Abstract
Bacteria have developed several mechanisms for iron uptake during colonization of mammalian hosts, where the availability of free iron is limiting for growth. Neisseria meningitidis expresses under iron-limiting conditions a receptor complex consisting of the lactoferrin-binding proteins A (LbpA) and LbpB to acquire iron from lactoferrin, which is abundantly present on the mucosal surfaces of the human nasopharynx. LbpA is an integral outer membrane-embedded iron transporter, whereas LbpB is a cell surface-exposed lipoprotein. In this study, we demonstrate that LbpB is also released into the culture medium. We identified NalP, an autotransporter known to be involved in the processing of other autotransporters, as the protease responsible for LbpB release. This release of LbpB reduced the complement-mediated killing of the bacteria when incubated with an LbpB-specific bactericidal antiserum. Since antibodies directed against LbpB are found in convalescent-patient sera, the release of an immunogenic protein as LbpB may represent a novel means for N. meningitidis to escape the human immune response.
The Gram-negative bacterial species Neisseria meningitidis and Neisseria gonorrhoeae are the only Neisseriaceae that are pathogenic to humans, who are also the only known reservoir of these bacteria. Normally, N. meningitidis behaves as a commensal and colonizes the upper respiratory tract without any obvious clinical symptoms. However, in rare cases, it crosses the mucosal barriers and causes sepsis and meningitis with a high mortality and morbidity. This occurs most frequently in children and young adults. In the human body, the concentration of free soluble iron is too low to support bacterial growth. Iron in the human body is bound intracellularly to heme, hemoglobin, or ferritin and in serum and on mucosal surfaces to transferrin and lactoferrin, respectively (13). Bacteria have developed several different mechanisms of iron utilization, one of which involves the synthesis and secretion of siderophores (25). N. meningitidis and N. gonorrhoeae do not produce siderophores (3, 38). However, when grown under iron limitation, they express surface-exposed receptors for human iron-binding compounds, including transferrin (10, 19), lactoferrin (6, 28, 30), hemoglobin (34), and haptoglobin (20).
The lactoferrin receptor is thought to be an important virulence factor of N. meningitidis. The main site of entry into the human body is the nasopharynx, where lactoferrin is abundant and could provide a major source of iron (24). Using an affinity isolation procedure, a single lactoferrin-binding protein was originally identified (32). The gene encoding this receptor, designated LbpA (lactoferrin-binding protein A), was subsequently characterized (6, 28, 30). LbpA showed a high degree of similarity to the TbpA (transferrin-binding protein A)-component of the transferrin receptor, which further consists of a lipoprotein designated TbpB (19). Upstream of lbpA, an open reading frame was identified, the deduced amino acid sequence of which showed homology to TbpB (26). Subsequent analysis showed that, in analogy to the transferrin receptor, the lactoferrin receptor is composed of two proteins, LbpA and LbpB (31). LbpB is a surface-exposed lipoprotein, but the exact function of the protein in the receptor complex remains to be shown. The protein appears to be expressed during infection, since LbpB-specific antibodies were found present in convalescent-patient sera (27).
In the course of our studies, we observed that LbpB could be found also in culture supernatants of N. meningitidis. We investigated the secretion mechanism of LbpB and its effect on the immunological response to the protein.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The N. meningitidis strains used are listed in Table 1 . The strains were grown on GC agar plates (Oxoid) supplemented with Vitox (Oxoid) at 37°C in candle jars, or in tryptic soy broth (TSB) (Gibco-BRL) at 37°C with mild shaking. To impose iron limitation, bacteria, grown overnight on plates, were inoculated in TSB supplemented with 20 μg of ethylenediamine di-o-hydroxyphenylacetic acid (EDDHA; Sigma)/ml to an optical density at 550 nm (OD550) of 0.1 and grown for 5 h. For the growth curve experiment under iron limitation, a preculture grown for 3 h in TSB plus EDDHA was diluted in fresh medium to an OD550 of 0.2, and growth was monitored for 7 h. Except where noted, IPTG (isopropyl-β-d-thiogalactopyranoside) was added to a final concentration of 1 mM to induce nalP or lbpB expression from the lac promoter on the plasmids listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| HB-1 | Unencapsulated derivative of H44/76 | 8 |
| HB-1 lbpAB::kan | Deletion of lbpA and lbpB | J. Kortekaas |
| HB-1 nalP::kan | Insertion of Kanr cassette in nalP | 37 |
| HB-1 app::kan | Insertion of Kanr cassette in app | 37 |
| HB-1 ausI::kan | Insertion of Kanr cassette in ausI | 35 |
| HB-1 iga::kan | Insertion of Kanr cassette in iga | 37 |
| H44/76 | 17 | |
| H44/76 nalP::kan | Insertion of Kanr cassette in nalP | 37 |
| Plasmids | ||
| pEN300 | Camr; encodes wild-type NalP | 37 |
| pEN305 | Camr; encodes active-site mutant NalP | 37 |
| pENLbpB(BNCV) | Camr; encodes LbpB from strain BNCV | R. Voulhoux |
Camr, chloramphenicol resistance; Kanr, kanamycin resistance.
Collection of cells and culture supernatants.
Cells were harvested by centrifugation (4,500 × g, 5 min) and resuspended in water to an OD550 of 10. The culture supernatants were centrifuged again (16,000 × g, 5 min) to remove residual cells. The protein content was precipitated by adding ice-cold trichloroacetic acid to a final concentration of 5% and incubation for at least 30 min at 4°C. Samples were centrifuged (16,000 × g, 20 min), and the pellets were washed with 90% acetone and dissolved in water. Relative to the original cultures, the supernatant fractions were 10-fold more concentrated than the cell lysates. The protein preparations were then mixed with an equal volume of 2-fold-concentrated sample buffer and boiled for 10 min.
To obtain large quantities of released LbpB in its native conformation, the culture supernatant of an IPTG-induced 500-ml culture of the unencapsulated strain HB-1 containing pENLbpB(BNCV) was isolated and concentrated 40-fold with 10-kDa cutoff centrifugal filter units (Millipore). To avoid proteolytic degradation, a protease inhibitor cocktail (Complete EDTA free; Roche) was added to the concentrated supernatant. Blebs were removed from the concentrated supernatant by ultracentrifugation for 1 h at 150,000 × g.
Antisera.
The rabbit anti-LbpB antiserum R1 was described previously (27). The mouse antiserum directed against fHbp, the rabbit antiserum recognizing TbpA and TbpB, and the bactericidal monoclonal antibodies directed against PorA were generously provided by GlaxoSmithKline (Rixensart, Belgium), A. Schryvers (University of Calgary, Calgary, Alberta, Canada), and the Netherlands Vaccine Institute (Bilthoven, Netherlands), respectively.
Electrophoresis and immunoblotting.
For analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), protein samples were loaded on a gel with 8% (wt/vol) polyacrylamide in the running gel. To detect the folded form of the LbpB protein, sample buffer with only 0.1% SDS and no reducing agent and gels without SDS were used, and the sample was not heated before electrophoresis (seminative SDS-PAGE). After electrophoresis, proteins were blotted onto a 0.45-μm-pore-size Protran filter (Schleicher & Schuell, Dassel, Germany) using the Protean III minigel blotting system (Bio-Rad Laboratories, Veenendaal, Netherlands) at 100 V for 1 h. Nonspecific binding of antibodies to the filters was prevented by overnight incubation in phosphate-buffered saline (PBS; pH 7.0) supplemented with 0.5% Protifar (Nutricia, Zoetermeer, Netherlands) and 0.1% Tween 20 (Merck). The sera were diluted 1:5,000 or 1:20,000 in the same buffer and applied for 1 h to the blots. After extensive washing, the blots were incubated with goat anti-rabbit IgG or goat anti-mouse IgG secondary antibodies conjugated to horseradish peroxidase (Biosource, Camarillo, CA) at a dilution of 1:10,000 in the same buffer. Binding of antibodies was visualized by chemiluminescence using an ECL kit (Amersham, Buckinghamshire, United Kingdom).
In-gel trypsin digestion and mass spectrometry.
Selected protein bands were excised from a Coomassie R250-stained SDS-PAGE gel and cut into 1-mm3 cubes. The gel pieces were destained by repeated incubation in 25 mM NH4HCO3-50% acetonitrile and then dehydrated in 100% acetonitrile. Shrunken gel pieces were dried in a Speed-Vac (Eppendorf) and then resuspended in 15 μl of trypsin solution (20 mg of trypsin/ml in 50 mM NH4HCO3) (Promega) for 1 h, followed by the addition of 25 μl of 50 mM NH4HCO3 buffer to completely immerse the gel pieces. After incubation overnight at room temperature, the protein samples were eluted with two washes of 0.1% trifluoroacetic acid-50% acetonitrile, dried in a Speed-Vac, and resuspended in 10 μl of 50 mM NH4HCO3. The peptides were purified by using a C18 Ziptip (Millipore) and analyzed using an Applied Biosystems 4800 MALDI-TOF-TOF apparatus. For the matrix-assisted laser desorption ionization (MALDI) analysis, the samples were spotted using a matrix (7 mg of α-cyano-4-hydroxycinnamic acid/ml in 10 mM ammonium phosphate-50% acetonitrile) and analyzed in positive mode. Mass spectra were searched by using the Mascot engine against the GenBank NR database. For each peptide mass fingerprint search, the mass tolerance was set to 0.05 Da. One missed tryptic cleavage was allowed. The mass tolerance for database searching with tandem mass spectrometry (MS/MS) spectra was set to 0.3 Da. All of the proteins listed were identified with a confidence interval of 95% from the MS and/or MS/MS analysis.
Lactoferrin binding.
To study the binding of lactoferrin to LbpB on blots, concentrated neisserial culture supernatant was separated by using seminative SDS-PAGE and blotted onto a nitrocellulose membrane as described above. Nonspecific binding of proteins to the filters was prevented by overnight incubation in PBS containing 0.6% Protifar and 0.1% (vol/vol) Tween 20 (blocking buffer). The blots were subsequently incubated with peroxidase-conjugated human lactoferrin (3.4 μg/ml in blocking buffer) (30) for 1 h at room temperature. After three washing steps of 5 min each in blocking buffer, the activity of peroxidase was detected by chemiluminescence using an ECL kit (Amersham).
Serum bactericidal assay.
Serum bactericidal assays were performed as described previously (27) with some adaptations. Bacteria grown overnight on plates were inoculated in TSB containing 0.05 mM IPTG and grown with mild shaking at 37°C for ∼2 h until they reached an OD550 of at least 0.6. Sera were inactivated for 30 min at 56°C. The sera were subsequently diluted 100-fold in Hanks balanced salt solution (HBSS; Gibco)-0.3% bovine serum albumin (BSA) and then serially diluted in a volume of 50 μl in sterile U-bottom 96-well microtiter plates (Nunc). Bacteria were diluted in HBSS-0.3% BSA to yield ∼13,000 CFU/ml. Of this dilution, 37.5 μl was added to the serum dilutions. Subsequently, 12.5 μl of baby rabbit complement (generous gift from GlaxoSmithKline) or, as a control for toxicity of the sera, heat-inactivated (30 min at 56°C) complement was added to the wells. The plate was incubated for 1 h at 37°C with shaking (60 rpm). Of each well, 15 μl was spotted onto a GC plate. Plates were tilted to allow the drop to run down the plate. After overnight incubation at 37°C in candle jars, the colonies were counted, and the percentage of killing was calculated.
RESULTS
NalP-mediated release of LbpB from the cell surface.
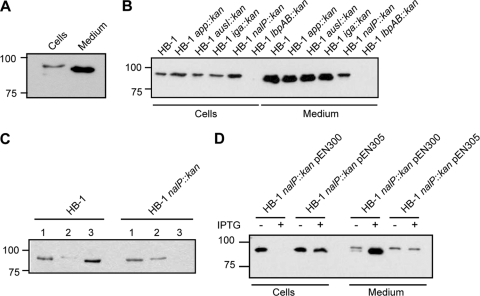
In the supernatant of iron-depleted cultures of N. meningitidis HB-1, an unencapsulated derivative of disease isolate H44/76, we often detected a secreted protein that was recognized by LbpB-specific antibodies (Fig. 1 A). This protein migrated slightly faster in SDS-PAGE than the LbpB found associated with the cells. Quantification of the blots revealed that ∼60% of the total amount of LbpB produced was in the medium. We assumed that a specific protease could mediate the proteolytic release of LbpB from the cell surface. A prime candidate would be the autotransporter NalP, since this is a cell-surface-exposed protease that is known to proteolytically cleave other cell-surface-exposed autotransporters, i.e., IgA protease, App, and AusI (35, 37). However, these NalP substrates themselves also contain serine-protease motifs and could, therefore, be responsible for the proteolytic release of LbpB as well. Therefore, we assessed whether any of these autotransporters was responsible for the release of LbpB. HB-1 and its nalP::kan, app::kan, iga::kan, and ausI::kan derivatives were grown in the presence of the iron chelator EDDHA to induce LbpB production. Cells and supernatants were collected and analyzed for the presence of LbpB by Western blotting with a polyclonal antiserum raised against LbpB. LbpB was detected in both the whole-cell lysates and the culture supernatants (Fig. 1B). In the whole-cell lysates, LbpB was detected as a band with an apparent molecular weight of ∼90,000 that was not detected in the lbpAB knockout strain (Fig. 1B). However, higher amounts of LbpB were detected in the whole-cell lysate of strain HB-1 nalP::kan than in those of the other strains. In the culture supernatants of HB-1 and most of its derivatives, two forms of LbpB were detected: a higher form of ∼90,000 corresponding to the LbpB form found in whole-cell lysates and a much more prominent band of ∼86,000 that likely represents a processed LbpB released into the medium (Fig. 1B). However, this ∼86K form appeared absent in the culture supernatant of the nalP::kan mutant, suggesting a role for NalP in the secretion of LbpB. The supernatant of the nalP::kan strain did contain the ∼90K form. This form could be pelleted from the supernatant by ultracentrifugation step at 150,000 × g (Fig. 1C), indicating that it corresponds to LbpB present in outer membrane blebs, which are abundantly shed off from the meningococcal cell surface. Overall, these results support our supposition that the release of LbpB from the cell surface is mediated by NalP.
FIG. 1.
(A) Western blot of whole-cell lysate and spent medium of strain HB-1. (B) Western blot of whole-cell lysates and spent media of strain HB-1 and various mutant derivatives indicated above the lanes. (C) Western blot of spent media of HB-1 and HB-1 nalP::kan (lanes 1), blebs isolated from that media by high-speed ultracentrifugation (lanes 2), and the supernatant after blebs isolation (lanes 3). (D) Western blot of whole-cell lysates and spent media of HB-1 nalP::kan overexpressing NalP or its active-site mutant derivative from the plasmids pEN300 and pEN305, respectively. Cells were grown in the presence or absence of IPTG as indicated. In all panels, cells were grown in the presence of EDDHA to impose iron limitation, and the blots were probed with anti-LbpB antiserum. Note that, relative to the original cultures, the supernatant samples were 10-fold more concentrated than the whole-cell lysates.
To assess directly whether the release of LbpB is mediated by NalP, we expressed wild-type NalP or its active-site mutant derivative NalPS427A from the neisserial expression plasmids pEN300 and pEN305, respectively, in the nalP::kan mutant strain. These plasmids contain the nalP alleles under the control of an IPTG-inducible lac promoter (37). Western blot analysis showed that all of the LbpB was released from the cell surface and processed into the ∼86K form when NalP was overproduced from pEN300 (Fig. 1D). In contrast, when the NalPS427A mutant protein was produced, LbpB was mainly detected in the whole-cell lysates in the ∼90K form, with minor leakage of that form into the medium presumably via blebs (Fig. 1D). Taken together, these results confirmed that LbpB of HB-1 is secreted into the medium as a result of the proteolytic activity of NalP.
Other neisserial lipoproteins are not released by NalP.
Both LbpB and NalP are lipoproteins containing a lipid moiety at the N terminus that anchors them to the outer membrane (29, 37). Both proteins are processed by NalP near their N termini, resulting in their release from the cell surface (37; see also above). Therefore, we considered the possibility that NalP-mediated cleavage is a general release mechanism for surface-exposed lipoproteins of N. meningitidis. To investigate this possibility, we studied the fate of two additional cell-surface-exposed lipoproteins, the transferrin-binding protein TbpB and the factor H-binding protein fHbp, which is a key inhibitor of the alternative complement-activation pathway mediating escape from killing by the innate immune system (22). Of note, fHbp has also been detected in the supernatant of the N. meningitidis strain MC58 (23). Western blot analysis with an antiserum that recognizes both the TbpA and TbpB proteins showed that the vast majority of TbpB was retained at the cell surface, regardless of the presence or absence of NalP (Fig. 2 A). Minor amounts of TbpB were detected in the culture supernatant (Fig. 2A, note the 10-fold loading difference), but this form had the same apparent molecular weight as the cell-associated form and, in addition, TbpA was released to a similar extent in the supernatant fraction (Fig. 2A). Therefore, this form of TbpB is probably associated with outer membrane blebs. As with TbpB, some fHbp was detected in the culture supernatant, but the amounts released were not affected by expression of NalP (Fig. 2B). These results showed that although NalP may process other lipoproteins, it certainly is not a general releasing factor for all of the lipoproteins exposed at the cell surface.
FIG. 2.
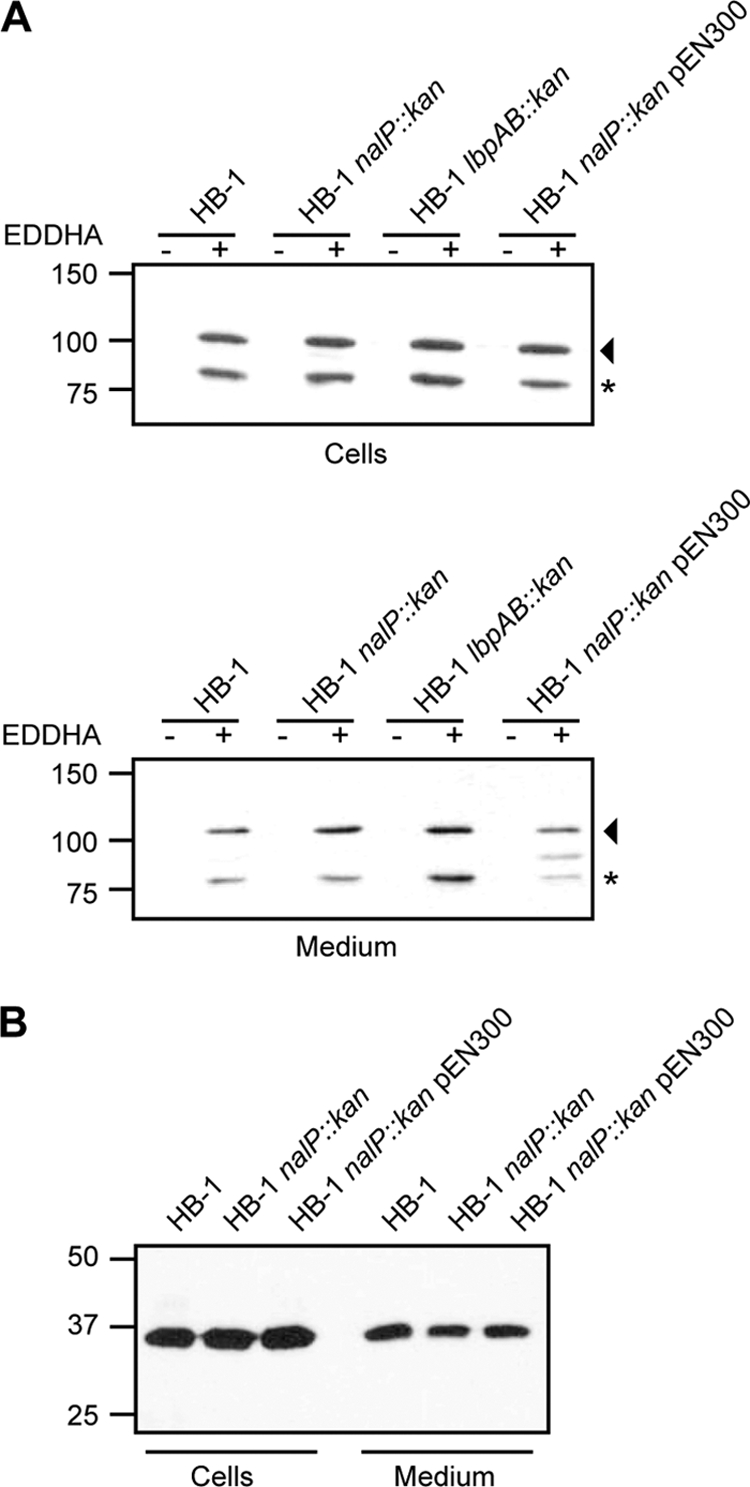
(A) Western blot analysis of whole-cell lysates (upper panel) and spent media (lower panel) of strains HB-1, HB-1 nalP::kan complemented or not with the plasmid pEN300, and HB-1 lbpAB::kan, using an antiserum recognizing both TbpA and TbpB. The arrowhead and the asterisk at the right of each panel mark TbpA and TbpB, respectively. The band in between TbpA and TbpB in the utmost right lane in the lower panel represents LbpB, with which the antiserum weakly cross-reacts (results not shown). (B) Western blot analysis of whole-cell lysates and spent media of strain HB-1 and its nalP knockout derivative complemented or not with plasmid pEN300. The blot was probed with mouse antiserum directed against fHbp. Note that, relative to the original cultures, the supernatant samples were 10-fold more concentrated than the whole-cell lysates.
The release of LbpB from the cell surface protects N. meningitidis against bactericidal antibodies.
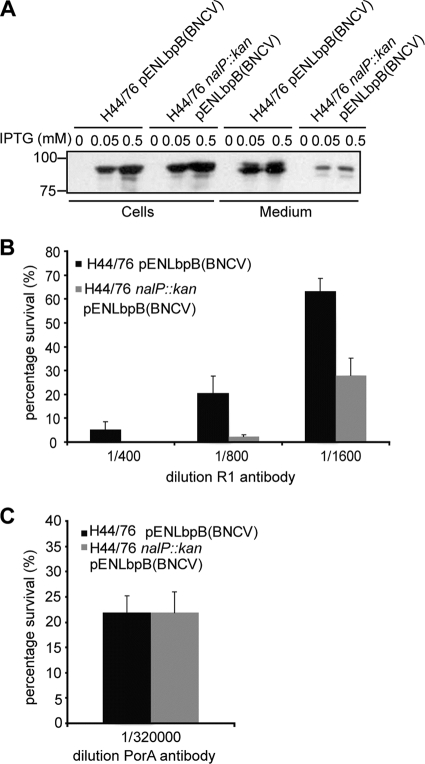
In view of the requirement of iron for growth, it seems counterintuitive that the bacteria release a factor that is thought to be part of the lactoferrin receptor complex. However, LbpB is immunogenic in humans (27), and its NalP-mediated release could potentially avert antibody-mediated host responses to LbpB. We tested this supposition by assessing the influence of NalP expression on the bactericidal activity of antibodies directed against LbpB. The anti-LbpB serum R1 that we used was raised against LbpB of strain BNCV. It does cross-react with H44/76-derived LbpB on Western blots but not in bactericidal assays (27). Therefore, we expressed BNCV-derived LbpB in the capsulated strain H44/76 to address whether NalP-mediated release of LbpB would protect the bacteria from the bactericidal activity of antibody plus complement. Thus, we transformed H44/76 and its nalP::kan derivative with pENLbpB(BNCV), which carries the lbpB gene of BNCV under the control of the IPTG-inducible lac promoter. Growing the cells under iron-replete conditions prevented expression of the chromosomally encoded lbpB. At both 0.05 and 0.5 mM IPTG, LbpB was released into the medium of the NalP+ strain, although considerable amounts of LbpB could still be detected in the whole-cell lysates, which suggests that the level of expression of the chromosomal nalP is rate-limiting for the release of LbpB (Fig. 3 A). As expected, hardly any LbpB was released into the medium of the nalP mutant strain (Fig. 3A). We performed a serum bactericidal assay on cells that had been induced for LbpB expression with 0.05 mM IPTG with the bactericidal anti-LbpB antiserum R1 (Fig. 3B). Killing of H44/76 by R1 required a higher concentration of the serum than was needed to obtain a similar degree of killing of H44/76 nalP::kan (Fig. 3B). A bactericidal anti-PorA monoclonal antibody was used as a positive control and resulted in equal killing of both strains (Fig. 3C). Apparently, the NalP-mediated release of LbpB into the medium resulted in sufficiently lower levels of cell surface-exposed LbpB to provide a considerable degree of protection against LbpB-specific bactericidal antibodies. The remaining bactericidal activity on the H44/76 cells could be explained by the considerable levels of LbpB that remained present at the cell surface under the LbpB overproduction conditions even when NalP was expressed (Fig. 3A). Overall, the results suggest that the release of LbpB by NalP could protect N. meningitidis from the immune system during infection and/or colonization.
FIG. 3.
(A) Western blot analysis of the whole-cell lysates and spent media of the strains used for the serum bactericidal assays, i.e., H44/76 pENLbpB(BNCV) and H44/76 nalP::kan pENLbpB(BNCV) induced or not with IPTG. The IPTG concentrations used are indicated above the panels. The blot was probed with anti-LbpB antiserum. (B) Results of the serum bactericidal assay on H44/76 pENLbpB(BNCV) (▪) and H44/76 nalP::kan pENLbpB(BNCV) (░⃞) with several dilutions of the polyclonal anti-LbpB antiserum R1. (C) Results of the serum bactericidal assay on H44/76 pENLbpB(BNCV) (▪) and H44/76 nalP::kan pENLbpB(BNCV) (░⃞) with a monoclonal antibody directed against PorA. The results shown in panels B and C are averages of three independent experiments performed in duplicate with the standard deviations indicated.
Released LbpB is cleaved near the N terminus.
The expression of BNCV-derived LbpB from plasmid yielded amounts of LbpB released into the medium that were detectable on a Coomassie blue-stained SDS-PAGE gel. We applied mass spectrometry to analyze the differences between this released LbpB and LbpB recombinantly produced in Escherichia coli (27). The LbpB bands were excised from SDS-PAGE gels and treated with trypsin, and the resulting peptides were analyzed by using MALDI-TOF-TOF. Both samples yielded peptide mass fingerprints that positively identified the proteins as LbpB from BNCV, which was confirmed by the sequence analysis of multiple peptide peaks. In the recombinant LbpB sample, however, peaks were detected that were absent in LbpB from the medium sample. These peaks contained peptides corresponding to residues 43 to 71 and residues 45 to 71 (S43KD45VPTPPPAKPSIEITPVNRPAVGAAMR71). A peak corresponding to residues 75 to 81 (RNTAFHR) was identified in both samples. Thus, the NalP-mediated cleavage targets a sequence between residue 19, i.e., the N-terminal lipidated cysteine after cleavage by signal peptidase II, and residue 75.
The release of LbpB occurs in the late growth phase.
N. meningitidis presumably needs lactoferrin as an iron source during colonization of the mucosal surfaces. Iron requirement might be more important early during colonization, while evasion from antibodies likely is important at later stages. To investigate whether LbpB release is influenced by the growth phase, we performed a growth experiment and took samples every hour for 7 h (Fig. 4 A). Western blot analysis showed that LbpB was released at later growth stages (Fig. 4B). Consistently, Western blot analysis with an antiserum that recognizes the translocator domain of NalP indicated that the levels of NalP also increased during growth (Fig. 4C). Particularly, cell-associated full-length NalP was found to accumulate during growth (Fig. 4C). This cell-associated form of NalP, rather than its secreted passenger domain, is probably responsible for LbpB release.
FIG. 4.
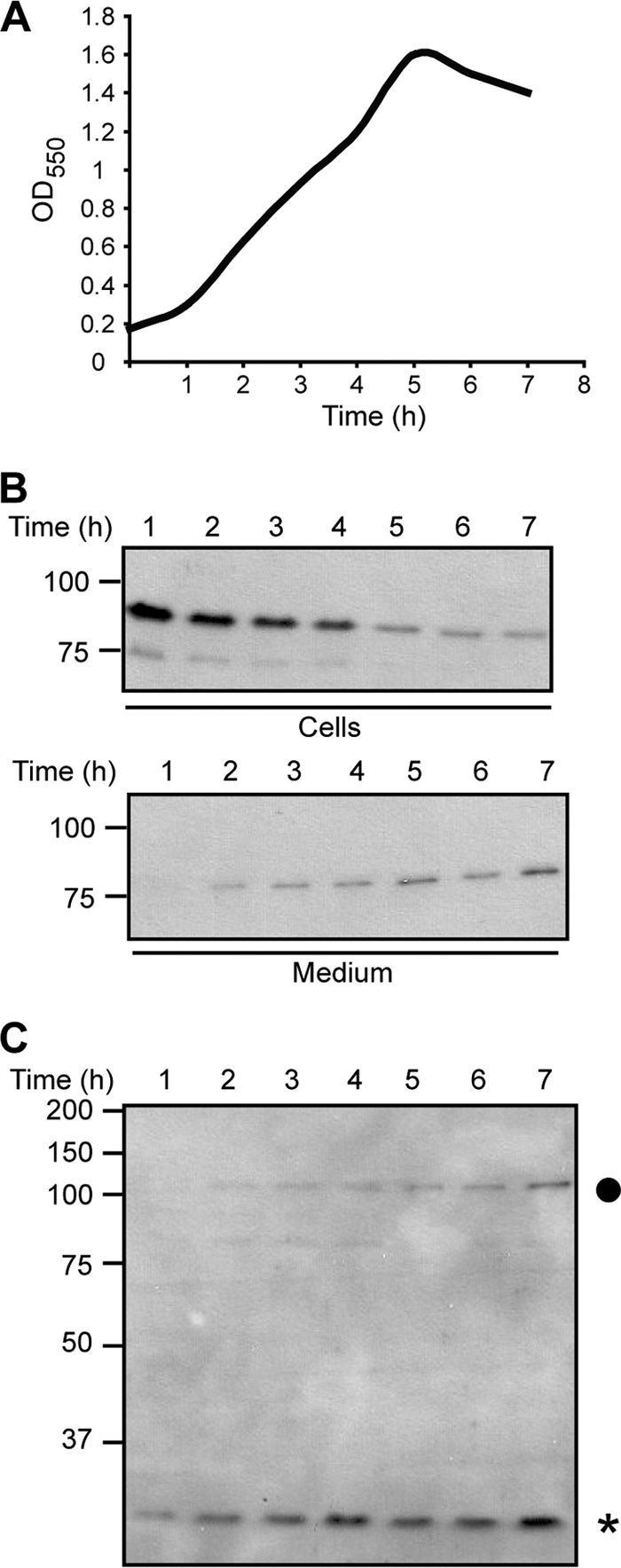
(A) Growth curve of strain HB-1. Cells were grown for 7 h in the presence of EDDHA to impose iron limitation. Samples were taken every hour for Western blot analysis. (B) Western blot analysis of whole-cell lysates (upper panel) and spent media (lower panel) using an antiserum recognizing LbpB. (C) Western blot analysis of whole-cell lysates using an antiserum recognizing the translocator domain of NalP. An asterisk indicates the NalP translocator domain, and a dot indicates full-length NalP.
Released LbpB is still able to bind lactoferrin.
We next sought to determine whether the truncated LbpB that is released into the medium could still bind lactoferrin. Previously, we demonstrated that the intact LbpB is heat modifiable, i.e., the folded form migrates faster during nondenaturing SDS-PAGE than the denatured form, and that lactoferrin can bind to the nondenatured form on a blot (29). To investigate whether the released truncated form of LbpB can bind lactoferrin, concentrated culture supernatant of strain HB-1 carrying pENLbpB(BNCV) was separated by SDS-PAGE either under denaturing conditions or under nondenaturing conditions. The proteins were blotted to nitrocellulose membranes, which were either incubated with LbpB-specific antiserum or with peroxidase-conjugated lactoferrin. The immunoblot revealed that the released LbpB is heat modifiable (Fig. 5) as reported previously for the intact LbpB (29), indicating this truncated form has retained its native folded conformation. Furthermore, lactoferrin preferentially bound to the nondenatured form (Fig. 5). Thus, the released LbpB has retained its ability to bind lactoferrin.
FIG. 5.
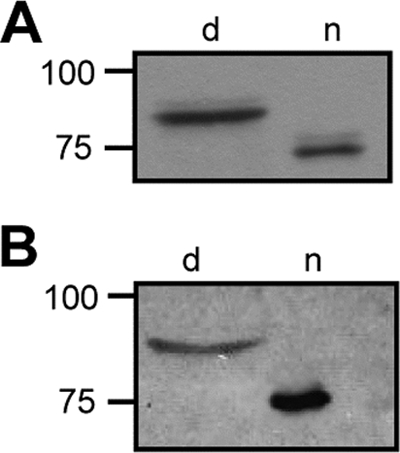
(A) Western blot analysis of concentrated supernatant of the strain HB-1 overexpressing LbpB(BNCV) from plasmid. Protein samples were incubated either at 100°C (lane d) or at room temperature (lane n) prior to seminative SDS-PAGE. The blot was probed with anti-LbpB antiserum. (B) Lactoferrin binding assay on the same samples as in panel A. The blot was incubated with peroxidase-coupled human lactoferrin.
DISCUSSION
Lactoferrin is found in phagocytic cells and in body fluids such as milk, mucus, and tears. To satisfy its need for iron, N. meningitidis expresses a lactoferrin receptor complex consisting of LbpA and LpbB. The receptor is specific for human lactoferrin and is, therefore, thought to be one of the reasons for the host specificity of these bacteria (18). The role of the LbpB protein in the complex is puzzling. The LbpB protein shares sequence similarity with the transferrin-binding protein B (TbpB). Both are lipoproteins and appear to facilitate a more efficient uptake of iron from their respective ligands (2, 7). LbpB is not essential for the acquisition of iron from lactoferrin in N. meningitidis since an lbpB mutant, in contrast to an lbpA mutant, can still grow on lactoferrin as an iron source (7, 29). Nevertheless, lactoferrin-binding activity of LbpB has been demonstrated in vitro (29) and is possibly mediated via its two long stretches of acidic amino acids, which could bind the positively charged ligand thereby bringing the lactoferrin in close contact with LbpA. In various meningococcal isolates, the gene cluster encoding the lactoferrin receptor is generally present, but this is not the case in the closely related gonococci (1). Half of the clinical isolates of N. gonorrhoeae lack the complete cluster, whereas the others possess lbpA, but only 30% of them possess lbpB (1). However, the overall presence of lbpB in N. meningitidis isolates suggests an important role for the protein in colonization or infection, which may be different from acting as an accessory factor for acquiring iron from lactoferrin.
In the present study we show that LbpB is released from the cell surface of N. meningitidis H44/76 through proteolytic cleavage by the autotransporter NalP and that this release protects the bacteria against the complement-mediated killing by anti-LbpB antibodies. Sera from convalescent patients contain antibodies that recognize LbpB, suggesting that LbpB is a target for the immune system during infection (27). Since LbpB is not essential for iron utilization from lactoferrin, we propose that the NalP-mediated release of LbpB could regulate the availability of LbpB on the cell surface in order to prevent killing of N. meningitidis during infection. It is interesting that the nalP gene is disrupted in all currently available gonococcal genome sequences (e.g., the open reading frames with locus tags NGK_0695 up to NGK_0699 in the genome sequence of N. gonorrhoeae strain NCCP11945). Hence, the absence of an lbpB gene in many gonococci (see above) may be related to the incapability of these bacteria to release the protein from their cell surface.
If LbpB is not essential for the utilization of lactoferrin as an iron source and if it is a target for the host immune response, why is the protein than expressed at all? What could be alternative functions for the protein? A clue might be that lactoferrin is known to have additional antimicrobial activities independent of its ability to chelate iron (4, 9, 24). For example, apolactoferrin interacts directly with the outer membrane of Gram-negative bacteria resulting in the release of lipopolysaccharides (11, 12). This bactericidal activity of lactoferrin is associated with a small positively charged peptide of approximately 47 amino acids, lactoferricin, that can be proteolytically released from the N terminus of the protein (5, 15). In Streptococcus pneumoniae, which is present in the same niche as N. meningitidis, it has been demonstrated that the surface-exposed protein PspA confers resistance to the bactericidal effects of lactoferrin by binding it, presumably on its active bactericidal site, thereby protecting the bacteria from the lethal action of lactoferrin and lactoferricin (33). Interestingly, it can also do so in soluble form. It is conceivable that LbpB could have a similar function in N. meningitidis (21), and our results indicate that the released LbpB retains its lactoferrin-binding properties.
Furthermore, lactoferrin was also shown to proteolytically cleave two factors, the autotransporters Hap and IgA1 protease, of Haemophilus influenzae important for the colonization of the nasopharynx (16). N. meningitidis expresses homologues of IgA1 protease and Hap (36), which also could be targets for the proteolytic activity of lactoferrin. The binding of lactoferrin to LbpB might prevent lactoferrin-mediated cleavage of these neisserial autotransporters.
Thus, even though Neisseria needs lactoferrin as an iron source during colonization of the mucosal surfaces, it should also be protected against its antimicrobial properties. Possibly, N. meningitidis needs to balance between protection against the bactericidal effect of lactoferrin and protection from antibody-mediated recognition of LbpB. NalP, the synthesis of which is phase-variable, can modulate the presence of LbpB at the bacterial cell surface. In the absence of NalP, all LbpB produced is at the cell surface where it binds lactoferrin, thereby presumably limiting its bactericidal effect. In the presence of NalP, most LbpB is removed from the cell surface rendering the bacteria less susceptible to bactericidal activity of antibodies directed against LbpB, but possibly more vulnerable against the bactericidal activity of lactoferrin. Alternatively, it is also possible that LbpB can still exert its postulated functions, both in protecting against lactoferrin and in iron acquisition, when it is present in the medium. In the latter case, LbpB could act like the hemophore HasA of Serratia marcescens, which captures free heme or extracts it from hemoglobin in the external medium and presents it to a specific outer membrane receptor (14). Similarly, released LbpB might bind lactoferrin in the medium and deliver it to the receptor LbpA in the outer membrane, for which it has affinity (31). Clearly, to fully understand the role of the NalP-mediated release of LbpB, we first need a better understanding of the role of LbpB in iron acquisition and other possible functions described above, which will be our next goal.
Acknowledgments
We thank Jeroen Kortekaas for providing strain HB-1 lbpAB::kan and Romé Voulhoux for providing the plasmid pENLbpB(BNCV). We thank Gregory Koningstein and Roel van der Schors for their help with the mass spectrometry.
This study was supported by the Research Council for Chemical Sciences with financial aid from the Netherlands Organization for Scientific Research and by the Netherlands Organization for Health Research and Development.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 26 April 2010.
REFERENCES
- 1.Anderson, J. E., M. M. Hobbs, G. D. Biswas, and P. F. Sparling. 2003. Opposing selective forces for expression of the gonococcal lactoferrin receptor. Mol. Microbiol. 48:1325-1337. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, J. E., P. F. Sparling, and C. N. Cornelissen. 1994. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J. Bacteriol. 176:3162-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archibald, F. S., and I. W. DeVoe. 1980. Iron acquisition by Neisseria meningitidis in vitro. Infect. Immun. 27:322-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold, R. R., M. Brewer, and J. J. Gauthier. 1980. Bactericidal activity of human lactoferrin: sensitivity of a variety of microorganisms. Infect. Immun. 28:893-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellamy, W., M. Takase, K. Yamauchi, H. Wakabayashi, K. Kawase, and M. Tomita. 1992. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta 1121:130-136. [DOI] [PubMed] [Google Scholar]
- 6.Biswas, G. D., and P. F. Sparling. 1995. Characterization of lbpA, the structural gene for a lactoferrin receptor in Neisseria gonorrhoeae. Infect. Immun. 63:2958-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonnah, R. A., and A. B. Schryvers. 1998. Preparation and characterization of Neisseria meningitidis mutants deficient in production of the human lactoferrin-binding proteins LbpA and LbpB. J. Bacteriol. 180:3080-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bos, M. P., and J. Tommassen. 2005. Viability of a capsule- and lipopolysaccharide-deficient mutant of Neisseria meningitidis. Infect. Immun. 73:6194-6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bullen, J. J. 1975. Iron-binding proteins in milk and resistance to Escherichia coli infection in infants. Postgrad. Med. J. 51(Suppl. 3):67-70. [PubMed] [Google Scholar]
- 10.Cornelissen, C. N., G. D. Biswas, J. Tsai, D. K. Paruchuri, S. A. Thompson, and P. F. Sparling. 1992. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J. Bacteriol. 174:5788-5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellison, R. T., III, T. J. Giehl, and F. M. LaForce. 1988. Damage of the outer membrane of enteric Gram-negative bacteria by lactoferrin and transferrin. Infect. Immun. 56:2774-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellison, R. T., III, F. M. LaForce, T. J. Giehl, D. S. Boose, and B. E. Dunn. 1990. Lactoferrin and transferrin damage of the Gram-negative outer membrane is modulated by Ca2+ and Mg2+. J. Gen. Microbiol. 136:1437-1446. [DOI] [PubMed] [Google Scholar]
- 13.Finkelstein, R. A., C. V. Sciortino, and M. A. McIntosh. 1983. Role of iron in microbe-host interactions. Rev. Infect. Dis. 5(Suppl. 4):S759-S777. [DOI] [PubMed] [Google Scholar]
- 14.Ghigo, J. M., S. Létoffé, and C. Wandersman. 1997. A new type of hemophore-dependent heme acquisition system of Serratia marcescens reconstituted in Escherichia coli. J. Bacteriol. 179:3572-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gifford, J. L., H. N. Hunter, and H. J. Vogel. 2005. Lactoferricin: a lactoferrin-derived peptide with antimicrobial, antiviral, antitumor, and immunological properties. Cell. Mol. Life Sci. 62:2588-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendrixson, D. R., J. Qiu, S. C. Shewry, D. L. Fink, S. Petty, E. N. Baker, A. G. Plaut, and J. W. St Geme III. 2003. Human milk lactoferrin is a serine protease that cleaves Haemophilus surface proteins at arginine-rich sites. Mol. Microbiol. 47:607-617. [DOI] [PubMed] [Google Scholar]
- 17.Holten, E. 1979. Serotypes of Neisseria meningitidis isolated from patients in Norway during the first six months of 1978. J. Clin. Microbiol. 9:186-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, B. C., and A. B. Schryvers. 1988. Specificity of the lactoferrin and transferrin receptors in Neisseria gonorrhoeae. Mol. Microbiol. 2:827-829. [DOI] [PubMed] [Google Scholar]
- 19.Legrain, M., V. Mazarin, S. W. Irwin, B. Bouchon, M. J. Quentin-Millet, E. Jacobs, and A. B. Schryvers. 1993. Cloning and characterization of Neisseria meningitidis genes encoding the transferrin-binding proteins Tbp1 and Tbp2. Gene 130:73-80. [DOI] [PubMed] [Google Scholar]
- 20.Lewis, L. A., E. Gray, Y. P. Wang, B. A. Roe, and D. W. Dyer. 1997. Molecular characterization of hpuAB, the haemoglobin-haptoglobin-utilization operon of Neisseria meningitidis. Mol. Microbiol. 23:737-749. [DOI] [PubMed] [Google Scholar]
- 21.Ling, J. M., and A. B. Schryvers. 2006. Perspectives on interactions between lactoferrin and bacteria. Biochem. Cell Biol. 84:275-281. [DOI] [PubMed] [Google Scholar]
- 22.Madico, G., J. A. Welsch, L. A. Lewis, A. McNaughton, D. H. Perlman, C. E. Costello, J. Ngampasutadol, U. Vogel, D. M. Granoff, and S. Ram. 2006. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J. Immunol. 177:501-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masignani, V., M. Comanducci, M. M. Giuliani, S. Bambini, J. Adu-Bobie, B. Arico, B. Brunelli, A. Pieri, L. Santini, S. Savino, D. Serruto, D. Litt, S. Kroll, J. A. Welsch, D. M. Granoff, R. Rappuoli, and M. Pizza. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J. Exp. Med. 197:789-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masson, P. L., J. F. Heremens, and C. H. Dives. 1966. An iron-binding protein common to many external secretions. Clin. Chim. Acta 14:735-739. [Google Scholar]
- 25.Neilands, J. B. 1981. Microbial iron compounds. Annu. Rev. Biochem. 50:715-731. [DOI] [PubMed] [Google Scholar]
- 26.Pettersson, A., V. Klarenbeek, J. van Deurzen, J. T. Poolman, and J. Tommassen. 1994. Molecular characterization of the structural gene for the lactoferrin receptor of the meningococcal strain H44/76. Microb. Pathog. 17:395-408. [DOI] [PubMed] [Google Scholar]
- 27.Pettersson, A., J. Kortekaas, V. E. Weynants, P. Voet, J. T. Poolman, M. P. Bos, and J. Tommassen. 2006. Vaccine potential of the Neisseria meningitidis lactoferrin-binding proteins LbpA and LbpB. Vaccine 24:3545-3557. [DOI] [PubMed] [Google Scholar]
- 28.Pettersson, A., A. Maas, and J. Tommassen. 1994. Identification of the iroA gene product of Neisseria meningitidis as a lactoferrin receptor. J. Bacteriol. 176:1764-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pettersson, A., T. Prinz, A. Umar, J. van der Biezen, and J. Tommassen. 1998. Molecular characterization of LbpB, the second lactoferrin-binding protein of Neisseria meningitidis. Mol. Microbiol. 27:599-610. [DOI] [PubMed] [Google Scholar]
- 30.Pettersson, A., P. van der Ley, J. T. Poolman, and J. Tommassen. 1993. Molecular characterization of the 98-kilodalton iron-regulated outer membrane protein of Neisseria meningitidis. Infect. Immun. 61:4724-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prinz, T., M. Meyer, A. Pettersson, and J. Tommassen. 1999. Structural characterization of the lactoferrin receptor from Neisseria meningitidis. J. Bacteriol. 181:4417-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schryvers, A. B., and L. J. Morris. 1988. Identification and characterization of the human lactoferrin-binding protein from Neisseria meningitidis. Infect. Immun. 56:1144-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaper, M., S. K. Hollingshead, W. H. Benjamin, Jr., and D. E. Briles. 2004. PspA protects Streptococcus pneumoniae from killing by apolactoferrin, and antibody to PspA enhances killing of pneumococci by apolactoferrin. Infect. Immun. 72:5031-5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stojiljkovic, I., V. Hwa, L. de Saint Martin, P. O'Gaora, X. Nassif, F. Heffron, and M. So. 1995. The Neisseria meningitidis haemoglobin receptor: its role in iron utilization and virulence. Mol. Microbiol. 15:531-541. [DOI] [PubMed] [Google Scholar]
- 35.van Ulsen, P., B. Adler, P. Fassler, M. Gilbert, M. van Schilfgaarde, P. van der Ley, L. van Alphen, and J. Tommassen. 2006. A novel phase-variable autotransporter serine protease, AusI, of Neisseria meningitidis. Microbes Infect. 8:2088-2097. [DOI] [PubMed] [Google Scholar]
- 36.van Ulsen, P., L. van Alphen, C. T. Hopman, A. van der Ende, and J. Tommassen. 2001. In vivo expression of Neisseria meningitidis proteins homologous to the Haemophilus influenzae Hap and Hia autotransporters. FEMS Immunol. Med. Microbiol. 32:53-64. [DOI] [PubMed] [Google Scholar]
- 37.van Ulsen, P., L. van Alphen, J. ten Hove, F. Fransen, P. van der Ley, and J. Tommassen. 2003. A neisserial autotransporter NalP modulating the processing of other autotransporters. Mol. Microbiol. 50:1017-1030. [DOI] [PubMed] [Google Scholar]
- 38.West, S. E., and P. F. Sparling. 1985. Response of Neisseria gonorrhoeae to iron limitation: alterations in expression of membrane proteins without apparent siderophore production. Infect. Immun. 47:388-394. [DOI] [PMC free article] [PubMed] [Google Scholar]