of PrfA-dependent uhpT transcription led to an increase to 90 transcripts (data not shown). To demonstrate that the P2 promoter is indeed SigB dependent during intracellular growth, we performed quantitative real-time PCR analysis for transcripts originating from this promoter by using RNA extracted from wild-type and isogenic ΔsigB bacteria following infection of P388D1 macrophages. There was a drastic reduction, ∼1,000-fold, in the number of transcripts originating from the P2 promoter in the mutant strain, confirming that SigB does indeed induce intracellular Frl expression (see Fig. S2C in the supplemental material).
Frl is required for optimal bacterial growth in blood.
In earlier studies, we have shown that Frl is required for efficient growth at early stages of listerial infection (25). We therefore speculated that Frl may be needed for efficient bacterial growth in blood prior to replication in spleen and liver. Using flow cytometry, we found that the expression of Frl on the bacterial cell surface was significantly upregulated in bacteria growing in blood compared to that in bacteria growing in BHI medium (Fig. 1D). These findings indicate upregulation of surface-associated Frl expression on bacteria in blood. To examine the functional implications of increased Frl expression, we compared the growth rates of wild-type L. monocytogenes and its isogenic Δfrl mutant in blood. We observed that the Δfrl mutant was significantly impaired for growth in blood compared to its wild-type counterpart (Fig. 1E). Complementation of the Δfrl mutant restored the ability of the Δfrl::pPL2-frl mutant to grow in this medium, implying that Frl is required for efficient listerial growth in blood.
Pretreatment with anti-Frl antibody enhances survival rates after L. monocytogenes infection.
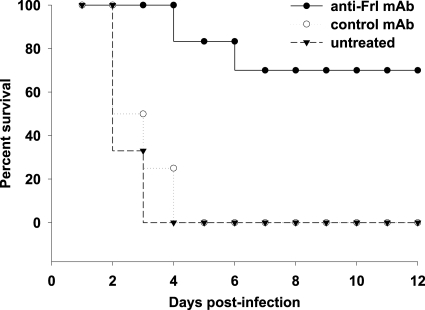
We reasoned that since Frl is significantly expressed on the cell surface of L. monocytogenes during early infection, this would make it a good target for the anti-Frl MAb. We therefore examined whether the prophylactic use of the anti-Frl MAb could prevent mice from succumbing to infection following a lethal dose of L. monocytogenes in vivo. Following injection of a lethal dose of 1 × 105 CFU of L. monocytogenes, all control mice receiving either control MAb (15/15 dead) or no treatment (15/15 dead) succumbed to infection (Fig. 2). In contrast, a single administration of anti-Frl MAb led to high survival rates (4/15 dead), with about 70% of all infected mice surviving (Fig. 2).
FIG. 2.
Induction of antilisterial resistance after passive administration of anti-Frl antibodies. Groups of mice (15 mice/group) received 500 μg anti-Frl MAb intraperitoneally (i.p.) 1 day before i.p. infection with a lethal dose (1 × 105 CFU) of L. monocytogenes. Controls included mice treated with control MAb and untreated mice. Survival rates were monitored for 12 days postinfection.
Reduction of organ-specific bacterial burden after Frl blockade.
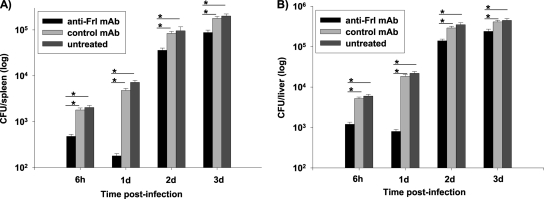
Correlation of enhanced survival rates with bacterial replication following treatment with the anti-Frl antibody was addressed by quantifying the bacterial burden in organs of mice pretreated with anti-Frl MAb following sublethal infection with L. monocytogenes (3 × 103 CFU) (Fig. 3). An early significant reduction of splenic bacterial counts at 6 h postinfection was observed in mice preimmunized with anti-Frl MAb in comparison to mice pretreated with control MAb (P = 0.0202) and untreated mice (P = 0.0133). Determinations of listerial growth at day 1 and day 2 postinfection were similar to those seen at 6 h postinfection with the anti-Frl MAb-treated mice showing a significantly decreased bacterial burden. Blockade of Frl led to inhibition of bacterial growth, as experiments determining bacterial growth at day 3 postinfection showed that mice treated with anti-Frl MAb had a splenic bacterial load which was still significantly less than that of controls (Fig. 3). A decrease in bacterial burden could also be evidenced in livers of anti-Frl MAb-treated mice compared to that of respective controls (Fig. 3).
FIG. 3.
Prior anti-Frl MAb treatment leads to organ-specific reduction of bacterial burden. Mice were treated with 500 μg anti-Frl MAb i.p. (12 mice/group) 1 day prior to i.p. infection with 3 × 103 CFU of L. monocytogenes. Three mice per group were killed at 6 h, 1 day, 2 days, and 3 days postinfection, and bacterial loads in spleens and livers were quantified. Controls included mice treated with 500 μg control MAb as well as untreated mice. Bars represent geometric means ± SEM (*, P < 0.05).
Frl MAb induces Fc-γ receptor-mediated phagocytosis and enhances bacterial killing.
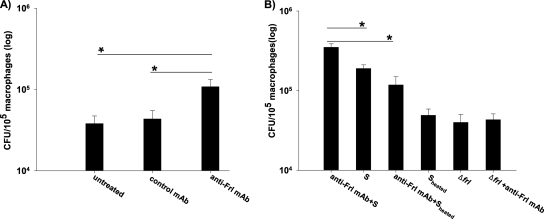
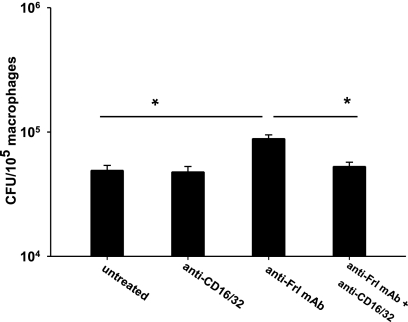
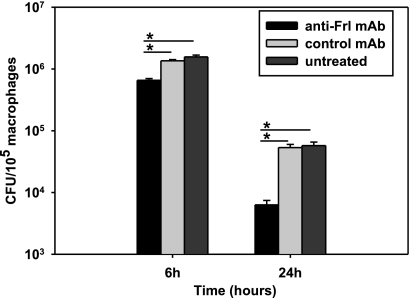
To investigate the mechanisms explaining how anti-Frl MAb interferes with the pathogenesis of an intracellular pathogen, macrophages were preincubated with anti-Frl MAb before exposure to L. monocytogenes. Treatment with anti-Frl MAb significantly increased the number of intracellular L. monocytogenes bacteria relative to treatment with controls (Fig. 4 A). This suggests that anti-Frl MAb increases listerial phagocytosis. Incubation of macrophages with naïve mouse serum alone also led to a significant increase in listerial phagocytosis (Fig. 4B). However, when cells were treated with a combination of anti-Frl MAb and mouse serum, the highest phagocytosis rates could be observed, indicating that both anti-Frl MAb and mouse serum increase the phagocytosis rate of Listeria (Fig. 4B). Heat treatment of mouse serum leading to deactivation of serum-associated complement significantly reduced phagocytosis rates (Fig. 4B). For the isogenic L. monocytogenes Δfrl mutant strain, no effect of anti-Frl MAb on phagocytosis rates could be observed (Fig. 4B). After blockade of the Fc-γ receptors (Fc-γRII and Fc-γRIII) by using the rat anti-mouse CD32/CD16 antibody, a significant reduction in macrophage uptake of L. monocytogenes was observed (Fig. 5). These results implicate enhanced phagocytosis of L. monocytogenes as a protective mechanism of the anti-Frl monoclonal antibody. To investigate whether anti-Frl MAb-mediated phagocytosis leads to enhanced bacterial killing and thereby translates into a reduction of the bacterial burden, we incubated peritoneum-derived macrophages with anti-Frl MAb or control MAb or left them untreated. Although P388D1 macrophages preincubated with the anti-Frl MAb showed enhanced phagocytosis of Listeria at early time points postinfection (up to 2 h) (Fig. 4), a distinct reduction of listerial CFU was seen in peritoneum-derived macrophages at later time points (6 h and 24 h) postinfection, compared to control MAb-treated or untreated macrophages (Fig. 6). This argues for a lag phase between phagocytosis and subsequent killing of bacteria. The experimental data therefore show that anti-Frl MAb-mediated phagocytosis is associated with subsequent killing of bacteria.
FIG. 4.
Anti-Frl MAb enhances bacterial phagocytosis. (A) The P388D1 macrophage cell line was incubated for 1 h with 5 μg of anti-Frl MAb or control MAb or left untreated. (B) Additional experiments were also performed with or without 5% fresh-frozen mouse serum that was either active (S) or heat inactivated (Sheated). Infection was performed with the wild-type L. monocytogenes or its isogenic Δfrl mutant strain (MOI of 10) for 2 h. Cells were lysed with 0.2% (vol/vol) NP-40. Viable intracellular bacteria were plated and enumerated following incubation at 37°C. Bars represent geometric means ± SEM (*, P < 0.05).
FIG. 5.
Antibody-mediated blockade of Frl leads to opsonization and subsequent ingestion of L. monocytogenes. P388D1 cells were incubated with or without 2 μg of rat anti-mouse CD32/CD16 for 1 h. Cells were washed twice and incubated with the anti-Frl MAb for 1 h or left untreated, followed by infection with wild-type L. monocytogenes as described for Fig. 4. Bars represent geometric means ± SEM (*, P < 0.05).
FIG. 6.
Anti-Frl MAb inhibits listerial survival in primary macrophages. The peritoneum-derived mouse macrophages were incubated for 1 h with 5 μg of anti-Frl MAb or control MAb or left untreated. Cells were lysed with 0.2% (vol/vol) NP-40 at the indicated time points. Viable intracellular bacteria were plated and enumerated following incubation at 37°C. Bars represent geometric means ± SEM (*, P < 0.05).
DISCUSSION
Here, we have demonstrated that Frl is expressed by all listerial species and is present in both cytoplasmic and cell wall fractions. Even though Frl is expressed under different growth conditions, transcriptional mapping revealed that its regulation is complex. The expression of Frl is greatly enhanced in bacteria grown in the presence of blood, and an isogenic mutant strain lacking the frl gene was defective for growth in this medium. An anti-Frl MAb conferred in vivo antilisterial resistance based on enhanced phagocytosis and subsequent bacterial killing.
We previously demonstrated that specific anti-Frl antibodies are detectable in antilisterial serum following immunization with L. monocytogenes (30), suggesting that Frl may be a surface-exposed protein. Previous studies of the ferritin-like protein have described it as a cytoplasmically expressed protein (20). However, some ferritin-like proteins have also been found to be associated with the bacterial cell wall and have been found in culture supernatants (39). In the studies presented in this work, we used an anti-Frl MAb and could show that Frl is detectable in both cytoplasmic and cell wall fractions of all Listeria spp. Cell wall localization of Frl was also demonstrated using flow cytometry as well as confocal laser scanning microscopy for bacteria grown in BHI medium or within infected eukaryotic host cells. Even though a previous report found that Frl was present in supernatants from bacterial cultures (39), we have been unable to detect it in culture fluids of bacteria grown under different growth conditions (data not shown). Also, as Frl lacks a signal peptide, it is not likely to be actively secreted. In infected cells, Frl aggregates were also observed distal to bacteria; the pathogenetic consequence of bacterial ferritin proteins present in the host cell is presently unknown. These dots were not present in macrophages infected with the Δfrl isogenic mutant or in uninfected macrophages.
The first ferritin-like protein isolated in the genus Listeria was from L. innocua (7) and then subsequently from L. monocytogenes (32). Comparison of the ferritin-like protein sequence from L. monocytogenes, L. innocua, and L. welshimeri showed strong conservation of this protein family in these species (21, 24). The sequenced ferritin-like proteins from the remaining three Listeria species, namely, L. ivanovii, L. seeligeri, and L. grayi, revealed that this protein is highly conserved across the three hemolytic (L. monocytogenes, L. ivanovii, and L. seeligeri) as well as the three nonhemolytic (L. innocua, L. welshimeri, and L. grayi) species. Interestingly, Frl proteins encoded by the pathogenic species L. monocytogenes and L. ivanovii are identical. The other ferritins, including that from L. grayi, have substitutions at either one or two sites, viz., Gln114 and/or Asn126. Recently, the X-ray crystal structures of the Frl proteins of L. monocytogenes and L. innocua (Gln114→Lys and Asn126→Asp in Frl from L. innocua) have been solved, and their biochemical properties have been compared (5). These studies indicate that differences in the amino acids at these two sites render the multimeric protein assemblages produced by Frl of L. monocytogenes both more thermostable and resistant to low pH than those formed by Frl of L. innocua.
Even though the frl gene is monocistronically transcribed, three transcriptional start sites have previously been identified in bacteria grown in BHI medium at 37°C or subjected to heat or hydrogen peroxide stress (32). The data presented in this study confirmed the presence of two SigA-dependent promoters (P1 and P3) at nucleotides (nt) −154 and −40, respectively, and a SigB-dependent promoter (P2) located at nt −123, upstream of the ATG start codon. Comparative analysis revealed that while the SigA-dependent promoters P1 and P3 were transcribed at equal frequencies during growth in BHI medium and intracellularly, transcription from the SigB-dependent promoter P2 was greatly induced during intracellular growth. These data support the notion that Frl is required for efficient bacterial growth under various conditions and that different sets of promoters are utilized under normal or stress (heat, hydrogen peroxide, and intracellular growth) conditions (32; this study) to ensure its expression.
Frl expression has also been shown to be repressed by Fur, the ferric uptake regulator, as well as Per, the hydrogen peroxide regulator (19, 32).
We have previously shown that Frl is required for efficient growth at early stages of listerial infections (30). Here, we demonstrate that the expression of Frl during intracellular growth is dependent on the alternative sigma factor SigB, which has recently been shown to regulate the expression of virulence genes required during early interactions of bacteria with the host (9). We therefore postulated that the expression of Frl might be required for effective growth of the bacteria in the blood prior to entry in cells of organs such as the spleen and liver. Using flow cytometry and staining with the anti-Frl MAb, we detected significant expression of Frl in bacteria grown in the presence of blood. To obtain a functional correlate for this upregulation, we assessed the growth properties of the EGD-eΔfrl mutant (30) and compared them with those of the parental EGD strain. The Frl mutant was clearly impaired for growth in blood, and complementation of the frl gene in the EGD-eΔfrl mutant restored its ability to grow in blood to the level of the wild-type strain, therefore proving a requirement of Frl expression for effective bacterial growth in blood (Fig. 1E).
Since Frl is an antigen targeted for immune responses expressed during in vivo L. monocytogenes infection (9) and, as shown here, is required for the growth of bacteria in blood, we considered that it might be a suitable target for prophylactic passive immunization. Using the MAb generated against Frl, we conducted experiments in which the MAb was administered prior to infection, reflecting passive preimmunization, to impair Frl function at the most early time point of pathogenesis. Our experimental work showed that blocking Frl via anti-Frl MAb prior to infection significantly impaired L. monocytogenes pathogenesis after infection with a lethal dose of L. monocytogenes. This was evidenced in both survival rates and bacterial burdens in the organs of infected mice. Mice preimmunized with anti-Frl MAb showed enhanced survival after L. monocytogenes infection. Regarding bacterial load, there was a significant reduction of bacteria in all groups already at 6 h postinfection, suggesting that innate defense mechanisms are efficiently mobilized to contain infection. However, in mice preimmunized with anti-Frl MAb, splenic bacterial counts were significantly reduced compared to those from mice treated with control MAb and untreated mice, indicating a rapid contribution of Frl blockade to limit early listerial growth. The blockade of Frl via antibodies led to a sustainable inhibition of bacterial growth, as experiments determining bacterial growth at day 3 postinfection showed that mice treated with anti-Frl MAb had significantly lower numbers of CFU than respective controls. This level of inhibition in bacterial load was enough to confer protection in anti-Frl MAb-treated mice. However, the rates of bacterial replication in the organs of anti-Frl MAb-treated mice and respective controls were identical, indicating that while the anti-Frl MAb significantly reduced listerial load early after infection, leading to lower bacterial numbers in spleen and liver, the anti-Frl MAb had no significant effect on bacterial growth once the bacteria had reached the organs. Indeed, if the anti-Frl MAb was given postinfection, no effect on survival or bacterial load could be seen (data not shown), giving further evidence to the role of Frl in the blood phase of infection prior to bacterial growth in organs.
We considered several mechanisms that could explain how antibodies could interfere with the pathogenesis of an intracellular pathogen. Possible routes of action would include opsonization of bacteria and complement activation prior to the intracellular life cycle or, alternatively, interference with the natural function of the protein, which in turn affects pathogenesis. Here, we demonstrated that treatment with anti-Frl MAb significantly increased the number of intracellular L. monocytogenes bacteria relative to the respective controls, indicating that the presence of anti-Frl MAb promotes listerial phagocytosis by these cells. Recent experimental work has shown that naïve human serum increases listerial macrophage-associated phagocytosis, most probably due to serum-associated complement components (35). Indeed, when naïve mouse serum was used, comparable results could be observed, with a reproducible increase in listerial phagocytosis rates after incubation with naïve mouse serum. However, when cells were treated with anti-Frl MAb and naïve mouse serum, the highest phagocytosis rates could be observed, indicating that both anti-Frl MAb and serum-associated complement act together to increase the phagocytosis rate of Listeria. Taken together, the experiments indicate a role for anti-Frl MAb in enhancing listerial phagocytosis, thereby leading to enhanced bacterial killing and subsequent reduction of organ-specific bacterial load and increased survival rates. To investigate this mode of action, the receptors associated predominantly with uptake of opsonized bacteria, the Fc-γRII (CD32) and Fc-γRIII (CD16) receptors (26), were blocked using the rat anti-mouse CD32/CD16 antibody. Fc-γRII (CD32) and Fc-γRIII (17) are the receptors associated predominantly with the uptake of opsonized bacteria and are widely distributed in different antigen-presenting cells. The uptake of Listeria by macrophages was previously shown to be mediated by Fc-γRIII (26). Blockade of the Fc-γ receptor led to a clear reduction in uptake of L. monocytogenes, lending support to the hypothesis that anti-Frl MAb-mediated opsonization is the most likely mechanism of antilisterial immune resistance. Indeed, some recent studies have focused on using the Fc-γ receptors as vaccine targets to induce both cellular and humoral immune responses against extracellular and intracellular pathogens (1, 22). Importantly, it has been shown that the targeting of the Fc-γ receptor with Francisella tularensis, an intracellular bacterium, combined with an anti-Francisella tularensis lipopolysaccharide MAb, led to enhancement of protective immunity against subsequent challenge with this pathogen (34).
Based on data from the studies described above, a likely scenario would be that the iron storage and protective properties of Frl are required for L. monocytogenes growth during the blood phase, therefore explaining the upregulation of Frl expression. Hence, preimmunization with anti-Frl MAb targets increased Frl expression and promotes phagocytic uptake and subsequent killing in host cells following ingestion. This killing could be achieved coordinately by innate immune cells such as macrophages, natural killer cells, and neutrophils, thus controlling bacterial access to host organs and reducing systemic infection. Indeed, activated macrophages are highly effective for killing of L. monocytogenes (6, 11, 17).
Our data supplement previous work using a MAb against listeriolysin (18) by showing that a MAb against specific targets can control listerial infection. Here, we identify an additional listerial antigen that, when targeted by antibody, provides enhanced resistance against listerial infection. The fact that the polyclonal serum taken after infection or immunization with L. monocytogenes does not offer protection against L. monocytogenes infection (14, 28, 33) even though specific anti-Frl antibodies have been detected (30) is a well-described phenomenon. In infection models of other intracellular pathogens, it has been demonstrated that MAbs can induce resistance whereas the respective polyclonal serum cannot (12). This can be explained either by the presence of low levels of antibodies against protective antigens or as a result of an ineffective antibody isotype. Our work shows that humoral immunity is capable of mediating protection against listerial infection. These results support and extend prior work (18) which demonstrated the effects of a monoclonal antibody directed against listeriolysin on Listeria infection, as they provide further evidence of a protective role for antibodies and they define a further monoclonal antibody with this capability. The effects on phagocytosis and organ load are comparable to those in prior studies (18), and we therefore believe that they are indicative of a protective role for antibody in Listeria infection.
In this study, we identify a role for Frl in virulence during in vivo L. monocytogenes infection and indicate that Frl is important for growth of L. monocytogenes in the blood phase of infection. In keeping with its role at this early time point in infection, preimmunization with a MAb against Frl inhibits bacterial growth and thereby controls L. monocytogenes infection. As our experimental model shows that antigen recognized by our MAb is required for bacterial survival in blood, we believe that prophylactic passive immunization using our antibody might prevent listeriosis in susceptible populations.
Supplementary Material
Acknowledgments
We acknowledge Sylvia Krämer for excellent technical assistance.
The work reported herein was supported by the Deutsche Forschungsgemeinschaft by grants through the Special Priority Program “Life in Cells” and the Excellence Cluster for Cardiopulmonary Systems (ECCPS) to T.C.
Editor: S. M. Payne
Footnotes
Published ahead of print on 3 May 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Adamova, E., M. C. Walsh, D. R. Gosselin, K. Hale, M. T. Preissler, R. F. Graziano, and E. J. Gosselin. 2005. Enhanced antigen-specific antibody and cytokine responses when targeting antigen to human FcGAMMA receptor type I using an anti-human FcGAMMA receptor type I-streptavidin fusion protein in an adjuvant-free system. Immunol. Invest. 34:417-429. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, S. C. 1998. Iron storage in bacteria. Adv. Microb. Physiol. 40:281-351. [DOI] [PubMed] [Google Scholar]
- 3.Baker, H. M., B. F. Anderson, and E. N. Baker. 2003. Dealing with iron: common structural principles in proteins that transport iron and heme. Proc. Natl. Acad. Sci. U. S. A. 100:3579-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauler, L. D., C. S. Duckett, and M. X. O'Riordan. 2008. XIAP regulates cytosol-specific innate immunity to Listeria infection. PLoS Pathog. 4:e1000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellapadrona, G., R. Chiaraluce, V. Consalvi, A. Ilari, S. Stefanini, and E. Chiancone. 2007. The mutations Lys 114 → Gln and Asp 126 → Asn disrupt an intersubunit salt bridge and convert Listeria innocua Dps into its natural mutant Listeria monocytogenes Dps. Effects on protein stability at low pH. Proteins 66:975-983. [DOI] [PubMed] [Google Scholar]
- 6.Bermudez, L. E. 1993. Differential mechanisms of intracellular killing of Mycobacterium avium and Listeria monocytogenes by activated human and murine macrophages. The role of nitric oxide. Clin. Exp. Immunol. 91:277-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozzi, M., G. Mignogna, S. Stefanini, D. Barra, C. Longhi, P. Valenti, and E. Chiancone. 1997. A novel non-heme iron-binding ferritin related to the DNA-binding proteins of the Dps family in Listeria innocua. J. Biol. Chem. 272:3259-3265. [DOI] [PubMed] [Google Scholar]
- 8.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 9.Camejo, A., C. Buchrieser, E. Couve, F. Carvalho, O. Reis, P. Ferreira, S. Sousa, P. Cossart, and D. Cabanes. 2009. In vivo transcriptional profiling of Listeria monocytogenes and mutagenesis identify new virulence factors involved in infection. PLoS Pathog. 5:e1000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell, D. J., and N. Shastri. 1998. Bacterial surface proteins recognized by CD4+ T cells during murine infection with Listeria monocytogenes. J. Immunol. 161:2339-2347. [PubMed] [Google Scholar]
- 11.Campbell, P. A. 1994. Macrophage-Listeria interactions. Immunol. Ser. 60:313-328. [PubMed] [Google Scholar]
- 12.Casadevall, A. 1998. Antibody-mediated protection against intracellular pathogens. Trends Microbiol. 6:102-107. [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee, S. S., H. Hossain, S. Otten, C. Kuenne, K. Kuchmina, S. Machata, E. Domann, T. Chakraborty, and T. Hain. 2006. Intracellular gene expression profile of Listeria monocytogenes. Infect. Immun. 74:1323-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheers, C., and M. Ho. 1983. Resistance and susceptibility of mice to bacterial infection. IV. Functional specificity in natural resistance to facultative intracellular bacteria. J. Reticuloendothel. Soc. 34:299-309. [PubMed] [Google Scholar]
- 15.Chiaraluce, R., V. Consalvi, S. Cavallo, A. Ilari, S. Stefanini, and E. Chiancone. 2000. The unusual dodecameric ferritin from Listeria innocua dissociates below pH 2.0. Eur. J. Biochem. 267:5733-5741. [DOI] [PubMed] [Google Scholar]
- 16.Crouzet-Ozenda, L., H. Haas, E. Bingen, A. Lecuyer, C. Levy, and R. Cohen. 2008. Listeria monocytogenes meningitis in children in France. Arch. Pediatr. 15(Suppl. 3):S158-S160. (In French.) [DOI] [PubMed] [Google Scholar]
- 17.Davies, W. A. 1983. Kinetics of killing Listeria monocytogenes by macrophages: rapid killing accompanying phagocytosis. J. Reticuloendothel. Soc. 34:131-141. [PubMed] [Google Scholar]
- 18.Edelson, B. T., P. Cossart, and E. R. Unanue. 1999. Cutting edge: paradigm revisited: antibody provides resistance to Listeria infection. J. Immunol. 163:4087-4090. [PubMed] [Google Scholar]
- 19.Fiorini, F., S. Stefanini, P. Valenti, E. Chiancone, and D. De Biase. 2008. Transcription of the Listeria monocytogenes fri gene is growth-phase dependent and is repressed directly by Fur, the ferric uptake regulator. Gene 410:113-121. [DOI] [PubMed] [Google Scholar]
- 20.Frazier, B. A., J. D. Pfeifer, D. G. Russell, P. Falk, A. N. Olsen, M. Hammar, T. U. Westblom, and S. J. Normark. 1993. Paracrystalline inclusions of a novel ferritin containing nonheme iron, produced by the human gastric pathogen Helicobacter pylori: evidence for a third class of ferritins. J. Bacteriol. 175:966-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couve, A. de Daruvar, P. Dehoux, E. Domann, G. Dominguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K. D. Entian, H. Fsihi, F. Garcia-del Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gomez-Lopez, T. Hain, J. Hauf, D. Jackson, L. M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueno, A. Maitournam, J. M. Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. de Pablos, J. C. Perez-Diaz, R. Purcell, B. Remmel, M. Rose, T. Schlueter, N. Simoes, A. Tierrez, J. A. Vazquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 22.Gosselin, E. J., C. Bitsaktsis, Y. Li, and B. V. Iglesias. 2009. Fc receptor-targeted mucosal vaccination as a novel strategy for the generation of enhanced immunity against mucosal and non-mucosal pathogens. Arch. Immunol. Ther. Exp. (Warsz.) 57:311-323. [DOI] [PubMed] [Google Scholar]
- 23.Grenningloh, R., A. Darji, J. Wehland, T. Chakraborty, and S. Weiss. 1997. Listeriolysin and IrpA are major protein targets of the human humoral response against Listeria monocytogenes. Infect. Immun. 65:3976-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hain, T., C. Steinweg, C. T. Kuenne, A. Billion, R. Ghai, S. S. Chatterjee, E. Domann, U. Karst, A. Goesmann, T. Bekel, D. Bartels, O. Kaiser, F. Meyer, A. Puhler, B. Weisshaar, J. Wehland, C. Liang, T. Dandekar, R. Lampidis, J. Kreft, W. Goebel, and T. Chakraborty. 2006. Whole-genome sequence of Listeria welshimeri reveals common steps in genome reduction with Listeria innocua as compared to Listeria monocytogenes. J. Bacteriol. 188:7405-7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison, P. M., and P. Arosio. 1996. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta 1275:161-203. [DOI] [PubMed] [Google Scholar]
- 26.Kolb-Maurer, A., S. Pilgrim, E. Kampgen, A. D. McLellan, E. B. Brocker, W. Goebel, and I. Gentschev. 2001. Antibodies against listerial protein 60 act as an opsonin for phagocytosis of Listeria monocytogenes by human dendritic cells. Infect. Immun. 69:3100-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lingnau, A., E. Domann, M. Hudel, M. Bock, T. Nichterlein, J. Wehland, and T. Chakraborty. 1995. Expression of the Listeria monocytogenes EGD inlA and inlB genes, whose products mediate bacterial entry into tissue culture cell lines, by PrfA-dependent and -independent mechanisms. Infect. Immun. 63:3896-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackaness, G. B. 1962. Cellular resistance to infection. J. Exp. Med. 116:381-406. [PubMed] [Google Scholar]
- 29.Meng, J., and M. P. Doyle. 1997. Emerging issues in microbiological food safety. Annu. Rev. Nutr. 17:255-275. [DOI] [PubMed] [Google Scholar]
- 30.Mohamed, W., A. Darji, E. Domann, E. Chiancone, and T. Chakraborty. 2006. The ferritin-like protein Frm is a target for the humoral immune response to Listeria monocytogenes and is required for efficient bacterial survival. Mol. Genet. Genomics 275:344-353. [DOI] [PubMed] [Google Scholar]
- 31.Nato, F., K. Reich, S. Lhopital, S. Rouyre, C. Geoffroy, J. C. Mazie, and P. Cossart. 1991. Production and characterization of neutralizing and nonneutralizing monoclonal antibodies against listeriolysin O. Infect. Immun. 59:4641-4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olsen, K. N., M. H. Larsen, C. G. Gahan, B. Kallipolitis, X. A. Wolf, R. Rea, C. Hill, and H. Ingmer. 2005. The Dps-like protein Fri of Listeria monocytogenes promotes stress tolerance and intracellular multiplication in macrophage-like cells. Microbiology 151:925-933. [DOI] [PubMed] [Google Scholar]
- 33.Osebold, J. W., and M. T. Sawyer. 1957. Immunization studies on listeriosis in mice. J. Immunol. 78:262-268. [PubMed] [Google Scholar]
- 34.Rawool, D. B., C. Bitsaktsis, Y. Li, D. R. Gosselin, Y. Lin, N. V. Kurkure, D. W. Metzger, and E. J. Gosselin. 2008. Utilization of Fc receptors as a mucosal vaccine strategy against an intracellular bacterium, Francisella tularensis. J. Immunol. 180:5548-5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Remer, K. A., T. Reimer, M. Brcic, and T. W. Jungi. 2005. Evidence for involvement of peptidoglycan in the triggering of an oxidative burst by Listeria monocytogenes in phagocytes. Clin. Exp. Immunol. 140:73-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaumburg, J., O. Diekmann, P. Hagendorff, S. Bergmann, M. Rohde, S. Hammerschmidt, L. Jansch, J. Wehland, and U. Karst. 2004. The cell wall subproteome of Listeria monocytogenes. Proteomics 4:2991-3006. [DOI] [PubMed] [Google Scholar]
- 37.Smith, B., M. Kemp, S. Ethelberg, P. Schiellerup, B. G. Bruun, P. Gerner-Smidt, and J. J. Christensen. 2009. Listeria monocytogenes: maternal-foetal infections in Denmark 1994-2005. Scand. J. Infect. Dis. 41:21-25. [DOI] [PubMed] [Google Scholar]
- 38.Suarez, M. M., R. M. Bautista, M. Almela, A. Soriano, F. Marco, J. Bosch, J. A. Martinez, A. Bove, A. Trilla, and J. Mensa. 2007. Listeria monocytogenes bacteremia: analysis of 110 episodes. Med. Clin. (Barc.) 129:218-221. (In Spanish.) [DOI] [PubMed] [Google Scholar]
- 39.Trost, M., D. Wehmhoner, U. Karst, G. Dieterich, J. Wehland, and L. Jansch. 2005. Comparative proteome analysis of secretory proteins from pathogenic and nonpathogenic Listeria species. Proteomics 5:1544-1557. [DOI] [PubMed] [Google Scholar]
- 40.Vazquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.