Abstract
Borrelia burgdorferi, the Lyme disease pathogen, dramatically alters its protein profile when it is transmitted between ticks and mammals. Several differentially expressed proteins have been shown to be critical for the enzootic cycle of B. burgdorferi. In this study, we demonstrated that expression of the surface lipoprotein-encoding gene bba07 is induced by an elevated temperature and a reduced pH during in vitro cultivation, as well as during nymphal tick feeding. Expression of bba07 is regulated by the Rrp2-RpoN-RpoS pathway, a central regulatory network that is activated during nymphal feeding. By generating a bba07 mutant of an infectious strain of B. burgdorferi, we demonstrated that although BBA07-deficient spirochetes were capable of infecting mice via needle inoculation and surviving in ticks, they were defective in infection of mammals via tick transmission. Complementation of the bba07 mutant with a wild-type copy of bba07 partially restored the transmission defect of the bba07 mutant. Based on these findings, we concluded that the surface lipoprotein BBA07 is produced during tick feeding and facilitates optimal transmission of B. burgdorferi from the tick vector to a mammalian host.
Borrelia burgdorferi, a spirochete pathogen that causes Lyme disease, has a complex enzootic life cycle involving ticks (e.g., Ixodes scapularis) and various vertebrate hosts (e.g., Peromyscus leucopus) (6, 44, 47). This spirochete adapts to these two disparate environments by altering its gene expression (39, 43, 46). Many of the genes that are differentially regulated encode known or predicted lipoproteins, some of which, including OspA, OspC, DbpB/A, BBK32, BptA, BB0365, BBA64, and lp6.6, have been shown to be important to the enzootic cycle of B. burgdorferi (1, 11, 18, 26, 31, 32, 34, 36, 41, 42, 56, 57). Thus, elucidating the functions of differentially expressed lipoproteins is important for understanding the infectious cycle of B. burgdorferi.
bba07 is one of the putative lipoprotein genes located on plasmid lp54 of B. burgdorferi. Previous microarray analyses by Revel et al. identified bba07 as a highly differentially expressed gene. When B. burgdorferi was cultivated at an elevated temperature (37°C) and a lower pH (pH 6.8) (surrogate conditions for the midgut of feeding ticks), bba07 expression increased more than 10-fold compared to the expression in spirochetes grown at a lower temperature (23°C) and normal culture pH (pH 7.5) (in vitro conditions mimicking the conditions in unfed ticks) (37). More recently, microarray analyses conducted by us and other workers showed that bba07 is one of the candidate genes under control of the Rrp2-RpoN-RpoS pathway (2, 8, 30). The Rrp2-RpoN-RpoS pathway, also called the σ54-σS sigma factor cascade, is a central pathway that is activated at the onset of nymphal tick feeding and is indispensable for transmission of spirochetes from ticks to mice, as well as for mammalian infection (2, 7, 12, 30). In this pathway, a two-component response regulator and σ54-dependent transcriptional activator, Rrp2, controls transcriptional activation of rpoS from a typical σ54-type −24/−12 promoter. RpoS (σS), a global regulator, then modulates expression of more than 100 genes, including the two major virulence genes, ospA and ospC (2, 7, 12, 15, 19, 30, 55). Global gene expression analyses showed that mutations in rrp2, rpoN, or rpoS resulted in 7- to 138-fold decreases in the number of bba07 transcripts (2, 7, 30).
Given the importance of the Rrp2-RpoN-RpoS pathway in the enzootic cycle of B. burgdorferi, we have focused on elucidating the functions of the Rrp2-RpoN-RpoS pathway-controlled lipoproteins as a strategy to identify new virulence factors in B. burgdorferi. In this study, we showed that bba07 is indeed an Rrp2-RpoN-RpoS-dependent gene and that its expression is induced by an elevated temperature and a reduced culture pH. Furthermore, we found that bba07 expression is induced during nymphal tick feeding and plays a role in the transmission of B. burgdorferi from ticks to mammals.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Low-passage, virulent B. burgdorferi strain B31 clone A3 (B31 A3), which contains all of the plasmids described for parental strain B31 MI except cp9, was kindly provided by P. Rosa, Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, National Institutes of Health (10). Low-passage, virulent B. burgdorferi strain 297 and isogenic rrp2 [rrp2(G239C)] and rpoS mutants mutants have been described previously (19, 56). Borreliae were cultivated in Barbour-Stoenner-Kelly (BSK-H) medium (Sigma, St. Louis, MO) supplemented with 6% normal rabbit serum (Pel Freez Biologicals, Rogers, AR) at 23°C or 37°C with 5% CO2 unless indicated otherwise. Relevant antibiotics were added to the cultures at the following final concentrations: 100 μg/ml streptomycin and 50 ng/ml erythromycin. The suicide vectors that were constructed were maintained in Escherichia coli strain TOP10.
Construction of the bba07 mutant.
To construct a suicide vector for generating the bba07 mutant by homologous recombination, the 1.5-kb upstream region and the 1.5-kb downstream region of bba07 were PCR amplified from strain B31 clone A3 genomic DNA with primer pairs bba7-UF/bba7-UR and bba7-DF/bba7-DR (Table 1), respectively. The resulting DNA fragments were cloned upstream and downstream of an erythromycin resistance cassette, respectively, in the pCR-XL-TOPO cloning vector (Invitrogen). The resulting suicide vector was confirmed by sequencing and was designated pHX07. pHX07 plasmid DNA was then transformed into B. burgdorferi strain B31 clone A3 using a previously described protocol (38, 56). Erythromycin-resistant transformants were analyzed by reverse transcription-PCR (RT-PCR) and immunoblotting to confirm the loss of bba07. Plasmid profiles of the bba07 mutant clones were determined by performing PCR with 21 pairs of primers specific for the endogenous plasmids (10, 23, 28, 35).
TABLE 1.
Primers used in this study
| Primer | Sequence (5′ → 3′) | Use |
|---|---|---|
| bba07-DF | GGATCCATCTGCTTCGTTTTAACTTTT | Amplify bba07 downstream region |
| bba07-DR | GGTACCGATTTACGTGATTTCTCCATT | Amplify bba07 downstream region |
| bba07-UF | GCGGCCGCTGTAATCAAGCTCATTGCAAAT | Amplify bba07 upstream region |
| bba07-UR | CTGCAGTTCATACGTCTCCCACACACAT | Amplify bba07 upstream region |
| Combba07-F | GCGGCCGCAGTAAAAGCTGTAGTCAAGCCCAT | Amplify bba07 complement region |
| Combba07-R | AGATCTAAAAAACAAAAACAAGCACAAACG | Amplify bba07 complement region |
| Qbba07-F | ATGGTGCATCAAATAAAGAACTTA | qRT-PCR primer for bba07 |
| Qbba07-R | GGATTGCTATCAAGAACTACCTGT | qRT-PCR primer for bba07 |
| Bba07-cDNA-R | AGAGCCATTTTAGCCTTTCTTTTG | cDNA synthesis for bba07 |
| QflaB-F | ACCAGCATCACTTTCAGGGTCTCA | qRT-PCR primer for flaB |
| QflaB-R | CAGCAATAGCTTCATCTTGGTTTG | qRT-PCR primer for flaB |
| qFlaB-cDNA-R | GATTCAAGTCTATTTTGGAAAGCACC | cDNA synthesis for flaB |
Complementation of the bba07 mutant.
The bba07 mutant was transcomplemented by inserting a wild-type copy of bba07 into the intergenic region of the bb0445 and bb0446 chromosomal genes, a strategy that was developed previously by Li et al. (25). The pXLF14601 suicide vector, which contains a streptomycin resistance cassette (aadA) inserted in the intergenic region between bb0445 and bb0446 along with ∼3 kb of flanking regions, was kindly provided by X. Li and E. Fikrig (Yale University School of Medicine) (13, 25). A wild-type copy of bba07 along with its putative native promoter region (322 bp) was then cloned upstream of the aadA marker in pXLF14601. The suicide vector constructed was then transformed into the bba07 mutant. Streptomycin- and erythromycin-resistant transformants were selected and subjected to PCR and Western blot analyses to confirm that bba07 expression was restored in these clones.
Mouse infection via needle inoculation.
Groups of 3- to 4-week-old immunocompetent C3H/HeN mice (Harlan, Indianapolis, IN) were subcutaneously inoculated with wild-type B. burgdorferi strain B31 clone A3, the bba07 mutant, or the complemented bba07 mutant strain at a dose of either 1 × 103 or 1 × 105 spirochetes per mouse. Two weeks postinoculation, ear punch biopsy specimens were collected and cultured in BSK-H medium supplemented with an antibiotic mixture specific for Borrelia growth (Sigma, St. Louis, MO). Four weeks postinoculation, mice were sacrificed, and mouse tissues (ear, skin, heart, and joint) were collected and cultured for spirochete growth. A single growth-positive culture was used as the criterion for infection of each mouse. All animal experiments and tick protocols described below were approved by the Institutional Animal Care and Use Committee at Indiana University.
Tick-mouse cycle of B. burgdorferi.
Pathogen-free Ixodes scapularis larvae were purchased from the Tick-Rearing Center at Oklahoma State University, Stillwater, OK. The tick-mouse experiments were conducted in the Vector-borne Diseases Laboratory at Indiana University School of Medicine, Indianapolis, IN. Groups of two C3H/HeN mice were first needle infected with wild-type strain B31 clone A3, bba07 mutant, or complemented bba07 mutant spirochetes (1 × 105 spirochetes per mouse). Two weeks postinoculation, mouse infection was confirmed by cultivation of ear punch biopsy specimens for spirochete growth. Then unfed larvae were placed on the infected mice (150 to 200 larvae/mouse). The ticks were allowed to feed, and fed ticks were collected within 24 h after repletion. Ten fed larvae from each group were subjected to immunofluorescence assay (IFA) analysis. The rest of the fed larvae were maintained in the tick incubator and allowed to molt to the nymphal stage (about 5 weeks). One month after molting, unfed nymphs were then allowed to feed on naïve C3H/HeN mice (12 ticks/mouse). Fully engorged nymphal ticks were collected within 24 h after repletion and subjected to IFA analysis. Two and 4 weeks after tick infestation, mouse tissues were collected and cultivated for spirochete growth as described above.
IFA.
The IFA was performed as described previously (2). Briefly, the entire contents of a fed tick were smeared and fixed on a silylated microscope slide (CEL Associates, Pearland, TX). The slides were incubated with BacTrace fluorescein isothiocyanate-conjugated goat anti-B. burgdorferi antibody (Kirkegaard and Perry Laboratories, Gaithersburg, MD) at 37°C. Samples were observed using an Olympus BX50 fluorescence microscope. Ten ticks from each group were examined by the IFA.
Quantitative RT-PCR (qRT-PCR).
RNA samples were extracted from either B. burgdorferi cultures or ticks using an RNeasy mini kit (Qiagen, Vanelcia, CA) according to the manufacturer's protocols. For RNA analysis of in vitro-cultivated spirochetes, three independent culture samples were used for each strain. For RNA analysis of tick samples, 10 groups of fed larvae (three ticks/group), three groups of unfed nymphs (40 ticks/group), or 10 groups of fed nymphs (one tick/group) were utilized. Mouse tissue RNAs isolated from Borrelia-infected mice were kindly provided by F. T. Liang (Louisiana State University, Baton Rouge, LA) (51). For RNA analysis of spirochetes in mouse tissues, four RNA samples from four mice were used. Contaminating genomic DNA in RNA samples was digested using RNase-free DNase I (Promega, Madison, WI), and the absence of DNA was confirmed by PCR amplification using primers specific for the B. burgdorferi flaB gene. For cDNA synthesis, SuperScript III reverse transcriptase and random primers (Invitrogen, Carlsbad, CA) were used for RNA samples isolated from in vitro cultures. Because of the low levels of B. burgdorferi RNA that were present in tick and mouse samples, cDNA was synthesized using a mixture of primers specific for bba07 (bba7-cDNA-R) and flaB (qFlaB-cDNA-R) (Table 1). Absolute quantitative PCR was performed in triplicate using the ABI 7000 sequence detection system and SYBR Green PCR master mixture (ABI, Pleasanton, CA). Briefly, a cloning vector containing an unrelated DNA sequence was used as the standard template. A series of 10-fold dilutions of the standard template (concentrations, 100 to 107 copies per μl) was prepared. qPCR was performed using 1 μl of the standard template in each reaction mixture. A standard curve was then generated by plotting the number of copies of the standard template against the cycle threshold (CT) values. The numbers of copies of the bba07 and flaB genes were extrapolated from the standard curve based on the CT values obtained for each reaction.
Proteinase K accessibility experiment.
A proteinase K accessibility experiment was performed as previously described (5, 50). Briefly, wild-type B. burgdorferi B31 clone A3 was cultivated at 37°C and harvested at a density of 1 × 108 spirochetes per ml. The sample was divided into two parts; one part was treated with 200 μg proteinase K (PK) (Sigma, St. Louis, MO) for 1 h at room temperature, and the other part was incubated with 1× phosphate-buffered saline (PBS) as a control. Samples were then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting.
SDS-PAGE and immunoblotting.
SDS-PAGE and immunoblotting were performed as previously described (54). Monoclonal antibodies directed against OspC (182-105-D2) and FlaB (8H3-33) have also been described previously (53). A polyclonal rat antibody against BBA07 was kindly provided by J. Blevins and M. Norgard (University of Texas Southwestern Medical Center, Dallas, TX). To determine serological responses of mice infected with various strains of B. burgdorferi (“seroconversion” test) (20), serum was collected from each C3H/HeN mouse at 4 weeks after tick infestation, and each mouse serum was used for immunoblotting against whole-cell lysates of wild-type B. burgdorferi B31 clone A3 to determine seroreactivity.
Statistical analyses.
Data were analyzed by Fisher's exact test (for infectivity) or by an unpaired t test (for qRT-PCR and numbers of spirochetes). The P values for the tests are indicated below.
RESULTS
Characterization of bba07 expression under in vitro cultivation conditions.
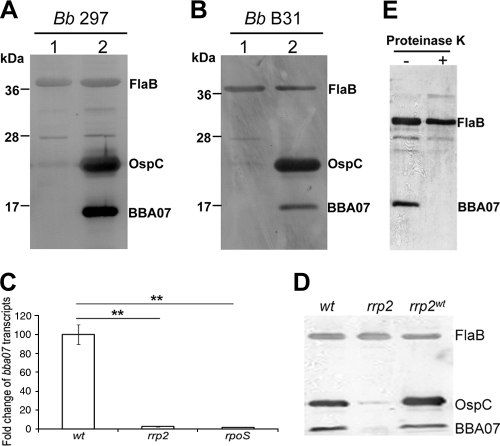
Workers have described several microarray analyses in which more than 100 differentially expressed B. burgdorferi genes were identified by cultivating spirochetes under various temperature or pH conditions, in the presence of host blood, or in dialysis membrane chambers transplanted into peritoneal cavities of rats (4, 29, 37, 48). Remarkably, only one study identified bba07 as a differentially regulated gene (37); Revel et al. showed that bba07 expression is upregulated more than 10-fold by an elevated culture temperature and lower pH. Therefore, we first investigated whether the culture temperature and pH affect BBA07 production. Wild-type B. burgdorferi 297 was cultivated either at 23°C and pH 7.5 or at 37°C and pH 7.0. Cells were harvested at late logarithmic phase (5 × 107 cells per ml) and then subjected to Western blot analysis. As shown in Fig. 1 A, similar to OspC expression, BBA07 expression was induced by an elevated temperature and reduced culture pH, a result that is consistent with previous microarray results described by Revel et al. (37). The same experiment was performed using another B. burgdorferi strain, strain B31 clone A3, and the results were similar to the results obtained for strain 297 (Fig. 1B), suggesting that the effect of temperature and pH on BBA07 production is not strain specific.
FIG. 1.
Regulation of BBA07 expression under in vitro cultivation conditions. (A and B) BBA07 expression is induced by elevated temperature and lower pH. Either B. burgdorferi 297 (Bb 297) (A) or B31 clone A3 (Bb B31) (B) was cultivated in BSK-H medium at 23°C and pH 7.5 (lane 1) or at 37°C and pH 7.0 (lane 2). Spirochetes were harvested at the late logarithmic growth phase, and whole-cell lysates were separated by SDS-PAGE and subjected to Western blot analysis using a mixture of antibodies against FlaB, OspC, and BBA07. The positions of proteins are indicated on the right. (C and D) Control of bba07 expression by the Rrp2-RpoN-RpoS pathway. Wild-type B. burgdorferi 297 (wt), an isogenic rrp2 mutant (rrp2), a complemented rrp2 strain (rrp2wt), and an rpoS mutant (rpoS) were cultivated at 37°C in BSK-H medium (pH 7.5), harvested at the late logarithmic growth phase, and subjected to either qRT-PCR analysis (C) or Western blot analysis using a combination of antibodies against FlaB, OspC, and BBA07 (D). Levels of the bba07 transcripts were normalized with the flaB transcript in each sample, and relative levels of bba07 expression were determined. The data represent the results for three independent culture samples. **, P < 0.01. (E) Surface localization of BBA07. Wild-type B. burgdorferi strain B31 clone A3 was incubated with either PBS alone (lane −) or PBS containing proteinase K (lane +). The treated cells were then subjected to immunoblot analysis using a combination of antibodies against FlaB and BBA07. FlaB was used as a negative control.
The Rrp2-RpoN-RpoS pathway is a central pathway that modulates differential gene expression in B. burgdorferi. Recent microarray analyses by us and other workers indicated that bba07 is one of the genes whose expression is under control of the Rrp2-RpoN-RpoS pathway (2, 8, 30). To confirm this microarray result, wild-type B. burgdorferi 297, an isogenic rrp2(G239C) mutant (55), and an rpoS mutant (19) were cultivated in BSK-H medium to late logarithmic phase at 37°C. RNAs were isolated from these spirochetes and subjected to qRT-PCR analyses. Similar to the findings for ospC expression, a mutation in rrp2 or inactivation of rpoS virtually abolished bba07 expression at both the transcription and protein levels (Fig. 1C and 1D). Thus, BBA07 is an Rrp2-RpoN-RpoS pathway-dependent protein.
Sequence analysis predicted that BBA07 is a putative lipoprotein (14). To determine whether BBA07 is surface exposed, wild-type B. burgdorferi B31 clone A3 was treated with proteinase K and subjected to immunoblot analysis. As shown in Fig. 1E, BBA07 was sensitive to proteinase K treatment, whereas the periplasmic protein FlaB remained intact upon treatment, suggesting that BBA07 is a surface-exposed lipoprotein.
Expression of bba07 is induced during nymphal tick feeding.
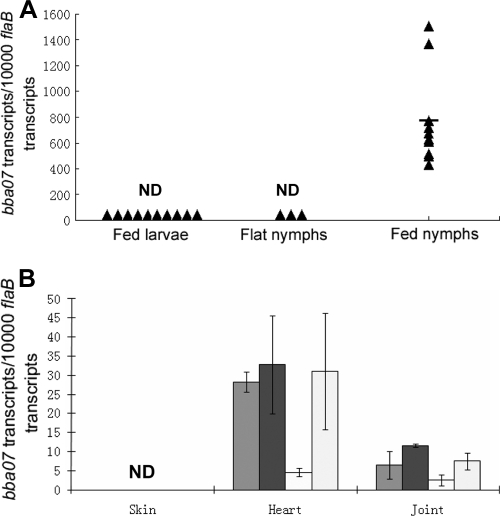
Given that bba07 is differentially expressed, we examined bba07 expression in the enzootic cycle of B. burgdorferi. Pathogen-free I. scapularis larvae were fed on C3H/HeN mice infected with wild-type B. burgdorferi strain B31 clone A3. Infected fed larvae were allowed to molt to nymphs. One month after molting, unfed nymphs were allowed to feed on naïve mice in order to produce fed nymphs. Fed larvae, unfed nymphs, and fed nymphs were collected and subjected to qRT-PCR analysis. As shown in Fig. 2 A, the bba07 transcript was not detected in either fed larvae or unfed nymphs. In contrast, bba07 expression was drastically induced in fed nymphs, the stage when spirochetes are transmitted from ticks to mammals. This observation is consistent with the observation that bba07 expression is controlled by the Rrp2-RpoN-RpoS pathway, the pathway that is activated at the onset of nymphal tick feeding (8).
FIG. 2.
Expression of bba07 in ticks and mice. (A) Relative levels of the bba07 transcript in fed larvae, unfed nymphs, and fed nymphs determined by qRT-PCR analysis and expressed as the number of bba07 transcripts per 10,000 copies of the flaB transcript. The triangles indicate the values for data points. For fed larvae, each data point was generated using three fed larvae (a total of 30 ticks for 10 data points); for flat nymphs, each data point was generated using 40 flat nymphs (a total of 120 ticks for three data points); and for fed nymphs, each data point was generated using one tick (a total of 10 ticks for 10 data points). The bar indicates the mean. (B) Relative levels of the bba07 transcript in joint, heart, and skin tissues harvested from mice that were needle infected with B. burgdorferi. Four mice were examined for each group. Each bar indicates the data for one tissue sample harvested from one mouse. For each RNA sample, three replicates were used for qRT-PCR analysis, and the error bars represent standard deviations for the three reactions. ND, not detected.
To determine the expression profile of bba07 during mammalian infection, qRT-PCR analyses were performed with RNAs extracted from various mouse tissues (skin, heart, and joint) obtained from mice that had been needle inoculated with wild-type spirochetes (4 weeks postinoculation). As shown in Fig. 2B, bba07 transcripts were readily detected in heart and joint tissues but were not detected in skin samples. The undetectable level of bba07 transcripts in the skin samples was not due to low numbers of spirochetes in the samples, as flaB transcripts were readily detected in the same samples. These results suggest that the bba07 gene is expressed in spirochetes while they are replicating in the mammalian host, at least in some tissues.
Construction of the bba07 mutant and complemented strains.
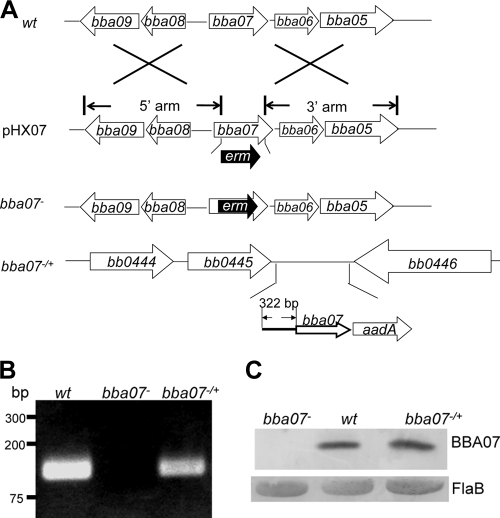
To investigate the role of BBA07 in the enzootic cycle of B. burgdorferi, we constructed a bba07-deficient mutant by the allele exchange method, as shown in Fig. 3 A. Briefly, suicide vector pHX07 carrying a disrupted bba07 gene along with intact surrounding regions was transformed into strain B31 clone A3. The lack of bba07 expression in the transformant clones was confirmed at both the RNA and protein levels (Fig. 3B and 3C). Disruption of bba07 did not appear to affect the cell morphology or growth kinetics of spirochetes in BSK-H medium (data not shown). Further plasmid profile analyses using PCR identified one bba07 mutant clone that contained all of the endogenous plasmids present in the parental wild-type strain; this clone was selected for complementation. However, as described below, this clone lost lp28-4 during storage.
FIG. 3.
Construction of the bba07 mutant and the complement strain. (A) Strategy used to construct the bba07 mutant and the complemented strain. wt, genomic structure of bba07 and the surrounding regions in wild-type B. burgdorferi B31 clone A3. Plasmid pHX07 was the suicide construct used for generating the bba07 mutant. Only the relevant portion of the plasmid is shown. The 5′ arm and the 3′ arm are the two PCR fragments used for cloning upstream and downstream of the erythromycin resistance (erm) gene. bba07−, genomic structure of the bba07 mutant; bba07−/+, bb0445-bb0446 region in the complemented bba07 mutant strain, where a wild-type copy of bba07 along its 322-bp upstream putative promoter region and streptomycin resistance gene (aadA) were inserted into the intergenic region between bb0445 and bb0446. (B and C) RT-PCR analysis (B) and Western blot analysis (C) of the wild type (wt), the bba07 mutant (bba07−), and the complemented strain (bba07−/+). Spirochetes were cultured at 35°C and harvested at the late logarithmic growth phase. RT-PCRs were performed using the primers specific for bba07, and Western blot analysis was performed using rat polyclonal antibody against BBA07. FlaB was used as a loading control.
To complement the bba07 mutant, we employed a strategy previously described by Li et al. (25), in which a wild-type copy of bba07 (driven by its native promoter) was inserted into the intergenic region between chromosomal genes bb0445 and bb0446 (Fig. 3A). Restoration of bba07 expression in the complemented clones was confirmed by RT-PCR and immunoblot analysis (Fig. 3B and 3C). Plasmid profile analysis of three randomly picked complemented clones showed that none of them contained lp28-4. Subsequent analysis revealed that this was due to loss of lp28-4 in the bba07 mutant clone used for complementation during storage. lp28-4 is one of the 21 endogenous plasmids that are present in the B. burgdorferi genome. Previous studies using clones in which there had been spontaneous loss of lp28-4 showed that this plasmid is not essential for infection of mice by B. burgdorferi via needle inoculation, but it partially contributes to infection of mammals since loss of lp28-4 leads to an increase in the 50% infective dose (ID50) (23, 35). The role of lp28-4 in the tick-mouse cycle has not been fully examined.
Multiple efforts to obtain a bba07 mutant or a complemented strain that contains all of the endogenous plasmids have not been successful. Thus, we chose a bba07 mutant clone and a complemented strain that both lack lp28-4 for further study. Our rationale was that comparison of the bba07 mutant with its complemented strain should allow us to assess the impact of inactivation of bba07 on spirochete behavior. In addition, we believed that a comparison of the complemented strain lacking lp28-4 and the wild-type strain should provide insight into the role of lp28-4 in the enzootic cycle of B. burgdorferi.
The bba07 mutant is capable of infecting mice and causing a persistent infection when needle inoculation is used.
We first examined the bba07 mutant's ability to establish an infection in mice when needle inoculation was used. Groups of five immunocompetent C3H/HeN mice were inoculated with a high dose (1 × 105 spirochetes per mouse) or a low dose (1 × 103 spirochetes per mouse) of the wild-type, bba07 mutant, or complemented strain. Four weeks postinoculation, mice were sacrificed, and various mouse tissues (skin, heart, and joint) were collected and cultured for spirochete growth. When the high dose was used, all of the mice inoculated with wild-type, bba07 mutant, or complemented spirochetes were infected (Table 2), suggesting that BBA07 is not necessary for B. burgdorferi to establish an infection in mice when needle inoculation is used. When the low dose was used, both the bba07 mutant and the complemented strain showed reduced infectivity, which is consistent with the previous observation that lp28-4 contributes to infection of mammals (23, 35). Since the infectivities of the bba07 mutant and the complemented strain were virtually identical (P = 1), this result suggests that inactivation of bba07 did not affect mouse infection via needle inoculation.
TABLE 2.
Infectivity of the bba07 mutant for mice via needle inoculation
| Time (days postinoculation) | Strain | Inoculum (spirochetes/ml) | No. of cultures positive/total no. of cultures |
No. of mice positive/total no. of mice | ||||
|---|---|---|---|---|---|---|---|---|
| Eara | Skin | Joint | Heart | All sites | ||||
| 28 | B31 clone A3 | 105 | 5/5 | 5/5 | 5/5 | 5/5 | 15/15 | 5/5 |
| 103 | 9/10 | 9/10 | 9/10 | 9/10 | 27/30 | 9/10 | ||
| bba07 mutant | 105 | 5/5 | 5/5 | 5/5 | 5/5 | 15/15 | 5/5 | |
| 103 | 3/10 | 3/10 | 3/10 | 3/10 | 9/30 | 3/10 | ||
| Complemented bba07 mutant | 105 | 5/5 | 5/5 | 5/5 | 5/5 | 15/15 | 5/5 | |
| 103 | 2/5 | 2/5 | 2/5 | 2/5 | 6/15 | 2/5 | ||
| 180 | B31 clone A3 | 2/2 | 2/2 | 2/2 | 2/2 | 6/6 | 2/2 | |
| bba07 mutant | 2/2 | 2/2 | 2/2 | 2/2 | 6/6 | 2/2 | ||
| Complemented bba07 mutant | 2/2 | 2/2 | 2/2 | 2/2 | 6/6 | 2/2 | ||
Ear punch biopsy specimens obtained 14 days postinoculation were also examined, and the results were identical to those for specimens obtained 28 days postinoculation.
To further examine the bba07 mutant's ability to cause a persistent infection in mice, groups of two infected mice were kept for 180 days and then examined to determine if they were infected; all mouse tissues were still culture positive for spirochete growth. This result suggests that neither BBA07 nor lp28-4 is required for maintaining a persistent infection in mice.
The bba07 mutant can colonize ticks, but it does not infect mice via tick infestation.
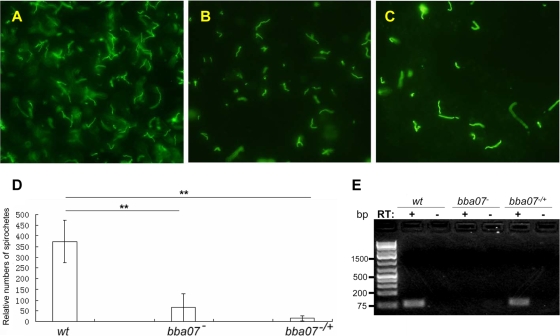
To examine whether BBA07 plays a role in the tick-mouse cycle of B. burgdorferi, larval ticks were allowed to feed on mice carrying wild-type, bba07 mutant, or complemented strain spirochetes. IFA analyses of fed larvae showed that spirochetes were present in all tick midguts examined, and there was no significant difference in the numbers of spirochetes in each group of ticks (data not shown). To determine the ability of spirochetes to survive during tick development (ecdysis), fed larvae were kept in a tick incubator and allowed to molt to nymphs (4 to 5 weeks). Flat (unfed) nymphs were then fed on groups of naïve C3H/HeN mice. Twenty-four hours after repletion, fed nymphs were collected and subjected to IFA analysis. Spirochetes were detected in all fed nymphs examined (Fig. 4 A, B, and C). Thus, neither BBA07 nor lp28-4 is essential for spirochete colonization of ticks and survival during tick ecdysis. However, significantly reduced numbers of spirochetes were observed in ticks harboring either the bba07 mutant or the complemented strain, but there was no significant difference in the number of spirochetes between the bba07 mutant and the complemented strain (Fig. 4D). The absence or presence of bba07 expression in the bba07 mutant- or complemented strain-infected nymphs was confirmed by RT-PCR analysis (Fig. 4E). This result suggests that lp28-4, but not bba07, is important for optimal survival of spirochetes in ticks.
FIG. 4.
IFA and RT-PCR analyses of spirochetes in fed nymphs. Unfed I. scapularis nymphs harboring either the wild type (wt) (A), the bba07 mutant (bba07−) (B), or the complemented bba07 mutant strain (bba07−/+) (C) were fed on naive C3H/HeN mice, and engorged nymphs were collected within 24 h and subjected to IFA analysis using fluorescein isothiocyanate-labeled anti-B. burgdorferi antibody. Seven ticks were examined for each group, and a representative image for each group of ticks is shown. (D) Relative number of spirochetes in each group of ticks. Spirochetes were counted with a fluorescence microscope. The error bars represent standard deviations for seven randomly picked fed nymphs. **, P < 0.01. (E) Confirmation of bba07 expression in fed nymphs. RNAs were extracted from six fed nymphs harboring wild-type, bba07 mutant, or complemented bba07 mutant strain spirochetes and subjected to RT-PCR in the presence (lanes +) or absence (lanes −) of reverse transcriptase (RT).
To further examine the bba07 mutant's ability to infect mice via tick infestation, tissue samples from mice that were bitten by ticks in the experiment described above were collected and cultured to determine the presence of spirochetes. All mice infested with ticks carrying wild-type spirochetes were infected (Table 3). In contrast, none of the 18 mice infested with ticks harboring the bba07 mutant were infected. Complementation of the bba07 mutant with the wild-type bba07 gene partially restored the noninfectious phenotype of the mutant when tick infestation was used; 3 of 10 mice in this group were culture positive for spirochete growth and positive for seroconversion (Fig. 5). The partial restoration of infectivity in the complemented strain was due to loss of lp28-4. This result suggests that inactivation of bba07 did not affect infection of mammals by needle inoculation, but it impaired the ability of spirochetes to infect mice via tick transmission. As the bba07 mutant does not have a defect in colonization of ticks, BBA07 likely plays a role in transmission of spirochetes from ticks to mammals.
TABLE 3.
Infectivity of the bba07 mutant for mice via tick transmission
| Strain | No. of cultures positive/total no. of cultures |
No. of mice positive/total no. of mice | ||||
|---|---|---|---|---|---|---|
| Ear | Skin | Joint | Heart | All sites | ||
| B31 clone A3 | 5/5 | 5/5 | 5/5 | 5/5 | 15/15 | 5/5 |
| bba07 mutant | 0/18 | 0/18 | 0/18 | 0/18 | 0/54 | 0/18a |
| Complemented bba07 mutant | 3/10 | 3/10 | 3/10 | 3/10 | 9/30 | 3/10b |
The P value for a comparison of the bba07 mutant and the complemented strain is <0.05, as calculated using Fisher's two-tailed exact test.
The P value for a comparison of strain B31 clone A3 and the complemented strain is <0.05, as calculated using Fisher's two-tailed exact test.
FIG. 5.
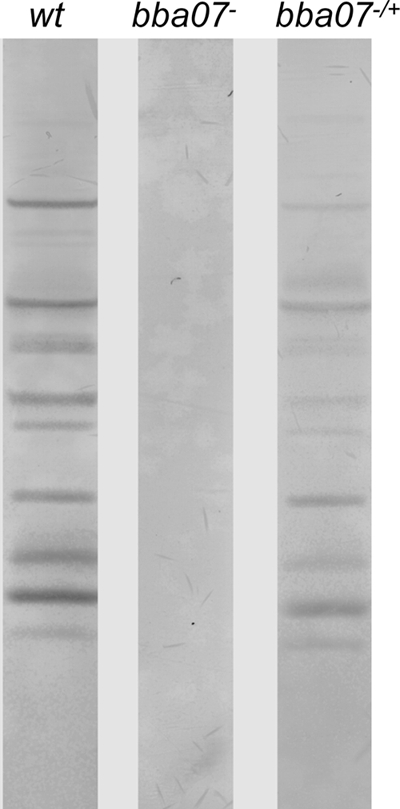
Serological responses of mice infected with various strains of B. burgdorferi. Serum was collected from each C3H/HeN mouse 4 weeks after infestation with ticks harboring wild-type spirochetes (lane wt), bba07 mutant spirochetes (lane bba07−), or complemented spirochetes (lane bba07−/+). Positive seroconversion for each mouse was determined by immunoblotting with the serum from each mouse (1:300 dilution) against whole-cell lysates of wild-type B. burgdorferi B31 clone A3. A representative immunoblot for each group is shown. For the group of mice infected with the complemented strain, an immunoblot for only one of the three infected mice is shown.
DISCUSSION
The Rrp2-RpoN-RpoS pathway is a central regulatory pathway that modulates differential gene expression in B. burgdorferi. Since it is indispensable for spirochete transmission and infection of mammals (2, 7, 8, 12, 30), this pathway must control expression of borrelial factors important for the enzootic cycle of B. burgdorferi. To date, only a few RpoS-dependent virulence factors have been identified, including OspC, DbpB/A, and BBK32 (1, 18, 32, 41, 42, 49). In this study, we showed that BBA07 is an Rrp2-RpoN-RpoS pathway-controlled surface lipoprotein that apparently plays a role in transmission of spirochetes from ticks to mammals.
The microarray analysis described by Revel et al. showed that bba07 is 1 of 79 B. burgdorferi genes whose expression is influenced by culture temperature and pH (37). The results of this study confirmed the finding of Revel et al. for bba07. We further demonstrated that expression of bba07 is under control of the Rrp2-RpoN-RpoS pathway. The pattern of bba07 expression in vitro correlated well with the subsequent in vivo observation that the bba07 transcripts were upregulated exclusively during nymphal feeding, the period when RpoS is induced (8, 40). In addition, bba07 transcripts were also expressed in mouse heart and joint tissues during infection of mammals. Interestingly, bba07 transcripts were not detectable in mouse skin samples, suggesting that there is a possible tissue tropism for bba07 expression.
B. burgdorferi has a segmented genome that is composed of a small linear chromosome that is approximately 900 kb long and at least 21 different linear and circular plasmids (9, 14). This unusual genome structure presents a major challenge for studying virulence determinants in B. burgdorferi. Some plasmids are prone to loss during in vitro manipulation (11, 23, 24, 35, 52). Three of these plasmids, lp25, lp28-1, and lp36, have been shown to be critical for B. burgdorferi to establish infection and cause disease in mice (16, 17, 20, 21, 23, 35, 52). In this study, the bba07 mutant and the complemented strain contained all of the important plasmids listed above, but they did not contain lp28-4. Unlike lp25, lp28-1, or lp36, lp28-4 is not essential for infection of mammals via needle inoculation of a medium or high dose of spirochetes (>1 × 104 bacteria per mouse) (3, 21, 23, 35, 52). However, lp28-4 partially contributes to infection of mammals since the spirochetes that lack lp28-4 have reduced infectivity when a small inoculum (≤1 × 103 bacteria per mouse) is used (3, 23). Our finding that the complemented bba07 mutant strain (lacking lp28-4) exhibited reduced infectivity for mammals in this study is consistent with this conclusion. There are conflicting reports about the contribution of lp28-4 in the tick vector. Strother et al. reported that loss of lp28-4 dramatically reduced the percentage of ticks infected by spirochetes when ticks were fed using the artificial capillary feeding method (45). However, in another study Elias et al. showed that when ticks were fed on infected mice (the natural route of acquisition), a B31 clone lacking lp28-4 infected ticks normally (10). In this study, we found that spirochetes lacking lp28-4 (both the bba07 mutant and the complemented strain) infected all of the ticks examined. This suggests that loss of lp28-4 does not affect the rate of infection of ticks by spirochetes. However, there was a reduced spirochete load in the ticks infected with spirochetes without lp28-4, suggesting that lp28-4 contributes to optimal survival of B. burgdorferi in the tick vector.
Despite the absence of lp28-4 in the bba07 mutant and the complemented strain, our studies provide important insights into the contribution of BBA07 to the enzootic cycle of B. burgdorferi. BBA07 does not appear to play a role in infection of mammals, since the infectivity of the bba07 mutant was similar to that of the isogenic complemented strain at either high or low doses (Table 2). In the tick vector, the bba07 mutant and the complemented strain colonize and survive similarly. However, the outcomes for infection of mice via tick infestation were significantly different for the bba07 mutant and the isogenic complemented strain. The bba07 mutant was not able to infect mice via tick infestation, while the complemented strain was partially infectious. Since both strains lacked lp28-4, the absence of lp28-4 could not fully account for the noninfectious phenotype observed for the bba07 mutant. Thus, abrogation of bba07 expression in the bba07 mutant must contribute to this phenotype. The phenotype of the bba07 mutant is similar to that of a recently reported bba52 mutant; while the bba52 mutant infects mice normally when needle inoculation is used, it is not infectious via tick bites because its transmission from the tick midgut to salivary glands is impaired (22). Since the bba07 mutant is capable of colonizing ticks and establishing an infection in mice via needle inoculation, its inability to infect mice via tick transmission suggests that BBA07 likely plays a role in the transmission from the tick midgut to the mammalian host. Future work to pinpoint the bba07 mutant's defect in transmission, such as a defect in its ability to interact with tick gut cells, migrate to the hemolymph, survive in the hemolymph, or enter the salivary glands, is warranted.
BBA07 is one of the few plasmid-encoded lipoproteins that do not belong to a paralogous family in B. burgdorferi (9, 14). BLAST analyses revealed that the BBA07 is highly conserved among Lyme disease Borrelia species (B. burgdorferi sensu stricto, B. garinii, and B. afzelii) (data not shown). However, sequence analysis did not provide any information about the putative function BBA07 since it does not share significant homology with any other protein. Curiously, BBA07 was annotated as ChpAI in the TIGR B. burgdorferi genome database (14). However, this assignment was based solely on the low level of homology (e value, >1) of the N-terminal portion of BBA07 to E. coli ChpAI (MazE, ChpR), an antitoxin. Along with the toxin ChpAK, ChpAI and ChpAK constitute a ChpAIK (MazEF) toxin-antitoxin system (27, 33). ChpAK kills cells by inhibiting translation and replication under nutritional stress conditions (bacterial programmed cell death). ChpAI counteracts ChpAK-mediated inhibition of translation and replication. However, several lines of evidence argue against annotation of BBA07 as a ChpAI homologue. First, the level of homology between BBA07 and ChpAI is low. Second, a BLAST search did not identify putative ChpAK genes in the B. burgdorferi genome. Third, unlike the cytosolic location of ChpAI, BBA07 is localized on the cell surface (Fig. 1E). Given that BBA07 plays a role in the enzootic cycle of B. burgdorferi, further study to elucidate the physiological function of this surface lipoprotein is warranted.
Acknowledgments
We thank Margaret Bauer, Bryan Troxell, and Tara Oman for comments on the manuscript. We also thank Michael Norgard and Jon Blevins for providing anti-BBA07 serum.
Funding for this work was provided in part by NIH grants R03 AR054942 and R01 AI083640, by an American Heart Association scientist development grant, and by Indiana INGEN and METACyt grants from Indiana University, funded by the Lilly Endowment, Inc. (to X.F.Y.). Part of this investigation was conducted in a facility constructed with support from research facilities improvement program grant C06 RR015481-01 from the National Center for Research Resources, NIH.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 26 April 2010.
REFERENCES
- 1.Blevins, J., K. E. Hagman, and M. V. Norgard. 2008. Assessment of decorin-binding protein A to the infectivity of Borrelia burgdorferi in the murine models of needle and tick infection. BMC Microbiol. 8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boardman, B. K., M. He, Z. Ouyang, H. Xu, X. Pang, and X. F. Yang. 2008. Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi. Infect. Immun. 76:3844-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Botkin, D. J., A. Abbott, P. E. Stewart, P. A. Rosa, H. Kawabata, H. Watanabe, and S. J. Norris. 2006. Identification of potential virulence determinants by Himar1 transposition of infectious Borrelia burgdorferi. Infect. Immun. 74:6690-6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks, C. S., P. S. Hefty, S. E. Jolliff, and D. R. Akins. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 71:3371-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks, C. S., S. R. Vuppala, A. M. Jett, and D. R. Akins. 2006. Identification of Borrelia burgdorferi outer surface proteins. Infect. Immun. 74:296-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease, a tick-borne spirochetosis? Science 216:1317-1319. [DOI] [PubMed] [Google Scholar]
- 7.Caimano, M. J., C. H. Eggers, K. R. O. Hazlett, and J. D. Radolf. 2004. RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect. Immun. 72:6433-6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caimano, M. J., R. Iyer, C. H. Eggers, C. Gonzalez, E. A. Morton, M. A. Gilbert, I. Schwartz, and J. D. Radolf. 2007. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol. Microbiol. 65:1193-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 10.Elias, A. F., P. E. Stewart, D. Grimm, M. J. Caimano, C. H. Eggers, K. Tilly, J. L. Bono, D. R. Akins, J. D. Radolf, T. G. Schwan, and P. Rosa. 2002. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect. Immun. 70:2139-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer, J. R., K. T. LeBlanc, and J. M. Leong. 2006. Fibronectin binding protein BBK32 of the Lyme disease spirochete promotes bacterial attachment to glycosaminoglycans. Infect. Immun. 74:435-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher, M. A., D. Grimm, A. K. Henion, A. F. Elias, P. E. Stewart, P. A. Rosa, and F. C. Gherardini. 2005. Borrelia burgdorferi σ54 is required for mammalian infection and vector transmission but not for tick colonization. Proc. Natl. Acad. Sci. U. S. A. 102:5162-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank, K. L., S. F. Bundle, M. E. Kresge, C. H. Eggers, and D. S. Samuels. 2003. aadA confers streptomycin resistance in Borrelia burgdorferi. J. Bacteriol. 185:6723-6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J.-F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert, M. A., E. A. Morton, S. F. Bundle, and D. S. Samuels. 2007. Artificial regulation of ospC expression in Borrelia burgdorferi. Mol. Microbiol. 63:1259-1273. [DOI] [PubMed] [Google Scholar]
- 16.Grimm, D., K. Tilly, D. M. Bueschel, M. A. Fisher, P. F. Policastro, F. C. Gherardini, T. G. Schwan, and P. A. Rosa. 2005. Defining plasmids required by Borrelia burgdorferi for colonization of tick vector Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol. 42:676-684. [DOI] [PubMed] [Google Scholar]
- 17.Grimm, D., C. H. Eggers, M. J. Caimano, K. Tilly, P. E. Stewart, A. F. Elias, J. D. Radolf, and P. A. Rosa. 2004. Experimental assessment of the roles of linear plasmids lp25 and lp28-1 of Borrelia burgdorferi throughout the infectious cycle. Infect. Immun. 72:5938-5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimm, D., K. Tilly, R. Byram, P. E. Stewart, J. G. Krum, D. M. Bueschel, T. G. Schwan, P. F. Policastro, A. F. Elias, and P. A. Rosa. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. U. S. A. 101:3142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hübner, A., X. Yang, D. M. Nolen, T. G. Popova, F. C. Cabello, and M. V. Norgard. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. U. S. A. 98:12724-12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jewett, M. W., K. Lawrence, A. C. Bestor, K. Tilly, D. Grimm, P. Shaw, F. Gherardini, and P. A. Rosa. 2007. The critical role of the linear plasmid lp36 in the infectious cycle of Borrelia burgdorferi. Mol. Microbiol. 64:1358-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawabata, H., S. J. Norris, and H. Watanabe. 2004. BBE02 disruption mutants of Borrelia burgdorferi B31 have a highly transformable, infectious phenotype. Infect. Immun. 72:7147-7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar, M., X. Yang, A. Coleman, and U. Pal. 2010. BBA52 facilitates Borrelia burgdorferi transmission from feeding ticks to murine hosts. J. Infect. Dis. 201:1084-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labandeira-Rey, M., and J. T. Skare. 2001. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect. Immun. 69:446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labandeira-Rey, M., J. Seshu, and J. T. Skare. 2003. The absence of linear plasmid 25 or 28-1 of Borrelia burgdorferi dramatically alters the kinetics of experimental infection via distinct mechanisms. Infect. Immun. 71:4608-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, X., U. Pal, N. Ramamoorthi, X. Liu, D. C. Desrosiers, C. H. Eggers, J. F. Anderson, J. D. Radolf, and E. Fikrig. 2007. The Lyme disease agent Borrelia burgdorferi requires BB0690, a Dps homologue, to persist within ticks. Mol. Microbiol. 63:694-710. [DOI] [PubMed] [Google Scholar]
- 26.Maruskova, M., M. D. Esteve-Gassent, V. L. Sexton, and J. Seshu. 2008. Role of the BBA64 locus of Borrelia burgdorferi in early stages of infectivity in a murine model of Lyme disease. Infect. Immun. 76:391-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masuda, Y., K. Miyakawa, Y. Nishimura, and E. Ohtsubo. 1993. chpA and chpB, Escherichia coli chromosomal homologs of the pen locus responsible for stable maintenance of plasmid R100. J. Bacteriol. 175:6850-6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDowell, J. V., S. Y. Sung, M. Labandeira-Rey, J. T. Skare, and R. T. Marconi. 2001. Analysis of mechanisms associated with loss of infectivity of clonal populations of Borrelia burgdorferi B31MI. Infect. Immun. 69:3670-3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ojaimi, C., C. Brooks, S. Casjens, P. Rosa, A. Elias, A. Barbour, A. Jasinskas, J. Benach, L. Katona, J. Radolf, M. Caimano, J. Skare, K. Swingle, D. Akins, and I. Schwartz. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 71:1689-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouyang, Z., J. S. Blevins, and M. V. Norgard. 2008. Transcriptional interplay among the regulators Rrp2, RpoN, and RpoS in Borrelia burgdorferi. Microbiology 154:2641-2658. [DOI] [PubMed] [Google Scholar]
- 31.Pal, U., J. Dai, X. Li, G. Neelakanta, P. Luo, M. Kumar, P. Wang, X. Yang, J. Anderson, and E. Fikrig. 2008. A differential role for BB0365 in the persistence of Borrelia burgdorferi in mice and ticks. J. Infect. Dis. 197:148-155. [DOI] [PubMed] [Google Scholar]
- 32.Pal, U., X. Yang, M. Chen, L. K. Bockenstedt, J. F. Anderson, R. A. Flavell, M. V. Norgard, and E. Fikrig. 2004. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Invest. 113:220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pedersen, K., S. K. Christensen, and K. Gerdes. 2002. Rapid induction and reversal of a bacteriostatic condition by controlled expression of toxins and antitoxins. Mol. Microbiol. 45:501-510. [DOI] [PubMed] [Google Scholar]
- 34.Promnares, K., M. Kumar, D. Y. Shroder, X. Zhang, J. F. Anderson, and U. Pal. 2009. Borrelia burgdorferi small lipoprotein Lp6.6 is a member of multiple protein complexes in the outer membrane and facilitates pathogen transmission from ticks to mice. Mol. Microbiol. 74:112-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purser, J. E., and S. J. Norris. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. U. S. A. 97:13865-13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Revel, A. T., J. S. Blevins, C. Almazan, L. Neil, K. M. Kocan, J. de la Fuente, K. E. Hagman, and M. V. Norgard. 2005. bptA (bbe16) is essential for the persistence of the Lyme disease spirochete, Borrelia burgdorferi, in its natural tick vector. Proc. Natl. Acad. Sci. U. S. A. 102:6972-6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Revel, A. T., A. M. Talaat, and M. V. Norgard. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. U. S. A. 99:1562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samuels, D. S. 1995. Electrotransformation of the spirochete Borrelia burgdorferi, p. 253-259. In J. A. Nickoloff (ed.), Methods in molecular biology. Humana Press, Totowa, NJ. [DOI] [PMC free article] [PubMed]
- 39.Schwan, T. G. 2003. Temporal regulation of outer surface proteins of the Lyme-disease spirochaete Borrelia burgdorferi. Biochem. Soc. Trans. 31:108-112. [DOI] [PubMed] [Google Scholar]
- 40.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. U. S. A. 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seshu, J., M. D. Esteve-Gassent, M. Labandeira-Rey, J. H. Kim, J. P. Trzeciakowski, M. Hook, and J. T. Skare. 2006. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol. Microbiol. 59:1591-1601. [DOI] [PubMed] [Google Scholar]
- 42.Shi, Y., Q. Xu, K. McShan, and F. T. Liang. 2008. Both decorin-binding proteins A and B are critical for the overall virulence of Borrelia burgdorferi. Infect. Immun. 76:1239-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh, S. K., and H. J. Girschick. 2004. Molecular survival strategies of the Lyme disease spirochete Borrelia burgdorferi. Lancet Infect. Dis. 4:575-583. [DOI] [PubMed] [Google Scholar]
- 44.Steere, A. C., J. Coburn, and L. Glickstein. 2004. The emergence of Lyme disease. J. Clin. Invest. 113:1093-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strother, K. O., A. Broadwater, and A. de Silva. 2005. Plasmid requirements for infection of ticks by Borrelia burgdorferi. Vector Borne Zoonotic Dis. 5:237-245. [DOI] [PubMed] [Google Scholar]
- 46.Templeton, T. J. 2004. Borrelia outer membrane surface proteins and transmission through the tick. J. Exp. Med. 199:603-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tilly, K., P. Rosa, and P. Stewart. 2008. Biology of infection with Borrelia burgdorferi. Infect. Dis. Clin. North Am. 22:217-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tokarz, R., J. M. Anderton, L. I. Katona, and J. L. Benach. 2004. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole-genome DNA array. Infect. Immun. 72:5419-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weening, E. H., N. Parveen, J. P. Trzeciakowski, J. M. Leong, M. Hook, and J. T. Skare. 2008. Borrelia burgdorferi lacking DbpBA exhibits an early survival defect during experimental infection. Infect. Immun. 76:5694-5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu, H., M. He, X. Pang, Z. C. Xu, J. Piesman, and X. F. Yang. 2009. Characterization of the highly regulated antigen BBA05 in the enzootic cycle of Borrelia burgdorferi. Infect. Immun. 15:1872-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu, Q., K. McShan, and F. T. Liang. 2008. Verification and dissection of the ospC operator by using flaB promoter as a reporter in Borrelia burgdorferi. Microb. Pathog. 45:70-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu, Q., S. V. Seemanapalli, L. Lomax, K. McShan, X. Li, E. Fikrig, and F. T. Liang. 2005. Association of linear plasmid 28-1 with an arthritic phenotype of Borrelia burgdorferi. Infect. Immun. 73:7208-7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang, X., M. S. Goldberg, T. G. Popova, G. B. Schoeler, S. K. Wikel, K. E. Hagman, and M. V. Norgard. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol. Microbiol. 37:1470-1479. [DOI] [PubMed] [Google Scholar]
- 54.Yang, X., T. G. Popova, K. E. Hagman, S. K. Wikel, G. B. Schoeler, M. J. Caimano, J. D. Radolf, and M. V. Norgard. 1999. Identification, characterization, and expression of three new members of the Borrelia burgdorferi Mlp (2.9) lipoprotein gene family. Infect. Immun. 67:6008-6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang, X. F., S. M. Alani, and M. V. Norgard. 2003. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc. Natl. Acad. Sci. U. S. A. 100:11001-11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang, X. F., U. Pal, S. M. Alani, E. Fikrig, and M. V. Norgard. 2004. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 199:641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang, X., A. S. Coleman, J. Anguita, and U. Pal. 2009. A chromosomally encoded virulence factor protects the Lyme disease pathogen against host-adaptive immunity. PLoS Pathog. 5:e1000326. [DOI] [PMC free article] [PubMed] [Google Scholar]