Abstract
Polyamines are biogenic polycationic molecules involved in key cellular functions. Extracellular polyamines found in bodily fluids or laboratory media can be imported by bacteria or bind to negatively charged bacterial surface structures, where they can impair binding of antimicrobials. We hypothesized that the presence of polyamines in fluids that bathe urogenital mucosal surfaces could alter the susceptibility of the sexually transmitted strict human pathogen Neisseria gonorrhoeae to mediators of the innate host defense. Herein we report that polyamines can significantly increase gonococcal resistance to two structurally diverse cationic antimicrobial peptides (polymyxin B and LL-37) but not to antibiotics that exert activity in the cytosol or periplasm (e.g., ciprofloxacin, spectinomycin, or penicillin). The capacity of polyamines to increase gonococcal resistance to cationic antimicrobial peptides was dose dependent, correlated with the degree of cationicity, independent of a polyamine transport system involving the polyamine permeases PotH and PotI, and was reversible. In addition, we found that polyamines increase gonococcal resistance to complement-mediated killing by normal human serum. We propose that polyamines in genital mucosal fluids may enhance gonococcal survival during infection by reducing bacterial susceptibility to host-derived antimicrobials that function in innate host defense.
Neisseria gonorrhoeae is a strict human pathogen that causes the sexually transmitted disease gonorrhea. Worldwide, gonorrhea remains a major public health problem, with more than 95 million new cases per year (G. Schmid, presented at the 18th International Society for Sexually Transmitted Disease Research [ISSTDR] Conference, London, United Kingdom, 2009). Unfortunately, the high prevalence of strains expressing clinically significant levels of resistance to inexpensive antibiotics has made treatment more expensive and reduced therapeutic options.
Gonococci and other bacterial pathogens have developed a number of strategies to resist both classical antibiotics and mediators of innate host defense, and some of these resistance mechanisms can provide cross-resistance to both classes of antimicrobials. For example, certain multidrug efflux pumps can recognize and export both clinically important antibiotics (e.g., beta-lactams, macrolides, and quinolones) (3) and host-derived antimicrobials that bathe mucosal surfaces (e.g., cationic antimicrobial peptides [CAMPs], long-chain fatty acids, bile salts, and progesterone) (14, 30). Additionally, decoration of lipid A by 4-aminoarabinose or phosphoethanolamine (PEA) can enhance the resistance of Gram-negative bacteria to polymyxin B (PMB) and host-derived CAMPs (15). In gonococci, there is evidence that a mechanism of antimicrobial resistance (e.g., antimicrobial export by the MtrC-MtrD-MtrE efflux pump) can increase bacterial survival and fitness in vivo (8, 39).
To understand how gonococci survive during infection, it is important to know if the activity of mediators of the innate host defense is impacted by the presence of other host compounds that are also present in mucosal fluids. In this respect, we asked if polyamines influence the antimicrobial capacity of the human cathelicidin LL-37 (29, 31) or complement-mediated killing by normal human serum (NHS), because certain polyamines, LL-37, and complement proteins can coexist during gonococcal infection (1, 5). Polyamines are cationic compounds synthesized by all living organisms and are involved in key cellular functions, such as growth (e.g., putrescine), DNA and RNA binding (e.g., spermidine and spermine), and resistance to oxidative stress (e.g., cadaverine) (for a review, see reference 33). Additionally, polyamines can modify the response of microbes to antibiotics, depending on their chemical characteristics, the bacterial target, or the antimicrobial agent under investigation (7, 11, 21); however, specific molecular mechanisms to explain how polyamines function in this capacity remain to be determined. Since previous studies with Escherichia coli and Pseudomonas aeruginosa showed that polyamines can bind to bacterial surfaces and influence the antimicrobial action of different antibiotics (13, 34), we hypothesized that similar relationships may hold for N. gonorrhoeae and host-derived antimicrobials. We now report that incubation of gonococci with sublethal levels of polyamines (e.g., spermine) reduces their susceptibility to the killing action of two structurally diverse CAMPs (PMB and LL-37) and to complement-mediated killing by NHS.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and chemicals.
The main bacterial strains used in this study are listed in Table 1. N. gonorrhoeae strains FA19 (17) and F62 (2) were the primary gonococcal strains employed and were routinely grown on gonococcal medium base (GCB) agar (Difco Laboratories, Detroit, MI) containing supplements I and II at 37°C under 4.6% (vol/vol) CO2 as described previously (28); additional strains of gonococci employed in certain experiments are described in Results and Discussion. N. gonorrhoeae strains were also grown in GCB broth containing supplements I and II and 0.043% (wt/vol) sodium bicarbonate (28). All antimicrobial assays (see below) employed nonpiliated, opacity-negative colony variants. E. coli strains DH5α and MC4100 were grown in Luria-Bertani (LB) broth or on LB agar plates at 37°C. All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO), unless otherwise noted. Polyamines were used in their hydrochlorated form and were dissolved in sterile MilliQ H2O. Ciprofloxacin was purchased from Fluka BioChemika (Steinheim, Germany), and nalidixic acid, PMB, spectinomycin, and beta-lactams were purchased from Sigma Chemical Co. (St. Louis, MO).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Reference |
|---|---|---|
| Strains | ||
| N. gonorrhoeae strains | ||
| FA19 | Wild-type; serum-resistant strain | 17 |
| FA19 lptA::spc | Spectinomycin resistance insertion in lptA gene | 15 |
| FA19 potHI::Kmr | potHI deletion with insertion of kanamycin cassette | This study |
| F62 | Clinical isolate; serum-sensitive strain | 27 |
| E. coli strains | ||
| DH5α | Cloning strain; serum-sensitive strain | Invitrogen |
| MC4100 | Serum-sensitive strain; strain CGSC 6152 | Coli Genetic Strain Center |
| Plasmids | ||
| pUC18K | aphA-3 (kanamycin resistance) cassette in pUC18 | 18 |
| pGEM-T Easy | Cloning vector | Promega |
Construction of gonococcal mutants deficient in polyamine permeases.
The following strategy was used to construct a deletion-insertion mutation in the potH and potI genes of strain FA19. Based on the annotation of the FA1090 genome sequence (www.genome.ou.edu), potH and potI encode putative polyamine permeases. A sequence of 600 bp, containing the first 50 bp of the potH coding sequence and 550 bp of the upstream region, was amplified by PCR from the genomic DNA of strain FA19, using specific primers (Table 2). A second sequence of 600 bp, containing the last 50 bp of potI and 550 bp of the downstream region, was amplified by a PCR using specific primers. Each set of primers contained suitable restriction enzyme sites to allow ligation of the amplicons into the vector pGEM-T Easy (Promega, Madison, WI), in a directional manner. After digestion of the purified amplicons and their insertion into and ligation to the plasmid, the constructs were used to transform E. coli DH5α and were plated on LB agar plates containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (20 μl at 50 mg/ml) and isopropyl-β-d-1-thiogalactopyranoside (IPTG) (100 μl at 0.1 M) spread over the surface prior to use. Blue-white screening allowed for the identification of transformants that contained the insert of interest, which was verified by PCR analysis of extracted plasmid DNA. The plasmid was double digested with EcoRI and HindIII (New England Biolabs, Ipswich, MA) to insert the nonpolar aphA-3 cassette, which encodes resistance to kanamycin (18). After ligation, the plasmid was used to transform E. coli DH5α. Putative transformants of E. coli DH5α resistant to kanamycin (100 μg/ml) were tested for the presence of potHI interrupted by the aphA-3 cassette by PCR. The plasmid was used to transform a piliated variant of strain FA19, and the mutant was selected on GCB agar containing kanamycin (50 μg/ml). Chromosomal DNAs were prepared from transformants and used in PCRs to confirm that the relevant gene was insertionally inactivated.
TABLE 2.
Primers used for gene deletionsa
| Name | Sequence (5′-3′) | Restriction enzyme site(s) |
|---|---|---|
| pHFNotI | AGCGCGGCCGCCGACTCCGTTGTTCCGTTG | N |
| pHREcoI | ACTGAATTCCACCGTCGTCGTCAGCG | E |
| pIE1H3F | ATAAGAATTCTACTAAGCTTATCGGCATCATCGGAACATT | E, H |
| pIPstR2 | ATAACTGCAGTGCTCCAAGCCGACCTGTT | P |
| aphA-3F | GGATGTGCTGCAAGGCGATTAAGTT | |
| aphA-3R | CCGGCTCGTATGTTGTGTGGAATT |
Restriction enzyme sites are underlined. E, EcoRI; H, HindIII; N, NotI; P, PstI.
Antimicrobial assays, growth of gonococci in the presence of polyamines, and serum bactericidal assay (SBA).
The minimal bactericidal concentration (MBC) for the test antimicrobials was defined as the lowest concentration of drug required to kill at least 99.9% of the input gonococci after 45 min of incubation at 37°C. For MBC determinations, overnight cultures of gonococci were resuspended in GCB broth plus supplements to an optical density at 600 nm (OD600) of approximately 0.05 and then incubated in a shaking water bath at 37°C until growth reached mid-log phase, equal to an OD600 of 0.4. Gonococci were diluted 1:100 in 0.2× GCB, and 80 μl of the diluted culture was distributed in a 96-well polypropylene plate that contained 10 μl of the test antimicrobial, previously serially diluted 2-fold, and 10 μl of polyamine in each well. We tested the pHs of the solutions used in the experiments and found the following values: pH 7.6 for 0.2× GCB, pH 7.5 for 0.2× GCB plus 1 mM spermine, and pH 7.0 for 0.2× GCB plus 8 mM spermine. It is important that there was no loss of gonococcal viability in these solutions for the length of the experiment (data not presented). Negative controls were carried out without antimicrobials and with or without polyamine. After 45 min of incubation at 37°C under 4.6% (vol/vol) CO2, 4 μl (4 × 105 CFU) of bacterial suspension from each well was spotted in triplicate on GCB agar plates. After 24 h of incubation at 37°C under 4.6% (vol/vol) CO2, the viability of the samples was determined. In PMB bactericidal experiments, gonococci were preexposed to spermine (8 mM) for 10 min at 37°C, pelleted by centrifugation, washed twice, and then tested against PMB (64 μg/ml). In this case, bacteria were exposed to PMB for 45 min at 37°C and then plated onto GCB agar to enumerate survivors. The MIC was defined as the lowest concentration of drug required to inhibit 99% of the growth of cells on a GCB agar plate containing the drug. N. gonorrhoeae strains (5 × 104 CFU) were spotted on GCB agar plates containing 2-fold serial dilutions of the target drug or polyamine and were incubated overnight at 37°C. The next day, the minimum concentration at which 99% of cells presented inhibited growth was considered the MIC. Antigonococcal assays using synthetic LL-37 (kindly provided by J. Pohl and P. Svoboda, Centers for Disease Control and Prevention, Atlanta, GA) to measure the MBCs of host-derived CAMPs have been described by Shafer et al. (32).
The capacity of gonococci to grow in the presence of polyamines was determined by resuspending an overnight, GCB agar-grown culture in 50 ml of GCB broth plus supplements (as described above) to an initial OD600 of 0.05. The cultures were incubated at 37°C in a shaking water bath, and the OD was monitored every hour until the culture reached stationary phase.
The SBA used pooled NHS from healthy adult donors not under antibiotic therapy and has been described previously (15); heat-inactivated NHS (heated for 30 min at 56°C) was used as a control. The SBA was also modified to test whether spermine-treated gonococci were susceptible to NHS. In this case, 80 μl of N. gonorrhoeae (ca. 108 CFU/ml) resuspended in Hanks' balanced salt solution (HBSS) containing 0.15 mM CaCl2 and 1 mM MgCl2 (HBSS2+) was incubated with 10 μl of spermine (final concentration of 0 mM, 1 mM, or 8 mM) for 10 min at 37°C. After preincubation, two experiments were performed. In the first experiment, 10 μl of NHS or HBSS2+ was added to the cell suspension, and the suspension was incubated for 30 min at 37°C. In the second experiment, the cells were washed (8 min of centrifugation at 7,000 × g) twice in 1 ml of HBSS2+, resuspended in 100 μl of 10% NHS in HBSS2+, and incubated for 30 min at 37°C. Cells were diluted and plated on GCB agar prior to and after incubation with NHS to monitor the viability of the samples. We also evaluated the antibacterial activity of NHS that had been pretreated with spermine (8 mM) or NHS recovered by centrifugation from spermine-treated or untreated gonococci. Data from all SBAs are reported as percent survival (± standard deviation [SD]), and the differences were considered significant when the P value was ≤0.05, as determined by Student's t test.
d-PMB binding to gonococci.
Gonococci were grown overnight on GCB agar as described above and then cultured in GCB broth with supplements to an OD600 of 0.4. The bacteria were washed twice with 0.9% (wt/vol) NaCl (pH 6.0) and resuspended in the same solution to give 1.5 × 108 CFU/ml. Cell suspensions were distributed in wells (40 μl) of a black, nonbinding-surface 96-well plate (Costar model 3650) that emitted a low fluorescence background at 460 nm. The bacteria were preincubated with or without spermine at 1 mM or 8 mM (total volume of 45 μl) at 37°C for 10 min, and the pHs of the solutions ranged from 5.5 to 5.7. Dansyl-polymyxin B (d-PMB) (Invitrogen-Molecular Probes, Eugene, OR) was resuspended in H2O and added to the corresponding wells, and the mixture (final volume of 50 μl) was incubated for 45 min at 37°C. A Victor X3 microplate reader (Perkin Elmer) was used to detect excited d-PMB at 340 nm, and the fluorescence emission was read at 460 nm and 535 nm (19) every 22.5 min, maintaining the cells at 37°C, during the length of the experiment. Control wells were assayed to monitor the fluorescence background levels of cells with or without spermine and with d-PMB, with or without spermine. Our control wells showed that d-PMB alone did not lose fluorescence intensity during the course of the experiment.
Gel electrophoresis and immunoblotting.
Immunoblotting was performed to analyze the influence of spermine on the binding capacity of complement component C8 and regulatory protein C4b-binding protein (C4BP) for whole gonococci. Briefly, 109 bacteria were preincubated with 0 mM, 1 mM, or 8 mM spermine in HBSS2+ to a final reaction volume of 90 μl for 10 min at 37°C. After preincubation with spermine, 10 μl of NHS was added to the suspension, and the mixture was incubated for 30 min at 37°C. Bacteria were washed twice with 900 μl of HBSS2+ by centrifugation for 8 min at 7,000 × g to remove unbound serum components, lysed with 1× sodium dodecyl sulfate (SDS)-Tris-acetate sample loading buffer, and heated for 30 min at 50°C. Samples were separated in a 3 to 8% Tris-acetate-SDS-polyacrylamide gel (Invitrogen, Frederick, MD), using 1× Tris-acetate running buffer under nonreducing conditions. Proteins were transferred to a pure nitrocellulose membrane (Bio-Rad, Hercules, CA) by immunoblotting for 75 min at 15 V on a semidry Trans-Blot system (Bio-Rad), using a transfer buffer (10 mM Tris, 100 mM glycine) containing 10% (vol/vol) methanol. After transfer, nonspecific binding sites on the membranes were blocked with TTBS (100 mM Tris-HCl, pH 7.5, 154 mM NaCl, 0.1% [vol/vol] Tween 20) containing 5% nonfat dry milk for 1 h at room temperature. After 3 washes in TTBS, membranes were incubated with mouse anti-human C8 monoclonal antibody (MAb) (Quidel Corporation, San Diego, CA) diluted 1/1,000 in phosphate-buffered saline (PBS) or with mouse anti-human C4BP MAb (Quidel Corporation) diluted 1/10,000 in PBS overnight at 4°C. After incubation with the appropriate horseradish peroxidase-conjugated anti-mouse IgG(H+L) secondary antibody (Bio-Rad) diluted 1/100,000 for 1 h at room temperature, anti-C8- and anti-C4BP-reactive bands were revealed with ECL Western blotting substrate (Pierce, Rockford, IL). A duplicate gel loaded with the same samples was stained with Coomassie blue to ensure that equal amounts of total protein were loaded for all samples.
Hemolysis assays.
Hemolysis assays were performed using a modified protocol of the EZ complement CH50 test (Diamedix, Miami, FL). Sensitized sheep erythrocytes (495 μl) were incubated with 60 μl of different dilutions of NHS (10% to 0.0098%) and with 10 μl of spermine at final concentrations in the assay mixture of 0 mM, 1 mM, and 8 mM. The final volume of the assay was 600 μl; when needed, HBSS2+ was used to complete the 600 μl. The assay mixture was incubated for 30 min at 37°C. Tubes were centrifuged at 2,000 rpm for 10 min at room temperature. Supernatants (530 μl) were transferred to cuvettes and read at 415 nm in a Genesys 10 Vis spectrophotometer (Thermo Scientific).
RESULTS AND DISCUSSION
The primary site of gonococcal infections is the human urogenital tract, where concentrations of certain polyamines (e.g., spermine and spermidine) can reach 1 to 15 mM (36); these values have been determined for males, but similar information for females is not known. Since polyamines have been reported to bind to bacterial surfaces and increase levels of antibiotic resistance expressed by E. coli and P. aeruginosa (13, 34, 35), we were interested in determining if they might also alter levels of gonococcal susceptibility to host-derived antimicrobials that participate in the innate host defense. Accordingly, we tested if incubation of gonococci in the presence of polyamines would modify their levels of susceptibility to CAMPs and complement-mediated killing by NHS. In preliminary experiments, we found that biosynthetic polyamines (spermine, spermidine, putrescine, and cadaverine) and two polyamine precursors (ornithine and arginine) lacked antigonococcal activity against strain FA19 (MBC > 512 mM and MIC > 64 mM [data not presented]). We also determined that growth of strain FA19 in GCB medium in the presence of increasing concentrations of spermine (1 to 8 mM) was identical to that of gonococci grown without spermine (data not presented). We propose that the relative resistance of the gonococcus to these polyamines is likely due to its evolution over the millennia as strict human pathogens that infect the polyamine-rich environment of the human genitourinary tract. Two commensal Neisseria species (N. lactamica and N. cinerea) that reside in the nasopharynx were found to be at least 8-fold more susceptible to spermine and spermidine, but the MIC values were 10 to 20 times greater than physiological levels (data not presented).
Polyamines increase gonococcal resistance to CAMPs.
In order to learn if exposure of gonococci to polyamines would alter their susceptibility to CAMPs, we determined the susceptibility of strain FA19 to PMB in the presence (4 mM) or absence of polyamines or polyamine precursors. We found that when strain FA19 was exposed to exogenous polyamines in an antimicrobial assay that evaluated the killing activity of PMB, gonococcal resistance to this CAMP increased (Table 3). Of the compounds tested, spermine had the greatest effect, as it increased PMB resistance (PMBr) 32-fold. We noted a relationship between the cationicity and length of these unbranched polyamines and their ability to increase PMBr (Table 3), which is consistent with an earlier report that described the capacities of different polyamines to increase bacterial resistance to antibiotics (11). Based on our initial results, obtained by incorporating a single concentration (4 mM) of polyamines or precursors in the CAMP antimicrobial assay, we next tested if increasing amounts of these compounds would increase gonococcal PMBr. As shown in Fig. 1 A, this was the case for spermine, spermidine, cadaverine, and putrescine but not for the polyamine precursors ornithine and arginine. Biosynthetic polyamines increased gonococcal resistance to PMB in a dose-dependent manner. We also tested various synthetic amines, including Tris (+1 charge), diaminopropane (+2), hexamethylenediamine (+2), and hexamethylenetetraamine (+4). All synthetic mono- and polyamines were tested at 0 and 8 mM; these compounds by themselves did not exert antigonococcal activity at these concentrations (Table 4). Similarly, a polyanion, pyrophosphate (−4), was tested at the same concentrations. When gonococci were pretreated with these compounds and then challenged with PMB, we noted that they were unable to increase bacterial resistance to PMB to the extent observed with spermine. In this respect, while 8 mM spermine increased the MBC of PMB 64-fold, the synthetic polyamines and the polyanion (8 mM) increased the MBC only 2- to 8-fold (Table 4).
TABLE 3.
Resistance levels of N. gonorrhoeae FA19 to PMB in the presence of a 4 mM concentration of various polyamines and polyamine precursors
 |
a Values observed in the human urogenital tract (36).
FIG. 1.
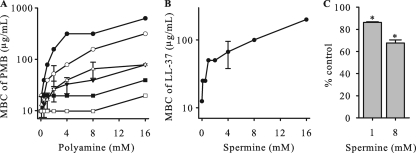
Polyamines increase the resistance of gonococci to CAMPs. The resistance level of strain FA19 to CAMPs in the presence of polyamines or polyamine precursors was determined as described in Materials and Methods. (A) MBCs of PMB with increasing concentrations of polyamines (spermine [•], spermidine [○], putrescine [▾], and cadaverine [▵]) or polyamine precursors (ornithine [▪] and arginine [□]). (B) MBCs of host-derived antimicrobial peptide LL-37 with increasing concentrations of spermine. (C) d-PMB binding to gonococci significantly decreased (*, P < 0.005) when gonococci were preincubated with spermine (1 mM or 8 mM) compared to that for cells without spermine, as measured by fluorescence emission at 460 nm.
TABLE 4.
Synthetic compounds are less effectual than spermine at increasing gonococcal resistance to PMB
| Compounda | Net charge | MBC of PMB (μg/ml) |
ΔMBCb | |
|---|---|---|---|---|
| 0 mM compound | 8 mM compound | |||
| Spermine | +4 | 9.76 | 625 | 64 |
| Propanediamine | +2 | 9.76 | 39.06 | 4 |
| Hexadiamine | +2 | 9.76 | 78.13 | 8 |
| Hexamethylene tetramine | 0 | 9.76 | 19.53 | 2 |
| Tris | +1 | 9.76 | 4.88 | 0.5 |
| Pyrophosphate | −4 | 9.76 | 39.06 | 4 |
Chemical names of compounds: spermine, spermine tetrahydrochloride; propanediamine, 1,3-diaminopropane dihydrochloride; hexadiamine, 1,6-diaminohexane; hexamethylene tetramine, 1,3,5,7-tetraazatricyclo-[3.3.1.13,7]decane; Tris, 2-amino-2-hydroxymethyl-1,3-propanediol; pyrophosphate, sodium diphosphate tetrabasic.
Fold change in PMB MBC in the presence versus absence of compound.
Using spermine as a model polyamine, we asked if it could increase gonococcal resistance to LL-37, a natural CAMP that is present in the human urogenital tract (16) and known to have antigonococcal activity in vitro (32). As shown in Fig. 1B, the resistance of strain FA19 to LL-37 increased with increasing concentrations of spermine in the antibacterial assay. According to these results, we found that the molar ratios required to observe spermine-mediated resistance to CAMPs were 17.7:1 for spermine and PMB and 12.5:1 for spermine and LL-37.
The capacity of spermine to increase gonococcal resistance to PMB and LL-37 was not restricted to strain FA19 or to gonococci, as results from other experiments that used three different gonococcal clinical isolates, i.e., F62, FA1090, and FA6140, or a strain of Neisseria meningitidis (M7) showed similar capacities, in that resistance to these CAMPs increased when spermine (8 mM) was added to the media (data not presented). In order to determine if changes in LOS structure impact gonococcal susceptibility to PMB or spermine or the ability of spermine to block PMB activity, we compared the susceptibilities of FA19, which produces a single LOS species of 3.6 kDa (26), and genetic derivatives of FA19 that produce 4.5- to 4.8-kDa LOS species. Briefly, we found that the extension of the LOS alpha-chain resulting in the 4.5- to 4.8-kDa LOS species did not alter the capacity of spermine to reduce PMB activity against gonococci. We also tested clinical isolates unrelated to strain FA19 that produce different LOS chemotypes and belong to different (Por1A or Por1B) serovars and obtained results similar to that with FA19 (data not presented). Thus, the length of the LOS or the Por serovar does not significantly influence the ability of spermine to reduce the antigonococcal action of PMB. Additionally, we tested genetic derivatives of FA19 (32; our unpublished observations) that have mutations in the mtr efflux pump operon (mtrC and mtrD), the transcriptional repressor of mtrCDE (mtrR), or the misR-misS operon, as these systems can influence the susceptibility of gonococci and meningococci to PMB (37) and LL-37 (32). These mutations did not significantly change the magnitude of the ability of spermine to reduce the antigonococcal action of PMB (data not presented). Thus, the MtrCDE efflux pump and the MisR/MisS two-component regulatory system are not likely to contribute to this property.
We also found that exposure of strain FA19 to spermine did not alter its level of susceptibility to antibiotics that act on cytosolic or periplasmic targets, such as DNA gyrase (e.g., ciprofloxacin and nalidixic acid), ribosomes (e.g., spectinomycin), or peptidoglycan synthesis (e.g., penicillin, ampicillin, and ceftriaxone) (data not presented). We next asked if transport of spermine into gonococci was required for its ability to enhance CAMP resistance. Based on analysis of the FA1090 genome sequence (www.genome.ou.edu), gonococci appear to have a single polyamine transporter system (PotFGHI). This polyamine transporter system is predicted to have two putative nucleotide-binding subunits, encoded by potA (NGO2039) and potG (NGO0192); three putative periplasmic polyamine-binding proteins, encoded by potF1 (NGO0206), potF2 (NGO1253), and potF3 (NGO1494); and a pair of permeases, encoded by potH (NGO0195) and potI (NGO0196). We constructed a nonpolar chromosomal deletion-insertion mutation in potH and potI, using the aphA-3 cassette (18), and transferred this mutation into the chromosome of strain FA19 by transformation. With this mutant (FA19 potHI::Kmr), we found that loss of the PotH and PotI permeases in strain FA19 did not influence the ability of spermine to increase gonococcal resistance to PMB (data not presented), suggesting that this mechanism of resistance does not require an influx of polyamines.
Having determined that the single polyamine transport system was not required for the ability of spermine to enhance the resistance of gonococci to PMB, we tested if such resistance was reversible. We considered this likely because polyamines are known to bind to the surfaces of Gram-negative bacteria (22, 34), where polyamines may be removed by washing. Briefly, we incubated gonococci with or without 8 mM spermine for 10 min at 37°C and then tested a sample for survival in the presence of 64 μg/ml of PMB, either directly or after the sample had been centrifuged and washed twice with 0.2× GCB. Unwashed gonococci displayed PMB resistance (data not presented), while the washed gonococci were as susceptible to PMB as gonococci not exposed to spermine (<1% survival). Thus, we concluded that the influence of spermine on CAMP resistance is reversible and likely due to its known ability to coat bacterial surfaces (22), where it can reduce binding of CAMPs.
In order to determine the influence of spermine on the binding of PMB to gonococci, we performed experiments using d-PMB. After preincubation of gonococci with spermine for 10 min, d-PMB was added, and the fluorescence intensity was monitored at 460 nm throughout the course of the 45-min experiment. Compared to the control (no spermine), the presence of 8 mM spermine resulted in significantly less emission of fluorescence (Fig. 1C), indicating that like the case for other Gram-negative bacteria (22, 34), spermine can reduce the binding of PMB to gonococci.
Polyamines increase gonococcal resistance to complement-mediated killing by NHS.
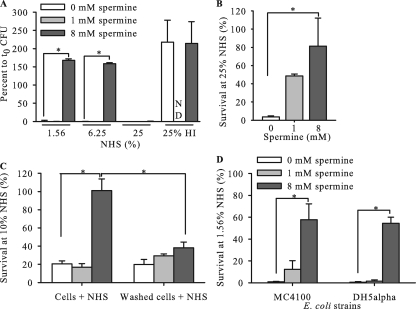
Since we observed that polyamines could increase gonococcal resistance to one class of mediators of innate immunity (e.g., CAMPs), we tested if a different host defense system might be influenced similarly. Accordingly, we examined the antigonococcal activity of NHS, which requires an active classical complement pathway (CCP) system and a natural IgM (4, 28), in the presence of spermine. For this purpose, we used strains F62, a highly NHS-sensitive clinical isolate (2, 28), and FA19 lptA::spc, a genetic derivative of the NHS-resistant strain FA19 containing a nonpolar insertional mutation in lptA that renders strain FA19 susceptible to complement-mediated killing by NHS (15). lptA encodes a PEA transferase that adds PEA to the 1 and 4′ positions of lipid A (15). Using the NHS-sensitive F62 strain (<2% survival in the presence of low [1.6%] and high [25%] concentrations of NHS), we found that the addition of spermine (8 mM) could significantly reduce the killing activity of NHS at low (1.6% and 6.2%) but not high (25%) concentrations of NHS (Fig. 2 A). Likewise, FA19 lptA::spc, which is intrinsically less NHS susceptible than F62, also displayed increased NHS resistance when it was incubated with 25% NHS in the presence of two concentrations (1 mM and 8 mM) of spermine (Fig. 2B). Like the case for PMB killing of gonococci, the influence of spermine on NHS-mediated killing of gonococci was reversible. Briefly, we found that when strain F62 was preincubated with 8 mM spermine, washed, and then tested against 10% (vol/vol) NHS, viability was reduced to the level observed with gonococci incubated in the absence of spermine (Fig. 2C), while unwashed, spermine-treated gonococci were NHS resistant. To determine if the effect of spermine on NHS killing of bacteria was exclusive to gonococci, we analyzed its effect on NHS killing of serum-sensitive E. coli strains. We observed <2% survival at 1.6% (vol/vol) NHS for E. coli MC4100 and DH5α in the absence of spermine, while 58% and 55% survival rates, respectively, were observed in the presence of 8 mM spermine in the same assay (Fig. 2D).
FIG. 2.
Spermine enhances NHS resistance of N. gonorrhoeae (in a reversible manner) and E. coli. The survival of gonococcal strains F62 (A) and FA19 lptA::spc (B) was monitored when NHS was coincubated with increasing concentrations of spermine. (C) When cells of strain F62 were washed after exposure to 8 mM spermine, the cells were susceptible; the increased level of resistance observed with spermine was reversible. (D) Survival of E. coli strains MC4100 and DH5α in NHS in the absence or presence (1 mM or 8 mM) of spermine. ND, not determined; HI, heat-inactivated NHS at 25%. *, significant differences between groups (P < 0.05).
Spermine can inhibit functional properties of the CCP.
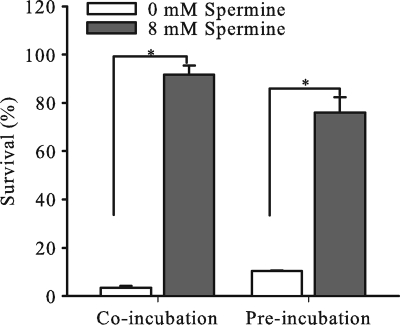
The capacity of spermine to inhibit NHS-mediated activity against gonococci and E. coli suggested that its presence in the SBA influenced CCP activity. Indeed, earlier studies that used 20 mM or greater concentrations of spermine showed that this polyamine can inhibit the formation of the C1 complex and prevent activation of the downstream CCP cascade (10, 20, 38). Using a modified protocol of the EZ complement CH50 test (Diamedix, Miami, FL), we found that 8 mM spermine, but not 1 mM spermine, significantly decreased the hemolytic activity of 1% (vol/vol) but not 10% (vol/vol) NHS against antibody-sensitized sheep erythrocytes (Fig. 3). Because antibody-coated sheep erythrocytes are highly sensitive to complement activity, we were concerned that although spermine-treated 10% (vol/vol) NHS retained substantial hemolytic potential, it might be insufficient for bactericidal activity. Accordingly, we asked if spermine-treated NHS retained antigonococcal activity. We found that 10% NHS pretreated with 8 mM spermine for 30 min at 37°C lacked the ability to kill strain F62 (survival equal to 75.9% ± 6.5%) compared to serum that was similarly incubated in the absence of spermine (survival equal to 10.3% ± 0.3%) (Fig. 4). This suggested to us that spermine reduced the activity of the CCP, as reported previously (10).
FIG. 3.
Spermine inhibits NHS hemolytic activity. We used a modified Diamedix EZ complement protocol to measure hemolysis of red blood cells in the presence of spermine. Sensitized sheep erythrocytes were incubated with increasing dilutions of NHS in the absence or presence of spermine (1 mM or 8 mM). We measured hemoglobin release by determining the OD415. At 1% NHS, hemolytic activity was significantly reduced in erythrocytes exposed to 8 mM spermine compared to that in unexposed erythrocytes or erythrocytes exposed to 1 mM spermine.
FIG. 4.
NHS pretreated with spermine loses its killing activity against N. gonorrhoeae. Serum bactericidal activity of NHS coincubated with spermine and gonococcal cells or of NHS preincubated with spermine and then added to gonococcal cells was tested. The NHS showed significantly reduced bactericidal activity against gonococci, whether it was coincubated or preincubated with 8 mM spermine.
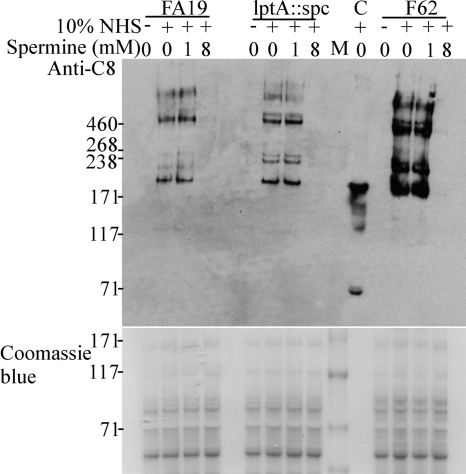
Based on our observation that spermine could reduce the antigonococcal activity of NHS, we decided to evaluate the deposition of complement proteins on the surfaces of gonococci in the presence of spermine. Accordingly, we asked if the binding to whole gonococci of specific proteins involved in the regulation of CCP activation (e.g., C4BP) or in the formation of the membrane attack complex (MAC) (e.g., C8) was influenced by the presence of spermine in the SBA. C4BP was previously shown to bind to the surface of N. gonorrhoeae, preventing C3b deposition and activation of downstream components of the CCP (23). C8 detection was preferred over C9 detection, since previous work by Harriman et al. demonstrated that C9 is not essential for complement-mediated killing of N. gonorrhoeae (6). To determine if spermine influences C4BP and/or C8 deposition on gonococci, NHS-resistant strain FA19 and NHS-sensitive strains FA19 lptA::spc and F62 were incubated in 10% NHS with (1 mM or 8 mM) or without spermine. The presence of C4BP and C8 associated with gonococci was determined by gel electrophoresis and immunoblotting, using anti-C4BP and anti-C8 MAbs. We noted that C8 deposition on all three strains was significantly reduced when bacteria and NHS were coincubated in the presence of 8 mM spermine (Fig. 5). Isogenic strains FA19 and FA19 lptA::spc bound greater levels of C4BP than that bound by strain F62. However, the level of C4BP bound to each strain did not seem to be enhanced by the presence of spermine (data not shown). We also noticed that cells preincubated with spermine and washed with HBSS2+ before incubation with NHS were as resistant to NHS as control cells and that they were as immunoreactive with anti-C8 MAbs (data not shown).
FIG. 5.
Deposition of C8 on gonococci. Gonococci were incubated in 10% NHS with increasing concentrations of spermine, as indicated at the top of the figure, and the immunoblot was developed using an anti-human C8 MAb. Anti-C8-immunoreactive bands were observed in the absence or at a low concentration of spermine (1 mM), while a high concentration of spermine (8 mM) impaired the detection of anti-C8-immunoreactive bands. C, NHS-only control; M, HiMark prestained molecular mass (kDa) marker (Invitrogen, Frederick, MD). The Coomassie blue-stained gel showed that all lanes were loaded with the same amount of proteins (NHS was not loaded in the stained gel).
In this study, we analyzed the influence of polyamines on the capacity of two mediators of innate host defense to exert antigonococcal action in vitro. Using spermine as a model polyamine, we found that the antigonococcal capacity of LL-37 and NHS could be reduced significantly in its presence. It is important that this inhibition of the bactericidal activities of both mediators of innate host defense occurred with physiologic levels of spermine, which has been reported to achieve levels of 15 mM in the human urogenital tract (36). Since spermine was the most effective polyamine in mediating this inhibition and was the most positively charged polyamine among the four tested, it is likely that ionic interactions between it and negatively charged bacterial surface structures are important in conferring resistance of gonococci to CAMPs, as suggested for the role of polyamines in resistance to antimicrobials in Gram-negative bacteria (22, 25). With respect to its ability to provide gonococci with increased resistance to CAMPs, we propose that spermine can displace divalent cations (Mg2+ and Ca2+) usually associated with the Gram-negative bacterial outer membrane and then coat the gonococcal cell surface and block sites that otherwise would bind CAMPs. While this hypothesis is consistent with and supported by results obtained by Peterson et al. for other Gram-negative bacteria, such as P. aeruginosa, E. coli, and Salmonella enterica serovar Typhimurium (22), additional post-surface-binding steps may be important for the ability of spermine to reduce the antimicrobial action of CAMPs. In this respect, while the results from experiments that measured the inhibition of d-PMB binding to gonococci in the presence of spermine (Fig. 1C) tend to support the model developed using other bacteria (22), additional processes could also explain why CAMP resistance is elevated in spermine-treated gonococci. We considered the possibility that spermine-treated gonococci may be generally deficient in transporting antimicrobials across the outer membrane. We do not think that this is the case, as antibiotics that function in the periplasm or cytosol, including positively charged antibiotics such as ceftriaxone (+1) and spectinomycin (+2), were not impaired in their antimicrobial activity. Clearly, more detailed studies are required to fully understand how spermine-treated gonococci express elevated CAMP resistance.
Previous work by Joiner et al. showed that although both NHS-sensitive and -resistant gonococci can activate the CCP and that C5b-9 MACs are established on their surfaces, the MACs formed on resistant gonococci are misformed and likely in a nonbactericidal state (9). Despite the ability of NHS-resistant gonococci to activate the CCP through C9, there is evidence that resistant gonococci can bind the complement regulatory protein C4BP, which results in downregulation of the CCP (23). The major outer membrane protein Por1A possessed by certain gonococci (e.g., strain FA19) binds C4BP to a greater extent than that by the Por1B protein possessed by other gonococci (e.g., F62), which are more frequently NHS sensitive. C4BP binding to N. gonorrhoeae can also be influenced by the structure of the inner core region of LOS, and possibly by the presence of lipid A substitutions, such as phosphoethanolamine (23, 24). The capacity of spermine to significantly reduce C8 binding to gonococci implies that a step in MAC formation can be impaired and that this is responsible for its ability to render NHS-sensitive gonococci resistant to complement-mediated killing. We do not yet know if early steps in CCP activation or steps in MAC formation are impaired by spermine, but we suggest that spermine masks sites on the bacterial surface which influence either one or both processes. Since previous work documented the ability of spermine to dissociate the C1 complex (38), it is likely that the capacity of 8 mM spermine to significantly reduce the bactericidal effect of NHS and to abolish C8 binding to whole gonococci can be explained by this mechanism. Clearly, however, the results from our hemolytic assays using antibody-sensitized sheep blood erythrocytes indicate that residual CCP activity remains in such serum but that the level is insufficient to kill bacteria. We are presently studying this issue in greater detail, and the results should provide new insights into how mucosal pathogens can develop resistance to complement-mediated killing in vivo.
Recently, Kwon and Lu (12) proposed that polyamines could be used in conjunction with antibiotics to sensitize pathogenic bacteria, as they showed that some bacteria are more susceptible to antibiotics in the presence of polyamines. Conversely, our findings suggest that this strategy could increase the resistance of certain human pathogens (e.g., N. gonorrhoeae) to innate immune defenses, which would enhance bacterial survival during early stages of infection and possibly accelerate manifestations of symptoms and disease.
Acknowledgments
We are sincerely thankful to Eun-Hee Lee-Delon and Yaramah Zalucki for helpful discussions, to Sanjay Ram for valuable advice regarding complement activity and for reading the paper before submission, to David Stephens for providing NHS, to Lane Pucko for help in manuscript preparation, and to Yih-Ling Tzeng for providing the DNA used to construct the FA19 misR-misS mutant. We are grateful to E.-H. Lee-Delon for constructing this strain.
This work was partially supported by National Institutes of Health grants AI-062755-05 (W.M.S.) and AI-031496-20 (P. F. Sparling, University of North Carolina) and by a VA Merit Review grant to W.M.S. W.M.S. is the recipient of a Senior Research Career Scientist Award from the VA Medical Research Service.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 3 May 2010.
REFERENCES
- 1.Bourgeon, F., B. Evrard, M. Brillard-Bourdet, D. Colleu, B. Jegou, and C. Pineau. 2004. Involvement of semenogelin-derived peptides in the antibacterial activity of human seminal plasma. Biol. Reprod. 70:768-774. [DOI] [PubMed] [Google Scholar]
- 2.Cannon, J. G., T. J. Lee, L. F. Guymon, and P. F. Sparling. 1981. Genetics of serum resistance in Neisseria gonorrhoeae: the sac-1 genetic locus. Infect. Immun. 32:547-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folster, J. P., and W. M. Shafer. 2005. Regulation of mtrF expression in Neisseria gonorrhoeae and its role in high-level antimicrobial resistance. J. Bacteriol. 187:3713-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffiss, J. M., G. A. Jarvis, J. P. O'Brien, M. M. Eads, and H. Schneider. 1991. Lysis of Neisseria gonorrhoeae initiated by binding of normal human IgM to a hexosamine-containing lipooligosaccharide epitope(s) is augmented by strain-specific, properdin-binding-dependent alternative complement pathway activation. J. Immunol. 147:298-305. [PubMed] [Google Scholar]
- 5.Hall, S. H., K. G. Hamil, and F. S. French. 2002. Host defense proteins of the male reproductive tract. J. Androl. 23:585-597. [PubMed] [Google Scholar]
- 6.Harriman, G. R., A. F. Esser, E. R. Podack, A. C. Wunderlich, A. I. Braude, T. F. Lint, and J. G. Curd. 1981. The role of C9 in complement-mediated killing of Neisseria. J. Immunol. 127:2386-2390. [PubMed] [Google Scholar]
- 7.Holtje, J. V. 1979. Regulation of polyamine and streptomycin transport during stringent and relaxed control in Escherichia coli. J. Bacteriol. 137:661-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jerse, A. E., N. D. Sharma, A. N. Simms, E. T. Crow, L. A. Snyder, and W. M. Shafer. 2003. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect. Immun. 71:5576-5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joiner, K. A., K. A. Warren, E. J. Brown, J. Swanson, and M. M. Frank. 1983. Studies on the mechanism of bacterial resistance to complement-mediated killing. IV. C5b-9 forms high molecular weight complexes with bacterial outer membrane constituents on serum-resistant but not on serum-sensitive Neisseria gonorrhoeae. J. Immunol. 131:1443-1451. [PubMed] [Google Scholar]
- 10.Kovacsovics, T. J., M. C. Peitsch, A. Kress, and H. Isliker. 1987. Antibody-independent activation of C1. I. Differences in the mechanism of C1 activation by nonimmune activators and by immune complexes: C1r-independent activation of C1s by cardiolipin vesicles. J. Immunol. 138:1864-1870. [PubMed] [Google Scholar]
- 11.Kwon, D. H., and C. D. Lu. 2007. Polyamine effects on antibiotic susceptibility in bacteria. Antimicrob. Agents Chemother. 51:2070-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon, D. H., and C. D. Lu. 2006. Polyamines increase antibiotic susceptibility in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 50:1623-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon, D. H., and C. D. Lu. 2006. Polyamines induce resistance to cationic peptide, aminoglycoside, and quinolone antibiotics in Pseudomonas aeruginosa PAO1. Antimicrob. Agents Chemother. 50:1615-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee, E. H., and W. M. Shafer. 1999. The farAB-encoded efflux pump mediates resistance of gonococci to long-chained antibacterial fatty acids. Mol. Microbiol. 33:839-845. [DOI] [PubMed] [Google Scholar]
- 15.Lewis, L. A., B. Choudhury, J. T. Balthazar, L. E. Martin, S. Ram, P. A. Rice, D. S. Stephens, R. Carlson, and W. M. Shafer. 2009. Phosphoethanolamine substitution of lipid A and resistance of Neisseria gonorrhoeae to cationic antimicrobial peptides and complement-mediated killing by normal human serum. Infect. Immun. 77:1112-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malm, J., O. Sorensen, T. Persson, M. Frohm-Nilsson, B. Johansson, A. Bjartell, H. Lilja, M. Stahle-Backdahl, N. Borregaard, and A. Egesten. 2000. The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations, and is attached to spermatozoa. Infect. Immun. 68:4297-4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maness, M. J., and P. F. Sparling. 1973. Multiple antibiotic resistance due to a single mutation in Neisseria gonorrhoeae. J. Infect. Dis. 128:321-330. [DOI] [PubMed] [Google Scholar]
- 18.Menard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore, R. A., N. C. Bates, and R. E. Hancock. 1986. Interaction of polycationic antibiotics with Pseudomonas aeruginosa lipopolysaccharide and lipid A studied by using dansyl-polymyxin. Antimicrob. Agents Chemother. 29:496-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muschel, L. H., and J. E. Jackson. 1966. Reversal of the bactericidal reaction of serum by magnesium ion. J. Bacteriol. 91:1399-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nastri, H. G., and I. D. Algranati. 1996. Effect of polyamines on plasmid-mediated kanamycin resistance and kanamycin phosphotransferase gene expression in Escherichia coli. Cell. Mol. Biol. (Noisy-le-Grand) 42:711-717. [PubMed] [Google Scholar]
- 22.Peterson, A. A., R. E. Hancock, and E. J. McGroarty. 1985. Binding of polycationic antibiotics and polyamines to lipopolysaccharides of Pseudomonas aeruginosa. J. Bacteriol. 164:1256-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ram, S., M. Cullinane, A. M. Blom, S. Gulati, D. P. McQuillen, B. G. Monks, C. O'Connell, R. Boden, C. Elkins, M. K. Pangburn, B. Dahlback, and P. A. Rice. 2001. Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae. J. Exp. Med. 193:281-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ram, S., J. Ngampasutadol, A. D. Cox, A. M. Blom, L. A. Lewis, F. St. Michael, J. Stupak, S. Gulati, and P. A. Rice. 2007. Heptose I glycan substitutions on Neisseria gonorrhoeae lipooligosaccharide influence C4b-binding protein binding and serum resistance. Infect. Immun. 75:4071-4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schindler, M., and M. J. Osborn. 1979. Interaction of divalent cations and polymyxin B with lipopolysaccharide. Biochemistry 18:4425-4430. [DOI] [PubMed] [Google Scholar]
- 26.Shafer, W. M., A. Datta, V. S. Kolli, M. M. Rahman, J. T. Balthazar, L. E. Martin, W. L. Veal, D. S. Stephens, and R. Carlson. 2002. Phase variable changes in genes lgtA and lgtC within the lgtABCDE operon of Neisseria gonorrhoeae can modulate gonococcal susceptibility to normal human serum. J. Endotoxin Res. 8:47-58. [PubMed] [Google Scholar]
- 27.Shafer, W. M., L. F. Guymon, I. Lind, and P. F. Sparling. 1984. Identification of an envelope mutation (env-10) resulting in increased antibiotic susceptibility and pyocin resistance in a clinical isolate of Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 25:767-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shafer, W. M., K. Joiner, L. F. Guymon, M. S. Cohen, and P. F. Sparling. 1984. Serum sensitivity of Neisseria gonorrhoeae: the role of lipopolysaccharide. J. Infect. Dis. 149:175-183. [DOI] [PubMed] [Google Scholar]
- 29.Shafer, W. M., L. E. Martin, and J. K. Spitznagel. 1984. Cationic antimicrobial proteins isolated from human neutrophil granulocytes in the presence of diisopropyl fluorophosphate. Infect. Immun. 45:29-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shafer, W. M., L. E. Martin, and J. K. Spitznagel. 1986. Late intraphagosomal hydrogen ion concentration favors the in vitro antimicrobial capacity of a 37-kilodalton cationic granule protein of human neutrophil granulocytes. Infect. Immun. 53:651-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shafer, W. M., V. Onunka, and P. J. Hitchcock. 1986. A spontaneous mutant of Neisseria gonorrhoeae with decreased resistance to neutrophil granule proteins. J. Infect. Dis. 153:910-917. [DOI] [PubMed] [Google Scholar]
- 32.Shafer, W. M., X. Qu, A. J. Waring, and R. I. Lehrer. 1998. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc. Natl. Acad. Sci. U. S. A. 95:1829-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabor, C. W., and H. Tabor. 1984. Polyamines. Annu. Rev. Biochem. 53:749-790. [DOI] [PubMed] [Google Scholar]
- 34.Tkachenko, A. G., O. N. Pozhidaeva, and M. S. Shumkov. 2006. Role of polyamines in formation of multiple antibiotic resistance of Escherichia coli under stress conditions. Biochemistry (Moscow) 71:1042-1049. [DOI] [PubMed] [Google Scholar]
- 35.Tkachenko, A. G., M. S. Shumkov, and A. V. Akhova. 2009. Adaptive functions of Escherichia coli polyamines in response to sublethal concentrations of antibiotics. Microbiology 78:25-32. [PubMed] [Google Scholar]
- 36.Tyms, A. S. 1989. Polyamines and the growth of bacteria and viruses, p. 368. In U. Bachrach and Y. M. Heimer (ed.), The physiology of polyamines, 1st ed., vol. 2. CRC Press, Boca Raton, FL. [Google Scholar]
- 37.Tzeng, Y. L., K. D. Ambrose, S. Zughaier, X. Zhou, Y. K. Miller, W. M. Shafer, and D. S. Stephens. 2005. Cationic antimicrobial peptide resistance in Neisseria meningitidis. J. Bacteriol. 187:5387-5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villiers, C. L., G. J. Arlaud, and M. G. Colomb. 1984. Diamine-induced dissociation of the first component of human complement, C1. Eur. J. Biochem. 140:421-426. [DOI] [PubMed] [Google Scholar]
- 39.Warner, D. M., J. P. Folster, W. M. Shafer, and A. E. Jerse. 2007. Regulation of the MtrC-MtrD-MtrE efflux-pump system modulates the in vivo fitness of Neisseria gonorrhoeae. J. Infect. Dis. 196:1804-1812. [DOI] [PubMed] [Google Scholar]