Abstract
Helminth infections have been associated with protection against allergy and autoimmune diseases. We investigated the effects of chronic infections with Ascaris lumbricoides and Trichuris trichiura (measured twice over a 5-year period) on cytokine and antibody responses. We collected blood from 1,060 children aged 4 to 11 years living in a poor urban area of Brazil and measured Th1 (gamma interferon [IFN-γ]) and Th2 (interleukin-5 [IL-5] and IL-13) cytokines and the regulatory cytokine IL-10 in unstimulated and stimulated (with mitogen or A. lumbricoides antigens) cultures of peripheral blood leukocytes and levels of total IgE and anti-A. lumbricoides IgG4 and IgE in serum. Intestinal helminth infections were associated with an increased proportion of children producing IL-5 in response to A. lumbricoides and producing IL-10 spontaneously, especially among coinfected and chronically infected children. Helminth infections were associated with a generalized suppression of cytokine responses to mitogen. Levels of total IgE and anti-A. lumbricoides IgG4 and IgE were especially elevated in chronically infected children. In conclusion, intestinal helminth infections were associated with a typical Th2 immune response profile and with the induction of immune hyporesponsiveness that was associated with greater frequencies of the production of spontaneous IL-10.
Among infectious agents, helminth parasites are regarded as master manipulators of the host immune response, being associated with chronic but generally asymptomatic infections. Although helminth infections induce strong Th2 responses, parasitic worms may survive in their mammalian hosts by switching off inflammatory immune responses and inducing a tolerant response to parasite antigens (38).
Atopy, characterized by raised immunoglobulin E (IgE) levels, is considered a major mediator of allergic diseases such as asthma, rhinoconjunctivitis, and eczema. The interaction of an environmental allergen with the innate immune system, uptake by antigen-presenting cells, and subsequent T-cell priming lead to the stimulation of Th2 cytokines, such as interleukin-4 (IL-4), IL-5, and IL-13. These cytokines stimulate IgE production and increased numbers of eosinophils and mast cells, which together may cause allergic inflammation in the respiratory tract (37).
The hygiene hypothesis has tried to explain the temporal trends of increased allergic disease prevalence over recent decades in industrialized countries by alterations in the host response to environmental allergens caused by decreased exposure to childhood infections through improvements in hygiene and greater access to antibiotics and vaccines (32). Such improved hygiene has been considered to alter the balance between type 1 (Th1) and type 2 (Th2) immune responses due to a failure of immune regulation resulting in allergy-mediating Th2 responses (22).
Exposure to pathogens and their products, and to helminths in particular, has been shown to protect against the development of autoimmune and allergic diseases in experimental animal models (7, 8, 10, 23, 25, 26, 28), and some evidence in support of this has been observed in human populations (3, 8, 10, 23, 29, 30, 31).
We previously demonstrated that children living in circumstances of poor hygiene without access to sanitation or clean water during the first 3 years of life have elevated spontaneous production of IL-10 up to 8 years later in life (13).
In the present study, we compared cytokine profiles from whole-blood cultures and antibody responses among children stratified by intestinal helminth infection status, determined at two separate time points in childhood.
MATERIALS AND METHODS
Study population and data collection.
This study was conducted in the city of Salvador in northeastern Brazil, which has a population of 2.5 million. The prevalence of wheezing in this city in the past 12 months in school children aged 12 to 13 years was reported to be very high (27.1%) (29). The design of this study has been reported elsewhere (6, 13, 29). In short, the study population included 1,445 children recruited in infancy for a prospective study measuring the impact of a citywide sanitation program on childhood morbidity (5). Data were collected from children born between 1994 and 2001 who lived in sentinel neighborhoods in the city. Standardized questionnaires were administered to the children's guardians between 1997 and 2003 (baseline) to collect data on demographic and social variables as well as on the home environment. In 2000, stool samples were collected to detect intestinal helminth infection (33). The children were surveyed again in 2005 to collect data on the same variables and to obtain stool and blood samples. Of the 1,445 children included in the study, we assayed 1,006 for gamma interferon (IFN-γ), 1,356 for IL-10, 1,289 for IL-13, and 1,243 for IL-5. For the present analysis, we used data on 1,060 children who had information for at least one of the cytokines and for whom data were also available on levels of total IgE and anti-Ascaris IgE and IgG4 and on the covariates of interest obtained by questionnaire. Ethical approval for the study was obtained from the Brazilian National Ethical Committee, and written informed consent was obtained from the legal guardian of each child.
Parasitological analysis.
Paired stool samples were collected from each child and analyzed for parasites at each of the two sampling times. Stools were analyzed using the gravitational sedimentation technique of Hoffman et al. to detect helminth eggs, protozoan cysts, and oocysts (17). Two slides were examined for each stool sample. Quantification of helminth eggs was performed using the Kato-Katz technique (18). All children with positive results were treated with appropriate antiparasitic drugs (6).
Ascaris lumbricoides antigens.
The A. lumbricoides extract was obtained by trituration of liquid nitrogen-frozen adult worms in phosphate-buffered saline (PBS), pH 7.4, by use of a blender (model 51BL30; Waring Commercial, Torrington, CT). The PBS-soluble fraction obtained by centrifugation was depleted of endotoxin by treatment with Triton X-114 (Sigma, St. Louis, MO), and protein content was determined by the Lowry method (20). Antigen was stored at −70°C until use.
Blood collection and whole-blood culture.
We collected venous blood into heparinized tubes and cultured the blood at a dilution of 1:4 in RPMI (Gibco, Auckland, New Zealand) containing 10 mM glutamine (Sigma-Aldrich, St. Louis, MO) and 100 μg/ml gentamicin (Sigma-Aldrich, St. Louis, MO). The cells were cultured within 6 h of collection and were maintained in a humidified environment of 5% CO2 at 37°C for 24 h for detection of IL-10 and for 5 days for the detection of IL-13, IL-5, and IFN-γ in the presence of Ascaris lumbricoides antigen (10 μg/ml), pokeweed mitogen (PWM; Sigma-Aldrich, St. Louis, MO) (2.5 μg/ml), or medium alone.
Cytokine production.
We measured the production of IL-5, IL-13, IFN-γ, and IL-10 in whole-blood culture supernatants, using commercially available antibody pairs and recombinant cytokine standards (BD Pharmingen, San Diego, CA), by sandwich enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions. Cytokine concentrations were determined by interpolation of standard curves. The detection limits (low/high) for each cytokine were as follows: for IL-5, 15.63/500 pg/ml; for IL-13, 62.5/4,000 pg/ml; for IFN-γ, 18.5/300 pg/ml; and for IL-10, 31.25/500 pg/ml. Responders were defined as those children with cytokine concentrations above the lower detection limits.
IgE and IgG4 production.
Determination of specific IgE serum concentrations was done for A. lumbricoides by use of the Immunocap assay (Phadia Diagnostics AB, Uppsala, Sweden). Children with an anti-Ascaris IgE level of ≥0.35 kilounits/liter were considered positive.
Anti-A. lumbricoides IgG4 was detected by indirect ELISA as follows. High-binding-level microassay plates (Costar, Cambridge, MA) were sensitized with 20 μg/ml of A. lumbricoides antigen diluted in carbonate-bicarbonate buffer, pH 9.6. Sera were diluted 1:50 in PBS containing 10% fetal calf serum (FCS) (Sigma Chemical Co., St. Louis, MO) and 0.1% Tween 20 (PBST). Plates were incubated with biotinylated anti-human IgG4 (Sigma Chemical Co., St. Louis, MO), followed by streptavidin-peroxidase (Pharmingen, San Jose, CA) and H2O2-OPD substrate (Merck & Co., Inc., White House Station, NJ), and were read using a 480-nm filter.
Total IgE was measured using high-binding-level microassay plates (Costar, Cambridge, MA) coated with 4 μg/ml of an anti-human IgE antibody (Pharmingen, San Diego, CA) overnight at 4°C. Plates were blocked with 0.15 M PBS, pH 7.2, containing 10% FCS and 0.05% Tween 20 (Sigma, St. Louis, MO) overnight at 4°C. Samples were diluted 1:10 in PBS containing 5% FCS and 0.05% Tween 20 and incubated overnight at 4°C. Plates were incubated with biotinylated anti-human IgE (Sigma Chemical Co., St. Louis, MO), followed by streptavidin-peroxidase (Pharmingen, San Jose, CA) and H2O2-OPD substrate (Merck & Co., Inc., White House Station, NJ), and were read using a 480-nm filter. A pool of parasite-infected patient sera was used as a positive control. Umbilical cord serum from a newborn of a nonatopic and nonparasitized mother was used as a negative control.
The assay cutoff for total IgE (0.2 μg/ml) was determined as the median plus the semi-interquartile deviation for negative controls (54 sera from children with 3 negative stool samples collected serially, specific IgE levels of <0.35, and <2% eosinophils in peripheral blood). The assay cutoff for IgG4 (optical density [OD] of 0.4) for A. lumbricoides was determined as the mean plus 3 standard deviations for negative controls (sera from children with 3 negative stool samples collected serially). The cutoff for Ascaris-specific IgE was 0.35 kilounits/liter, as recommended by the manufacturer. Antibody levels of anti-Ascaris IgG4 and total IgE were defined as positive or negative by using the above cutoffs.
Statistical analyses.
The occurrence and chronicity of infections with A. lumbricoides and T. trichiura were defined as follows: (i) past infection, infection with either parasite detected in early life (i.e., survey conducted in 2000); (ii) current infection, infection with either parasite detected later in childhood (i.e., survey conducted in 2005); (iii) chronic infection, infection with either A. lumbricoides or T. trichiura in both 2000 and 2005; and (iv) coinfection, infection with both helminths in 2005. Associations between cytokine responsiveness (responders versus nonresponders) and intestinal helminth infection status for the different stimuli (spontaneous [medium control], mitogen, or Ascaris) were assessed using the Pearson chi-square test or trend test, as appropriate. We also compared cytokine concentrations by helminth infection status. The proportion of individuals with cytokine concentrations greater than or equal to a certain value was estimated and is presented graphically (Fig. 1A to D). Measurements above the detection limits of the assays were censored at the threshold value, and standard statistical methods for analyses of censored (or nondetectable) data were used, including the Kaplan-Meier estimator and log rank test (11, 15, 16, 18). The Kaplan-Meier method is the standard nonparametric method for computing summary statistics for censored data (34). A proportion was estimated for each observed concentration, and the results are summarized in plots in Fig. 1A to D. Comparisons of cytokine concentrations according to infection groups were performed using the log rank test. The rejection of the null hypothesis for the log rank test means that not all groups have the same distribution. In addition, geometric means and bootstrap standard errors were computed for cytokine concentrations from PWM stimulations for each infection group. These results are presented graphically in Fig. 1E and F. We estimated odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for production of anti-Ascaris IgE, total IgE, and anti-Ascaris IgG4 by helminth infection status, using logistic regression (1). All models were adjusted for age and sex. Estimated ORs for A. lumbricoides infection were adjusted for T. trichiura infection and vice versa. Statistical analyses were performed using SPSS v15, R v2.9, and STATA v.9 software.
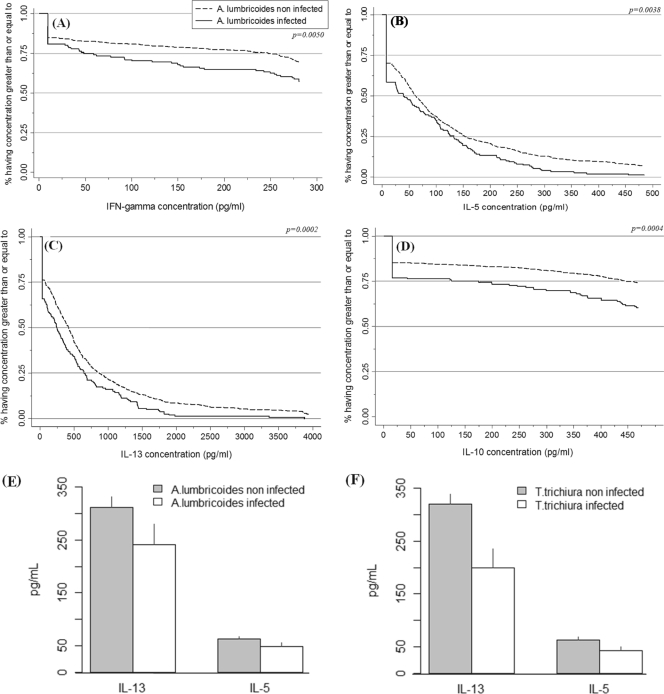
FIG. 1.
Comparison of cytokine concentrations from PWM-stimulated whole-blood cultures by presence (continuous line) or absence (dotted line) of current infection with Ascaris lumbricoides. Cytokine levels were compared using nonparametric Kaplan-Meier estimator and log rank tests for IFN-γ (A), IL-5 (B), IL-10 (C), and IL-13 (D), and cytokine concentrations are presented according to the presence or absence of Ascaris (E) and Trichuris (F) infections.
RESULTS
Study population.
The study population had an age range of 4 to 11 years, 41.1% of the population was 6 to 7 years of age, and the majority of participants were boys (54.3%). Ascaris lumbricoides and Trichuris trichiura were the most common helminth parasites in our population. The percentages of children with A. lumbricoides and T. trichiura infections in 2005 were 16.1% and 13.8%, respectively. In addition, 7% were chronically infected with A. lumbricoides and 6.3% were chronically infected with T. trichiura (infected in both surveys). A total of 7.0% of the children were coinfected with A. lumbricoides and T. trichiura in 2005.
Effects of A. lumbricoides and T. trichiura infections on whole-blood cytokine production.
To measure the impact of helminth infections on cytokine production in whole-blood cultures, we measured Th1 (IFN-γ), Th2 (IL-5 and IL-13), and Treg (IL-10) cytokines. The percentage of children producing IL-10 spontaneously in whole-blood cultures was higher among children with current A. lumbricoides (16%; P < 0.001) and T. trichiura (15.1%; P = 0.001) infections in the 2005 survey, as well as those with coinfections (21.6%; P < 0.001), than among noninfected children in the respective infection groups (Table 1). Furthermore, the proportion of children producing IL-10 was higher among children with chronic A. lumbricoides (23.3%; P < 0.001) and T. trichiura (22.4%; P < 0.001) infections than among nonchronically infected children (i.e., those classified into the “never,” “past,” and “present” groups). Children with current T. trichiura infections were associated more frequently with production of IL-13 than uninfected children were (P = 0.01).
TABLE 1.
Effect of helminth infection on spontaneous cytokine production in whole-blood cultures
| Type of infection | No. of individuals (% with spontaneous cytokine production in whole-blood cultures)a |
|||
|---|---|---|---|---|
| IFN-γ | IL-5 | IL-10 | IL-13 | |
| Overall | 774 (11.0) | 955 (6.1) | 1,045 (8.2) | 990 (34.3) |
| Current Ascaris infection | ||||
| Negative | 643 (10.7) | 806 (6.2) | 876 (6.7) | 829 (35.3) |
| Positive | 131 (12.2) | 149 (5.4) | 169 (16.0)* | 161 (29.2) |
| Chronic Ascaris infection | ||||
| Never | 539 (10.9) | 675 (6.8) | 728 (6.3) | 687 (35.5) |
| Past | 104 (9.6) | 131 (3.1) | 148 (8.8) | 142 (34.5) |
| Present | 74 (12.2) | 87 (4.6) | 96 (10.4) | 91 (23.1) |
| Both | 57 (12.3) | 62 (6.5) | 73 (23.3)+ | 70 (37.1) |
| Current Trichuris infection | ||||
| Negative | 665 (10.5) | 824 (5.9) | 899 (7.1) | 853 (32.7) |
| Positive | 109 (13.8) | 131 (6.9) | 146 (15.1)* | 137 (44.5)* |
| Chronic Trichuris infection | ||||
| Never | 539 (10.6) | 734 (6.3) | 809 (7.2) | 763 (32.9) |
| Past | 104 (10.1) | 90 (3.3) | 90 (6.7) | 90 (31.1) |
| Present | 74 (11.3) | 73 (5.5) | 79 (8.9) | 72 (54.2) |
| Both | 57 (16.1) | 58 (8.6) | 67 (22.4)+ | 65 (33.8) |
| Coinfection with Ascaris and Trichuris | ||||
| Negative for both | 593 (10.8) | 741 (6.3) | 804 (6.6) | 762 (34.0) |
| Positive for only one | 122 (9.0) | 148 (3.4) | 167 (10.2) | 158 (34.2) |
| Positive for both | 59 (16.9) | 66 (9.1) | 74 (21.6)+ | 70 (38.6) |
Differences between groups were assessed using the chi-square test or trend test, as appropriate. Numbers in bold show statistically significant results. *, P ≤ 0.05 (χ2); +, P ≤ 0.05 (χ2 trend test).
Current or chronic infections with A. lumbricoides (P ≤ 0.01) and T. trichiura (P < 0.05) were associated with larger proportions of children producing IL-5 (Table 2) upon A. lumbricoides stimulation. A similar pattern was observed for coinfected children (27.3%; P < 0.001). T. trichiura-infected children were also associated with IL-13 responsiveness (P < 0.05). However, elevated frequencies of IL-10 responder children were observed only among those with chronic T. trichiura infections, not among the groups of children without chronic T. trichiura infections (P = 0.02).
TABLE 2.
Effect of helminth infection on cytokine production in Ascaris lumbricoides-stimulated whole-blood cultures
| Type of infection | No. of individuals (% with cytokine production in A. lumbricoides-stimulated whole-blood cultures)a |
|||
|---|---|---|---|---|
| IFN-γ | IL-5 | IL-10 | IL-13 | |
| Overall | 774 (2.5) | 955 (12.1) | 1,045 (4.1) | 990 (20.5) |
| Current Ascaris infection | ||||
| Negative | 643 (2.2) | 806 (10.7) | 876 (3.8) | 829 (20.3) |
| Positive | 131 (3.8) | 149 (20.1)* | 169 (5.9) | 161 (21.7) |
| Chronic Ascaris infection | ||||
| Never | 539 (2.4) | 675 (11.0) | 728 (3.3) | 687 (20.2) |
| Past | 104 (1.0) | 131 (9.2) | 148 (6.1) | 142 (20.4) |
| Present | 74 (2.7) | 87 (16.1) | 96 (6.3) | 91 (16.5) |
| Both | 57 (5.3) | 62 (25.8)+ | 73 (5.5) | 70 (28.6) |
| Current Trichuris infection | ||||
| Negative | 665 (2.3) | 824 (10.4) | 899 (3.7) | 853 (19.3) |
| Positive | 109 (3.7) | 131 (22.9)* | 146 (6.8) | 137 (27.7)* |
| Chronic Trichuris infection | ||||
| Never | 539 (2.3) | 734 (10.5) | 809 (3.3) | 763 (19.4) |
| Past | 104 (1.4) | 90 (10.0) | 90 (6.7) | 90 (18.9) |
| Present | 74 (3.8) | 73 (21.9) | 79 (5.1) | 72 (29.2) |
| Both | 57 (3.6) | 58 (24.1)+ | 67 (9.0)+ | 65 (26.2)+ |
| Coinfection with Ascaris and Trichuris | ||||
| Negative for both | 593 (2.0) | 741 (10.0) | 804 (3.4) | 762 (19.9) |
| Positive for only one | 122 (4.1) | 148 (16.2) | 167 (7.2) | 158 (18.4) |
| Positive for both | 59 (3.4) | 66 (27.3)+ | 74 (5.4) | 70 (31.4) |
Differences between groups were assessed using the chi-square test or trend test, as appropriate. Numbers in bold show statistically significant results. *, P ≤ 0.05 (χ2); +, P ≤ 0.05 (χ2 trend test).
Following stimulation with PWM, we observed a generalized downregulation of the majority of the cytokines among children with intestinal helminth infections (Table 3). For all infection groupings, but especially chronic T. trichiura infections, we observed significant reductions in the proportions of cytokine responders among infected compared to noninfected children. The proportions of children with detectable IFN-γ, IL-5, IL-10, and IL-13 were lower among children with current A. lumbricoides infections than among noninfected children (P < 0.01; log rank test) (Fig. 1A to D). This is illustrated in Fig. 1 by the differences between the individual Kaplan-Meier graphs representing the proportions of children with each concentration of cytokine among those with and without A. lumbricoides infection. The proportions of children expressing each concentration of cytokine were lower for infected than noninfected children for all 4 cytokines measured. For example, 75% of the children infected by A. lumbricoides had IL-10 concentrations of ≥125 pg/ml, while 75% of the children not infected with A. lumbricoides had IL-10 concentrations of ≥425 pg/ml (Fig. 1C). We also observed that approximately 38% of noninfected children and 31% of infected children had IL-13 concentrations of ≥500 pg/ml (Fig. 1D). Similar effects were observed for children with chronic A. lumbricoides or T. trichiura infections and coinfections compared to the appropriate noninfected groups (P < 0.05 for all comparisons [see the supplemental material]). An alternative way of presenting the data, using geometric means and bootstrap-estimated standard errors, is shown in Fig. 1E (A. lumbricoides infection status) and Fig. 1F (T. trichiura infection status). Geometric mean cytokine concentrations for IL-5 and IL-13 were lower in infected than noninfected children for both A. lumbricoides and T. trichiura infections.
TABLE 3.
Effect of helminth infection on cytokine production in PWM-stimulated whole-blood cultures
| Type of infection | No. of individuals (% with cytokine production inPWM-stimulated whole-blood cultures)a |
|||
|---|---|---|---|---|
| IFN-γ | IL-5 | IL-10 | IL-13 | |
| Overall | 774 (84.2) | 955 (68.3) | 1,045 (83.9) | 990 (74.4) |
| Current Ascaris infection | ||||
| Negative | 643 (84.9) | 806 (70.1) | 876 (85.3) | 829 (76.1) |
| Positive | 131 (80.9) | 149 (58.4)* | 169 (76.9)* | 161 (65.8)* |
| Chronic Ascaris infection | ||||
| Never | 539 (85.0) | 675 (71.4) | 728 (86.7) | 687 (77.1) |
| Past | 104 (84.6) | 131 (63.4) | 148 (78.4) | 142 (71.1) |
| Present | 74 (78.4) | 87 (60.9) | 96 (80.2) | 91 (67.0) |
| Both | 57 (84.2) | 62 (54.8)+ | 73 (72.6)+ | 70 (64.3)+ |
| Current Trichuris infection | ||||
| Negative | 665 (85.9) | 824 (70.4) | 899 (86.1) | 853 (77.1) |
| Positive | 109 (74.3)* | 131 (55.0)* | 146 (70.5)* | 137 (57.7)* |
| Chronic Trichuris infection | ||||
| Never | 539 (86.1) | 734 (71.0) | 809 (86.4) | 763 (77.5) |
| Past | 104 (84.1) | 90 (65.6) | 90 (83.3) | 90 (74.4) |
| Present | 74 (79.2) | 73 (58.9) | 79 (73.4) | 72 (62.5) |
| Both | 57 (69.6)+ | 58 (50.0)+ | 67 (67.2)+ | 65 (52.3)+ |
| Coinfection with Ascaris and Trichuris | ||||
| Negative for both | 593 (86.0) | 741 (71.4) | 804 (86.8) | 762 (78.1) |
| Positive for only one | 122 (79.5) | 148 (58.8) | 167 (74.9) | 158 (62.7) |
| Positive for both | 59 (76.3)+ | 66 (54.5)+ | 74 (73.0)+ | 70 (61.4)+ |
Differences between groups were assessed using the chi-square test or trend test, as appropriate. Numbers in bold show statistically significant results. *, P ≤ 0.05 (χ2); +, P ≤ 0.05 (χ2 trend test).
Effects of Ascaris lumbricoides and Trichuris trichiura infections on total IgE and Ascaris-specific IgE and IgG4.
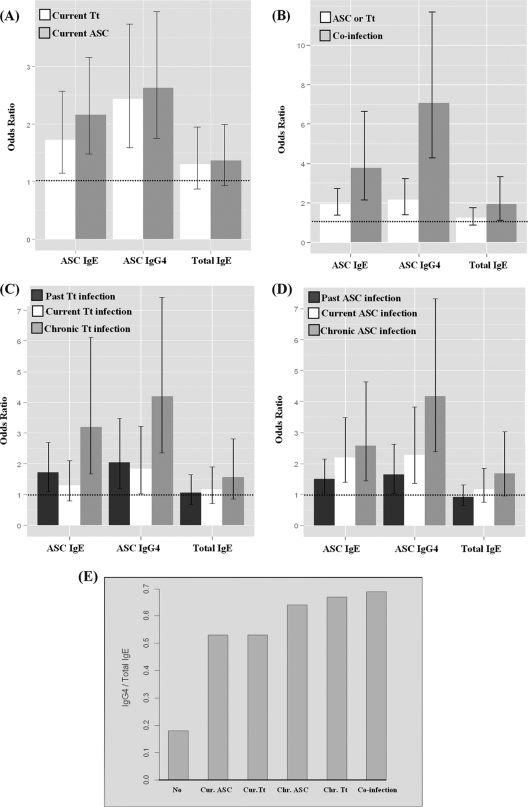
Among the mechanisms postulated to explain helminth-mediated modulation of allergy, the production of polyclonal IgE at levels that can saturate mast cells and of IgG4 blocking antibodies is commonly mentioned (10, 37). To address these mechanisms, we estimated the effects of A. lumbricoides and T. trichiura infections on the presence of total IgE and Ascaris-specific IgE and IgG4 (Fig. 2A to D). All infection groups (current and chronic infections and coinfections) were associated with increased proportions of children with these antibodies. Children with current A. lumbricoides infections had a greater chance of having IgE for A. lumbricoides (OR, 2.16; CI, 1.48 to 3.16), total IgE (OR, 1.36; CI, 0.93 to 1.99), or IgG4 for A. lumbricoides (OR, 2.63; CI, 1.75 to 3.95) than those without current A. lumbricoides infection (Fig. 2A). The same pattern of response was observed for coinfections (Fig. 2B) and chronic infections (Fig. 2C and D). The presence of coinfection increased the chance of producing antibodies compared to that for the group without current A. lumbricoides and T. trichiura infections (for anti-Ascaris IgE, OR = 3.78 and CI = 2.15 to 6.64; for anti-Ascaris IgG4, OR = 7.08 and CI = 4.28 to 11.71; and for total IgE, OR = 1.93 and CI = 1.12 to 3.31). Chronic infections were strongly associated with the presence of these antibodies. For example, the chance of producing anti-Ascaris IgE among children with T. trichiura infection in 2000 and 2005 was 3.20 times higher than that for individuals without T. trichiura infection in both periods (OR, 3.20; CI, 1.67 to 6.13) (Fig. 2C). In addition, the ratio of IgG4/total IgE was higher for children with chronic helminth infections or coinfections with T. trichiura and A. lumbricoides than for children with current infections. Ratios were lowest for noninfected children (Fig. 2E).
FIG. 2.
Effects of helminth infection on the presence of antibodies, represented by ORs and 95% CIs. (A) Effects of current helminth infections on anti-Ascaris IgE and IgG4 and on total IgE. White bars represent Trichuris infection (Tt), and gray bars represent Ascaris infection (ASC). (B) Effects of helminth infections on anti-Ascaris IgE and IgG4 and on total IgE during single helminth infections (white bars) and coinfections (gray bars). (C and D) Effects of chronic Trichuris (C) and Ascaris (D) infections on anti-Ascaris IgE and IgG4 and on total IgE. Dark gray bars represent early-life infection, white bars represent current infections, and light gray bars represent infection in both periods analyzed. (E) Bars represent the ratio of IgG4/IgE as percentages among responders for IgG4 and total IgE. No, no infection; Cur. ASC, current Ascaris infection; Cur. Tt, current Trichuris infection; Chr. ASC, chronic Ascaris infection; Chr. Tt, chronic Trichuris infection; co-infection, current infection with both parasites.
DISCUSSION
There is some evidence from experimental animal models and epidemiological studies of human populations indicating that the development of autoimmune and allergic pathologies could be modified by helminth infections (3, 7, 8, 10, 23, 28, 29, 31). Several mechanisms have been proposed to explain the anti-inflammatory effects of helminth infections, including the production of IL-10 and transforming growth factor beta (TGF-β) by regulatory T cells, production of IgG4 isotype blocking antibodies, and suppression of mast cells, basophils, and eosinophils (2, 10). Helminth infections induce strong Th2 responses, as described previously (10, 27). The findings of the present study support previous data and show the presence of Th2 immune polarization during intestinal helminth infection, in which the presence of anti-Ascaris IgE and the production of IL-5 upon stimulation of whole blood with parasite antigen were particularly prominent among children infected with both A. lumbricoides and T. trichiura (Table 1). Furthermore, we observed an upregulation of IL-10 by whole-blood cultures stimulated with A. lumbricoides antigens, especially during T. trichiura infections and coinfections. Survival of adult worms in the human host is thought to be facilitated by the induction of an immune regulatory network (23, 35).
We previously demonstrated that children whose household had no sewage system or potable tap water from early life were likely to produce more spontaneous IL-10 than those children living in households with access to a sewage system and clean drinking water, suggesting a possible homeostatic effect induced by these exposures (13) or factors associated with them. In this study, our data suggest that intestinal helminths may contribute to this immune regulation, as a large proportion of children with current infections with either or both A. lumbricoides and T. trichiura or with chronic infections showed evidence of spontaneous IL-10 production by unstimulated whole blood. Recently, Turner et al. (35) described that African children under conditions of hyperendemic exposure to the intestinal nematodes A. lumbricoides and T. trichiura constitutively secrete more immunoregulatory cytokines (IL-10 and TGF-β1) than do children living under conditions of mesoendemic exposure. The activation of this regulatory network induced by helminths has also been suggested to be responsible for preventing the elimination of the worms and for protection of the host from immunopathology that would otherwise result from excessive inflammation (21, 36). Looking at the effect of helminth infections on cytokine production induced by PWM, we did see a generalized suppression of cytokine production. This hyporesponsiveness occurred during current and chronic infections and during coinfections.
The hyporesponsiveness associated with helminths may extend to third-party antigens, such as vaccines, and has been suggested by the findings of several studies of humans (9, 25, 30, 35). Such suppression could also affect immunity to aeroallergens, as suggested by the observation of an inverse association between the production of parasite antigen-induced IL-10 by peripheral blood lymphocytes and skin test reactivity to house dust mites among children from Gabon living in an area where Schistosoma mansoni infection is endemic (35).
The upregulation of IL-10 during helminth infection has been described previously (3). A study from Brazil comparing asthmatic subjects infected with S. mansoni to uninfected subjects (from a different population) showed that Dermatophagoides pteronyssinus-stimulated peripheral blood mononuclear cells from infected asthmatics produced fewer Th2 cytokines and more IL-10 than control cells did (3). However, other studies conducted in areas where A. lumbricoides was the predominant helminth did not provide evidence for either enhanced IL-10 responses to aeroallergens (27, 28) or an increase in the frequency of regulatory T-cell populations induced by aeroallergen stimulation of peripheral blood lymphocytes (8). Based on our findings, we hypothesize that exposure to helminths and their products produces a homeostatic effect (through spontaneous IL-10 production) by inducing the generalized suppression of cytokine production, mainly during chronic infections but also during coinfection, which seems to have an additive effect.
Nondetectable data were obtained for a large number of assays, particularly for unstimulated and Ascaris antigen-stimulated cultures. A high frequency of nondetectable data for unstimulated cultures is not surprising, and the low level of detection for Ascaris-stimulated cultures may be explained by the relatively low prevalence of intestinal helminth infections in this population—the 2005 survey showed the prevalences of A. lumbricoides and T. trichiura infections to be 16.1% and 13.8%, respectively. Nondetectable data occur not only in immunology but also in other areas, such as astronomy, pharmacology, environmental control, and ecology (16). In order to take into account such a data structure, several statistical methods have been discussed in the literature (12, 15, 19), from descriptive methods to regression models. The most common approach used is to replace nondetectable data by some fraction of the assay's detection limit. However, such a procedure may lead to spurious results when frequencies of nondetectable data are high (16). To avoid this possibility, in the present analysis we favored a statistical approach designed for the analysis of nondetectable data (15, 16).
Looking at the effect of helminth infections on IgE and IgG4 antibodies, we observed that both intestinal helminth infections, especially chronic infections, were more likely to be associated with the presence of total IgE and anti-Ascaris IgG4 and IgE and may reflect cross-reactivity between antigens of different helminth parasites. Anti-Ascaris IgG4 may contribute to the suppression of skin prick test (SPT) reactivity and allergic reactions, as proposed by others (10, 21, 27, 29, 37). Furthermore, there was some evidence that chronic infections and coinfections were more strongly associated with the presence of anti-Ascaris IgG4 and with greater ratios of anti-Ascaris IgG4 to IgE than current infections were. IL-10 is considered to promote the production of IgG4 over IgE (23), and a positive but nonsignificant association was observed between spontaneous IL-10 production and the presence of anti-Ascaris IgG4 (data not shown).
In summary, our data from a population-based prospective study among children living in an urban region of Brazil provide evidence that chronic infections with intestinal helminths are associated with a generalized suppression of mitogen-induced cytokine production by peripheral blood leukocytes but with enhanced production of spontaneous IL-10. Such a regulated immune response phenotype induced by intestinal helminths may contribute to the development of an anti-inflammatory phenotype that may mediate the modulation of allergy that has been attributed to these infections.
Supplementary Material
Acknowledgments
We have no competing financial interests.
This study was funded by The Wellcome Trust, UK, HCPC Latin America Excellence Centre Programme (072405/Z/03/Z).
We thank the individuals who contributed directly or indirectly to this work, including laboratory technicians, field workers, and students.
The Wellcome Trust Foundation and the Brazilian agencies CAPES, CNPQ, and FAPESB are also acknowledged for funding and providing support for postdoctoral, master's degree, and doctoral fellowships.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 19 April 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Agresti, A. 2007. Introduction to categorical data analysis, 2nd ed. Wiley-Interscience, New Jersey, NJ.
- 2.Akdis, M. 2008. T cell tolerance to inhaled allergens: mechanisms and therapeutic approaches. Expert Opin. Biol. Ther. 8:769-777. [DOI] [PubMed] [Google Scholar]
- 3.Araujo, M. I., B. Hoppe, M. Medeiros, Jr., L. Alcântara, M. C. Almeida, A. Schriefer, R. R. Oliveira, R. Kruschewsky, J. P. Figueiredo, A. A. Cruz, and E. M. Carvalho. 2004. Impaired T helper 2 response to aeroallergen in helminth infected patients with asthma. J. Infect. Dis. 190:1797-1803. [DOI] [PubMed] [Google Scholar]
- 4.Reference deleted.
- 5.Barreto, M. L., B. Genser, A. Strina, M. G. Teixeira, A. M. Assis, R. F. Rego, C. A. Teles, M. S. Prado, S. M. Matos, D. N. Santos, L. A. dos Santos, and S. Cairncross. 2007. Effect of city-wide sanitation programme on reduction in rate of childhood diarrhea in northeast Brazil: assessment by two cohort studies. Lancet 370:1622-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barreto, M. L., S. S. Cunha, N. Alcântara-Neves, L. P. Carvalho, A. A. Cruz, R. T. Stein, B. Genser, P. J. Cooper, and L. C. Rodrigues. 2006. Risk factors and immunological pathways for asthma and other allergic diseases in children: background and methodology of a longitudinal study in a large urban center in northeastern Brazil (Salvador-SCAALA study). BMC Pulm. Med. 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burkhart, C., G. Y. Liu, S. M. Anderton, B. Metzler, and D. C. Wraith. 1999. Peptide-induced T cell regulation of experimental autoimmune encephalomyelitis: a role for IL-10. Int. Immunol. 11:1625-1634. [DOI] [PubMed] [Google Scholar]
- 8.Cooper, P. J., A. L. Moncayo, I. Guadalupe, S. Benitez, M. Vaca, M. Chico, and G. E. Griffin. 2008. Repeated treatments with albendazole enhance Th2 responses to Ascaris lumbricoides, but not to aeroallergens, in children from rural communities in the Tropics. J. Infect. Dis. 198:1237-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper, P. J., I. Espinel, W. Paredes, R. H. Guderian, and T. B. Nutman. 1998. Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: a possible role of interleukin-10. J. Infect. Dis. 178:1133-1138. [DOI] [PubMed] [Google Scholar]
- 10.Cooper, P. J. 2009. Interactions between helminth parasites and allergy. Curr. Opin. Allergy Clin. Immunol. 9:29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eastoe, E. F., C. J. Halsal, J. E. Heffernan, and H. Hun. 2006. A statistical comparison of survival and replacement analyses for the use of censored data in a contaminant air database: a case study from the Canadian Arctic. Atmos. Environ. 40:6528-6540. [Google Scholar]
- 12.Eriksson, M., E. Sartono, C. L. Martins, C. Balé, M. L. Garly, H. Whittle, et al. 2007. A comparison of ex vivo cytokine production in venous and capillary blood. Clin. Exp. Immunol. 150:469-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Figueiredo, C. A., N. M. Alcântara-Neves, R. Veiga, L. D. Amorim, V. Dattoli, L. R. Mendonça, S. Junqueira, B. Genser, M. Santos, L. C. de Carvalho, P. J. Cooper, L. Rodrigues, and M. L. Barreto. 2009. Spontaneous cytokine production in children according to biological characteristics and environmental exposures. Environ. Health Perspect. 117:845-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reference deleted.
- 15.Helsel, D. R. 2005. More than obvious: better methods for interpreting nondetect data. Environ. Sci. Technol. 39:419-423. [DOI] [PubMed] [Google Scholar]
- 16.Helsel, D. R. 2005. Nondetects and data analysis: statistics for censored environmental data, 1st ed. Wiley-Interscience, New Jersey, NJ.
- 17.Hoffman, W. A., J. A. Pons, and J. L. Janer. 1934. The sedimentation-concentration method in schistosomiasis mansoni. Puerto Rico J. Public Health 9:281-298. [Google Scholar]
- 18.Katz, N., A. Chaves, and J. Pellegrino. 1972. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Paulo 14:397-400. [PubMed] [Google Scholar]
- 19.Lee, L., and D. Helsel. 2007. Statistical analysis of water-quality data containing multiple detection limits. II. S-language software for nonparametric distribution modeling and hypothesis testing. Comput. Geosci. 33:696-704. [Google Scholar]
- 20.Lowry, O. H., N. J. Rosenbrough, A. L. Faar, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 21.Maizels, R. M., and M. Yazdanbakhsh. 2003. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat. Rev. Immunol. 3:733-744. [DOI] [PubMed] [Google Scholar]
- 22.Matricardi, P. M., and S. Bonini. 2000. High microbial turnover rate preventing atopy: a solution to inconsistencies impinging on the hygiene hypothesis? Clin. Exp. Allergy 30:1506-1510. [DOI] [PubMed] [Google Scholar]
- 23.Mingomataj, E. Ç., F. Xhixha, and E. Gjata. 2006. Helminths can protect themselves against rejection inhibiting hostile respiratory allergy symptoms. Allergy 61:400-406. [DOI] [PubMed] [Google Scholar]
- 24.Reference deleted.
- 25.Nookala, S., S. Srinivasan, P. Kaliraj, R. B. Narayanan, and T. B. Nutman. 2004. Impairment of tetanus-specific cellular and humoral responses following tetanus vaccination in human lymphatic filariasis. Infect. Immun. 72:2598-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacífico, L. G., F. A. Marinho, C. T. Fonseca, M. M. Barsante, V. Pinho, P. A. Sales-Junior, L. S. Cardoso, M. I. Araújo, E. M. Carvalho, G. D. Cassali, M. M. Teixeira, and S. C. Oliveira. 2009. Schistosoma mansoni antigens modulate experimental allergic asthma in a murine model: a major role for CD4+ CD25+ Foxp3+ T cells independent of interleukin-10. Infect. Immun. 77:98-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearce, E. J., C. M. Kane, J. Sun, J. J. Taylor, A. S. McKee, and L. Cervi. 2004. Th2 response polarization during infection with the helminth parasite Schistosoma mansoni. Immunol. Rev. 201:117-126. [DOI] [PubMed] [Google Scholar]
- 28.Ponte, E. V., F. Lima, M. I. Araújo, R. R. Oliveira, L. S. Cardoso, and A. A. Cruz. 2006. Skin test reactivity and Der p-induced interleukin 10 production in patients with asthma or rhinitis infected with Ascaris. Ann. Allergy Asthma Immunol. 96:713-718. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues, L. C., P. J. Newcombe, S. S. Cunha, N. M. Alcantara-Neves, B. Genser, A. A. Cruz, S. M. Simoes, R. Fiaccone, L. Amorim, P. J. Cooper, M. L. Barreto, and Social Change, Asthma and Allergy in Latin America. 2008. Early infection with Trichuris trichiura and allergen skin test reactivity in later childhood. Clin. Exp. Allergy 38:1769-1777. [DOI] [PubMed] [Google Scholar]
- 30.Sabin, E. A., M. I. Araujo, E. M. Carvalho, and E. J. Pearce. 1996. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J. Infect. Dis. 173:269-272. [DOI] [PubMed] [Google Scholar]
- 31.Smits, H. H., and M. Yazdanbakhsh. 2007. Chronic helminth infections modulate allergen-specific immune responses: protection against development of allergic disorders? Ann. Med. 39:428-439. [DOI] [PubMed] [Google Scholar]
- 32.Strachan, D. P. 1989. Hay fever, hygiene, and household size. Br. Med. J. 299:1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strina, A., S. Cairncross, M. L. Barreto, C. Larrea, and M. S. Prado. 2003. Childhood diarrhea and observed hygiene behavior in Salvador, Brazil. Am. J. Epidemiol. 157:1032-1038. [DOI] [PubMed] [Google Scholar]
- 34.Therneau, T., and P. Grambsch. 2000. Modeling survival data: extending the Cox model, 1st ed. Springer Verlag, New York, NY.
- 35.Turner, J. D., J. A. Jackson, H. Faulkner, J. Behnke, K. J. Else, J. Kamgno, M. Boussinesq, and J. E. Bradley. 2008. Intensity of intestinal infection with multiple worm species is related to regulatory cytokine output and immune hyporesponsiveness. J. Infect. Dis. 197:1204-1212. [DOI] [PubMed] [Google Scholar]
- 36.Wilson, M. S., M. D. Taylor, A. Balic, C. A. M. Finney, J. R. Lamb, and R. M. Maizels. 2005. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J. Exp. Med. 202:1199-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yazdanbakhsh, M., P. G. Kremsner, and R. Ree. 2002. Allergy, parasites, and the hygiene hypothesis. Science 296:490-494. [DOI] [PubMed] [Google Scholar]
- 38.Zaccone, P., Z. Fehervari, J. M. Phillips, D. W. Dunne, and A. Cooke. 2006. Parasitic worms and inflammatory diseases. Parasite Immunol. 28:515-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.