Abstract
Trypanosoma cruzi is a protozoan parasite that causes human Chagas’ disease, a leading source of congestive heart failure in Central and South America. CD8+ T cells are critical for control of T. cruzi infection, and CD8+ T cells recognizing the immunodominant trans-sialidase gene-encoded peptide TSKB20 (ANYKFTLV) account for approximately 30% of the total CD8+ T-cell population at the peak of infection in C57BL/6 mice. Type I interferons (IFN-I) are pleiotropic cytokines that play a critical role in both innate and adaptive immunity against a variety of infections, but their induction and their role in infection are dictated by the infectious agent. Because type I IFNs and IFN-responsive genes are evident early after T. cruzi infection of host cells, we examined the influence of IFN-I on the development of CD8+ T-cell responses during this infection. Mice lacking the receptor for IFN-I (IFNARKO) and their wild-type counterparts both developed chronic infections and generated similar frequencies of immunodominant TSKB20- and subdominant TSKB18-specific CD8+ T cells following T. cruzi infection. In contrast, peak TSKB20-specific CD8+ T-cell responses generated during infection with vaccinia virus engineered to express TSKB20 were approximately 2.5-fold lower in IFNARKO mice than B6 mice, although after viral clearance, the frequencies of TSKB20-specific CD8+ T cells stabilized at similar levels. Together, these data suggest that IFN-I induction and biology are dependent upon the microbial context and emphasize the need to investigate various infection models for a full understanding of CD8+ T-cell development.
CD8+ T cells are an essential contributor to the control of the intracellular protozoan pathogen Trypanosoma cruzi, the causative agent of human Chagas' disease. T. cruzi trypomastigotes can infect virtually any nucleated cell, and once in the host cell cytoplasm, transform into and replicate as amastigotes. The cytoplasmic localization of T. cruzi allows for parasite-derived proteins to be available for processing and presentation through the major histocompatibility complex class I (MHC-I) pathway, and obliteration of this pathway leads to early death for T. cruzi-infected mice (21, 22). T. cruzi-infected mice depleted of CD8+ T cells also succumb to infection during the acute phase (within 35 days after infection) (20). The annotated genome of T. cruzi contains more than 12,000 genes (5), yet despite this genetic complexity and the resultant enormous number of potential epitopes, the CD8+ T-cell response to T. cruzi is focused on only a few peptides. In B6 mice infected with the Brazil strain of T. cruzi, CD8+ T cells recognizing TSKB20 (ANYKFTLV) and TSKB18 (ANYDFTLV) expand significantly and represent over 40% of the total CD8+ T-cell population at the peak of the immune response (12).
Type I interferons (IFN-I) are a complex set of innate cytokines induced early after infection. IFN-β and the multiple IFN-α species all act through a heterodimeric receptor (IFNAR) and have pleiotropic effects on both innate and adaptive immune responses, including MHC upregulation, B-cell isotype switching, and NK cell activation, as well as providing direct survival signals to CD8+ and CD4+ T cells (16). Although type I IFNs appear to be essential for antiviral innate response (19), their necessity in other infections is less well documented. Recently, type I interferons have also been shown to govern the expansion of antiviral CD8+ T cells (1, 7, 8, 10, 18).
Global cytokine analysis of T. cruzi-infected cells has demonstrated that infection of host cells in vitro by T. cruzi is relatively “quiet,” with the most notable response being the production of IFN-I and IFN-I-responsive genes (ISGs) (24). In vivo studies have also shown early induction of IFN-I and ISGs (3, 17). Given the evidence for early production of IFN-I in T. cruzi infection and the role this cytokine has been shown to play in the generation of protective immunity in other infection models, in this study we have examined the contribution of IFN-I to the immunodominant CD8+ T-cell response to T. cruzi.
MATERIALS AND METHODS
Mice, parasites, and virus.
C57BL6 mice and IFNARKO mice were bred at the University of Georgia and were housed in microisolator cages under specific-pathogen-free conditions. Tissue culture trypomastigotes of T. cruzi (Brazil strain) were maintained by passage through Vero cells. Vaccinia virus expressing TSKB20 under a secretory signal (TSKB20-ES-VV; a kind gift of Jonathon Yewdell, NIH) were grown and titrated in thymidine kinase-deficient 143B cells. Mice were infected intraperitoneally (i.p.) with 1,000 tissue culture trypomastigotes of T. cruzi or 2 × 106 PFU of TSKB20-ES-VV. Mice were sacrificed by CO2 inhalation followed by cervical dislocation. All animal protocols were previously approved by the University of Georgia Institutional Animal Care and Use Committee.
MHC-I peptide tetramer staining and surface phenotyping.
MHC-I tetramers were synthesized at the Tetramer Core Facility (Emory University, Atlanta, GA). The tetramers used in these studies were TSKB18 (ANYDFTLV/Kb) and TSKB20 (ANYKFTLV/Kb). In kinetics experiments, 10 μl of mouse peripheral blood was obtained via the tail vein and collected in Na citrate solution. In other experiments, mouse splenocytes were analyzed. Single-cell suspensions of whole blood or splenocytes were washed in phosphate azide buffer (PAB) (1% sodium azide and 0.5% bovine serum in phosphate-buffered saline), stained for 45 min at 4°C with TSKB20/Kb-phycoerythrin (PE), TSKB18/Kb-allophycocyanin (APC), anti-CD8 Pacific Blue (BD Pharmingen, San Diego, CA), and a cocktail of Cy5-PE-labeled anti-CD4, anti-CD11b, and anti-B220 for use as an exclusion channel. For surface phenotyping, MHC-I tetramer staining was performed as described above, and at the same time, cells were incubated with CD44-fluorescein isothiocyanate (FITC), CD127-PE-Cy7, CD62L-APC, or KLRG-1- APC (all from BD Pharmingen). Whole blood was lysed in a hypotonic ammonium chloride solution after surface staining. At least 50,000 cells were acquired on a BD FacsCalibur flow cytometer (BD) and then analyzed with FlowJo (Tree Star, Ashland, OR) using a biexponential transformation.
ICCS.
For intracellular cytokine staining (ICCS), splenocytes were isolated from mice and plated at 106 cells/ml in a 96-well round-bottom plate with the indicated peptide(s) at a 10 μM concentration. GolgiPlug (BD Pharmingen) was added as per the manufacturer's instructions (1:1,000 dilution) to block cytokine secretion, and cells were incubated at 37°C and 5% CO2 for 5 h. Following stimulation, the cells were washed with phosphate azide buffer and then fixed and permeabilized and stained with fluorescent-conjugated antibodies anti-CD8-FITC and anti-IFN-γ-APC. The cells were stained on ice (4°C) in the dark for 30 min. Cell characteristics were then acquired on a BD FacsCalibur flow cytometer (San Diego, CA) or a Cyan flow cytometer (Cytomation, Fort Collins, CO), and the data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Statistical analysis.
Statistical analyses were performed using a Student's t test. P values of ≤0.05 were considered statistically significant.
RESULTS
Generation of T. cruzi-specific CD8+ T cells is unaffected in hosts deficient in IFN-I signaling.
IFNARKO mice and wild-type (WT) B6 mice control infection with 1,000 Brazil strain T. cruzi trypomastigotes (100% survival to >200 days postinfection), allowing for the analysis of T. cruzi-specific CD8+ T cells throughout the course of this chronic infection. The frequencies of CD8+ T cells recognizing the immunodominant TSKB20 peptide and the subdominant TSKB18 peptide in serially collected whole-blood samples were nearly identical throughout the course of infection (Fig. 1), clearly demonstrating that IFN-I is not required for normal expansion of T. cruzi-specific CD8+ T cells.
FIG. 1.
Type I interferons (IFN-I) are not required for generation of T. cruzi-specific CD8+ T cells. Eight-week-old B6 (triangles) and IFNARKO (circles) mice were infected intraperitoneally (i.p.) with 1,000 Brazil strain tissue culture trypomastigotes. Whole blood from serially bled mice was stained with H-2Kb MHC-I tetramers bearing TSKB20 (closed symbols) or TSKB18 (open symbols), anti-CD8, and an exclusion channel containing PE-Cy5.5-labeled anti-CD4, anti-B220, and anti-CD11b. Values represent the mean of 8 mice per group with P > 0.1 for all time points.
Effector function of T. cruzi-specific CD8+ T cells is unaffected by deficient IFN-I signaling.
To determine if the lack of IFN-I signaling in CD8+ T cells affected the maintenance and effector function of CD8+ T cells responding to T. cruzi infection, we examined the ability of CD8+ T cells from chronically infected IFNARKO and B6 mice to mount recall responses to the same TS peptides. B6 and IFNARKO mice at 180 days postinfection had similar frequencies of IFN-γ-producing CD8+ T cells after stimulation with TSKB20 or TSKB18 and also maintained the same immunodominance pattern of TSKB20 > TSKB18 (Fig. 2).
FIG. 2.
Peptide-specific IFN-γ-producing CD8+ T cells are generated in the absence of IFN-I signaling following T. cruzi infection. Spleen cells were harvested from uninfected B6 (left column), wild-type B6 (middle column), and IFNARKO (right column) mice at 180 days after infection with 1,000 Brazil strain T. cruzi parasites. Frequencies of IFN-γ-producing CD8+ T cells were determined by intracellular cytokine staining after 5 h of in vitro stimulation with T. cruzi peptides as described in Materials and Methods. Values represent the percentage of IFN-γ+ cells among CD8+ T cells and are representative of a total of 4 mice. P = 0.83 for TSKB20-stimulated IFN-γ production, and P = 0.36 for TSKB18-stimulated IFN-γ production.
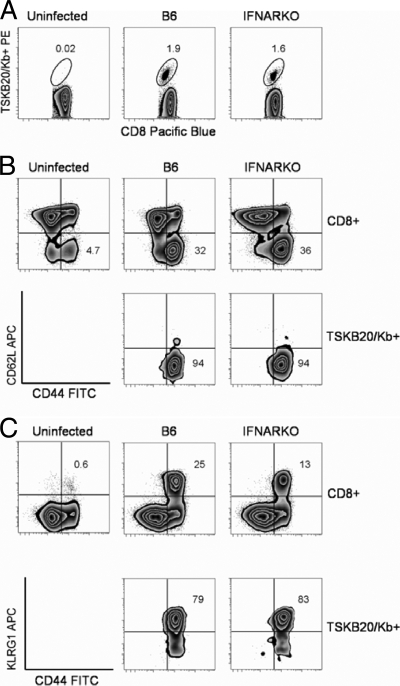
T. cruzi-specific CD8+ T cells from chronically infected mice display a primarily effector-memory (CD44hi CD62Llo CCR7lo CD127lo) phenotype (12). Using CD62L as a representative marker for effector/effector memory CD8+ T cells, we found that the same percentage of total CD8+ T cells (P = 0.77) and TSKB20-specific CD8+ T cells (P = 0.92) had a CD44hi CD62Llo phenotype in IFNARKO mice as in B6 mice (Fig. 3). However, a slightly greater percentage of TSKB20-specific cells expressed KLRG-1, an activation marker associated with chronic antigenic stimulation, in IFNARKO mice (80% CD44hi KLRG1hi) as compared to B6 mice (63% CD44hi KLRG1hi; P = 0.027) (Fig. 3), possibly suggesting a higher degree of antigen exposure in the IFNARKO mice.
FIG. 3.
The phenotype of T. cruzi-specific CD8+ T cells is unaltered in IFNARKO mice. Spleen cells were harvested from uninfected B6 (left column), wild-type B6 (middle column), and IFNARKO (right column) mice at 180 days after infection with 1,000 Brazil strain T. cruzi parasites. Surface staining with MHC-I tetramers and fluorochrome-conjugated antibodies was performed as described in Materials and Methods. (A) MHC-I tetramer staining of CD8+ T cells from uninfected mice, T. cruzi-infected B6 mice, or T. cruzi-infected IFNARKO mice. For B6 mice, the percentage of CD8+ IFN-γ+ cells was 3.1% ± 1.7%; for IFNARKO mice, the percentage of CD8+ IFN-γ+ cells was 3.4% ± 1.8% (P = 0.83; n = 4). Data are representative of two experiments. (B) Expression of CD62L (y axis) and CD44 (x axis) on total CD8+ T cells (top) or TSKB20-specific CD8+ T cells (bottom). (C) Expression of KLRG-1 (y axis) and CD44 (x axis) on total CD8+ T cells (top) or TSKB20-specific CD8+ T cells (bottom). The data presented show phenotyping for representative mice for each group (n = 5 or 6 mice per group).
IFN-I is critical for expansion of TSKB20-specific CD8+ T cells following TSKB20-VV infection.
To determine if the independence of the TSKB20-specific response from IFN-I signaling was specific for these epitopes or was related to the expression of these epitopes in T. cruzi, we examined the generation of TSKB20-specific T-cell responses to vaccinia virus expressing the TSKB20 epitope. In contrast to the equivalent response to TSKB20 seen in T. cruzi-infected mice, IFNARKO mice displayed a 2-fold-lower TSKB20-specific CD8+ T-cell response during acute TSKB20-VV infection (Fig. 4) relative to B6 mice. Following viral clearance in the mice infected with TSKB20VV, TSKB20-specific CD8+ T contract to approximately 1% of CD8+ T cells in both IFNARKO and B6 mice, as measured by MHC-I tetramer staining (Fig. 5 A) and peptide-induced IFN-γ responses (Fig. 5B). At this time point, the TSKB20-specific CD8+ T cells generated in TSKB20VV-infected IFNARKO and WT mice were also phenotypically indistinguishable with respect to CD44/CD62L expression (Fig. 5C).
FIG. 4.
IFNARKO mice develop lower peak TSKB20-specific responses following TSKB20-VV infection. Eight week old female B6 (triangles), male B6 (open circles), and male IFNARKO (diamonds) mice were infected i.p. with 2 × 106 PFU TSB20-VV. Because of the dearth of female IFNARKO mice in the colony, we used all males in the IFNARKO group and compared them to both male and female B6 mice. Mice were serially bled through the tail vein at the designated times, and whole blood was stained with H-2Kb MHC-I tetramers bearing TSKB20, anti-CD8, and an exclusion channel containing PE-Cy5.5-labeled anti-CD4, anti-B220-, and anti-CD11b. Values represent the mean of 4 to 6 mice per group and are representative of 2 experiments. P is <0.05 for time points before 30 days postinfection.
FIG. 5.
Peptide-specific IFN-γ-producing CD8+ T cells are generated in the absence of IFN-I signaling following TSKB20-VV infection. Spleen cells were harvested from uninfected B6 mice (left column) and B6 (middle column) and IFNARKO (right column) mice infected i.p. with 2 × 106 PFU TSB20-VV. (A) MHC-I tetramer staining of CD8+ T cells from uninfected mice, T. cruzi-infected B6 mice, or T. cruzi-infected IFNARKO mice. For B6 mice, the percentage of CD8+ IFN-γ+ mice is 1.0% ± 0.8%; for IFNARKO mice, the percentage of CD8+ IFN-γ+ mice is 1.2% ± 0.6% (P = 0.83; n = 12 mice). The data in the figure show one representative mouse per group. (B) Frequencies of IFN-γ-producing CD8+ T cells determined by intracellular cytokine staining after 5 h of in vitro stimulation with T. cruzi peptides as described in Materials and Methods. Values represent the percentage of IFN-γ+ cells among CD8+ T cells and are representative of a total of 4 mice (P > 0.1). (C) Expression of CD62L (y axis) and CD44 (x axis) on total CD8+ T cells (top) or TSKB20-specific CD8+ T cells (bottom). The data presented show phenotyping for representative mice for each group (n = 5 mice per group).
DISCUSSION
Type I interferons constitute a family of pleiotropic cytokines critical to host defense against a variety of pathogens. Virus-infected cells produce IFN-I, which acts in an autocrine or paracrine fashion to induce an antiviral state, a phenomenon described more than 50 years ago. IFNIRKO mice generally succumb to infection with highly virulent cytopathic viruses, but survive infection with less-cytopathic viruses such as lymphocytic choriomeningitis virus (LCMV), although with accompanying persistent infection and consequent functional exhaustion of T cells. In the case of infection with the intracellular bacterium Listeria monocytogenes, the absence of IFN-I signaling seems to be beneficial to the host (13).
More recently, the role of IFN-I in directing adaptive immune responses in hosts with intact IFN-I signaling has been appreciated (6). The immunomodulatory effects of IFN-I on T cells can occur indirectly through the effects of IFN-I on antigen-presenting cells (9, 11) or by the direct binding of IFN-I to receptors on T cells, generally enhancing but sometimes depressing T-cell function, depending on the context of exposure (16). IFN-I promotes T-cell expansion in several mouse models of infection, such as LCMV (1, 8, 23), VV (23), or Listeria monocytogenes (23). LCMV infection provides perhaps the most striking example of the dependence of CD8+ T-cell responses on IFN-I as CD8+ T cells specific for the immunodominant LCMV gp33 epitope fail to expand in the absence of IFN-I (1, 8, 23). Because IFN-β stands out as one of the few proteins secreted relatively early by T. cruzi-infected cells (24), we hypothesized that IFN-I would be critical for strong and immunodominant expansion of T. cruzi-specific CD8+ T cells observed in this infection.
The data presented herein demonstrate that, contrary to these expectations, T. cruzi-specific CD8+ T cells develop independently of IFN-I signaling. No differences in the frequencies or function of TSKB20- or TSKB18-specific CD8+ T cells between IFNARKO and B6 mice were observed throughout T. cruzi infection. In contrast, maximal expansion of TSKB20-specific CD8+ T cells in response to TSKB20-VV infection required type I IFN, similarly to the reported 2.5-fold-lower expansion of CD8+ T cells to either OVA-VV or GP33-VV infection in IFNARKO mice (23). These data confirm that responses against the same CD8+ T-cell epitope differ in their requirements for IFN-I signaling, depending on the pathogen expressing that epitope, and provide the first report that IFN-1 signaling is not critical for development of CD8+ T-cell responses against a protozoan parasite.
Analysis of high-dose infections with T. cruzi reveals upregulation of IFN-I and interferon-stimulated genes as the most prominent host cell response to infection (2-4, 24). If one excludes these cytokine-dependent responses, the host cell response to infection appears to be quite variable but with the common feature of changes in stress response genes and genes controlling cell growth (4). T. cruzi infection in vivo is also noteworthy in that systemic indicators of innate immune system stimulation are slow to appear and the expansion of pathogen-specific CD8+ T cells is quite sluggish relative to a number of other viral, bacterial, and parasitic infections (15). The fact that T. cruzi-specific CD8+ T cells do not appear to rely on IFN-I for their expansion and that IFNARKO mice survive T. cruzi infection implies that the fundamental mechanisms contributing to adaptive immunity in this system are independent of IFN-I. Furthermore, these results suggest that the some of the earliest events accompanying invasion (e.g., the production of type I IFN) are not sufficient to initiate what later becomes a very potent cellular and humoral response to T. cruzi. This result provides further support for the hypothesis that strong immune recognition and the generation of adaptive immune responses are delayed until a round of replication and the release of an amplified population of parasites occurs: some 4 to 5 days following the initial infection (14).
Acknowledgments
This work was supported by Public Health Service grants AI-022070 and AI-33106 from NIAID/NIH.
The assistance of Juan Bustamante with assembly of the figures is gratefully acknowledged.
Editor: J. H. Adams
Footnotes
Published ahead of print on 10 May 2010.
REFERENCES
- 1.Aichele, P., H. Unsoeld, M. Koschella, O. Schweier, U. Kalinke, and S. Vucikuja. 2006. CD8 T cells specific for lymphocytic choriomeningitis virus require type I IFN receptor for clonal expansion. J. Immunol. 176:4525-4529. [DOI] [PubMed] [Google Scholar]
- 2.Chessler, A. D., L. R. Ferreira, T. H. Chang, K. A. Fitzgerald, and B. A. Burleigh. 2008. A novel IFN regulatory factor 3-dependent pathway activated by trypanosomes triggers IFN-beta in macrophages and fibroblasts. J. Immunol. 181:7917-7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chessler, A. D., M. Unnikrishnan, A. K. Bei, J. P. Daily, and B. A. Burleigh. 2009. Trypanosoma cruzi triggers an early type I IFN response in vivo at the site of intradermal infection. J. Immunol. 182:2288-2296. [DOI] [PubMed] [Google Scholar]
- 4.Costales, J. A., J. P. Daily, and B. A. Burleigh. 2009. Cytokine-dependent and-independent gene expression changes and cell cycle block revealed in Trypanosoma cruzi-infected host cells by comparative mRNA profiling. BMC Genomics 10:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Sayed, N. M., P. J. Myler, D. C. Bartholomeu, D. Nilsson, G. Aggarwal, A. N. Tran, E. Ghedin, E. A. Worthey, A. L. Delcher, G. Blandin, S. J. Westenberger, E. Caler, G. C. Cerqueira, C. Branche, B. Haas, A. Anupama, E. Arner, L. Aslund, P. Attipoe, E. Bontempi, F. Bringaud, P. Burton, E. Cadag, D. A. Campbell, M. Carrington, J. Crabtree, H. Darban, J. F. da Silveira, P. de Jong, K. Edwards, P. T. Englund, G. Fazelina, T. Feldblyum, M. Ferella, A. C. Frasch, K. Gull, D. Horn, L. Hou, Y. Huang, E. Kindlund, M. Klingbeil, S. Kluge, H. Koo, D. Lacerda, M. J. Levin, H. Lorenzi, T. Louie, C. R. Machado, R. McCulloch, A. McKenna, Y. Mizuno, J. C. Mottram, S. Nelson, S. Ochaya, K. Osoegawa, G. Pai, M. Parsons, M. Pentony, U. Pettersson, M. Pop, J. L. Ramirez, J. Rinta, L. Robertson, S. L. Salzberg, D. O. Sanchez, A. Seyler, R. Sharma, J. Shetty, A. J. Simpson, E. Sisk, M. T. Tammi, R. Tarleton, S. Teixeira, S. Van Aken, C. Vogt, P. N. Ward, B. Wickstead, J. Wortman, O. White, C. M. Fraser, K. D. Stuart, and B. Andersson. 2005. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science 309:409-415. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki, A., and R. Medzhitov. 2010. Regulation of adaptive immunity by the innate immune system. Science 327:291-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katze, M. G., Y. He, and M. Gale, Jr. 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2:675-687. [DOI] [PubMed] [Google Scholar]
- 8.Kolumam, G. A., S. Thomas, L. J. Thompson, J. Sprent, and K. Murali-Krishna. 2005. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202:637-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Bon, A., N. Etchart, C. Rossmann, M. Ashton, S. Hou, D. Gewert, P. Borrow, and D. F. Tough. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4:1009-1015. [DOI] [PubMed] [Google Scholar]
- 10.Le Bon, A., and D. F. Tough. 2002. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 14:432-436. [DOI] [PubMed] [Google Scholar]
- 11.Luft, T., K. C. Pang, E. Thomas, P. Hertzog, D. N. Hart, J. Trapani, and J. Cebon. 1998. Type I IFNs enhance the terminal differentiation of dendritic cells. J. Immunol. 161:1947-1953. [PubMed] [Google Scholar]
- 12.Martin, D. L., D. B. Weatherly, S. A. Laucella, M. A. Cabinian, M. T. Crim, S. Sullivan, M. Heiges, S. H. Craven, C. S. Rosenberg, M. H. Collins, A. Sette, M. Postan, and R. L. Tarleton. 2006. CD8+ T-cell responses to Trypanosoma cruzi are highly focused on strain-variant trans-sialidase epitopes. PLoS Pathog. 2:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Connell, R. M., S. K. Saha, S. A. Vaidya, K. W. Bruhn, G. A. Miranda, B. Zarnegar, A. K. Perry, B. O. Nguyen, T. F. Lane, T. Taniguchi, J. F. Miller, and G. Cheng. 2004. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J. Exp. Med. 200:437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padilla, A. M., J. M. Bustamante, and R. L. Tarleton. 2009. CD8+ T cells in Trypanosoma cruzi infection. Curr. Opin. Immunol. 21:385-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padilla, A. M., L. J. Simpson, and R. L. Tarleton. 2009. Insufficient TLR activation contributes to the slow development of CD8+ T cell responses in Trypanosoma cruzi infection. J. Immunol. 183:1245-1252. [DOI] [PubMed] [Google Scholar]
- 16.Pestka, S., C. D. Krause, and M. R. Walter. 2004. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 202:8-32. [DOI] [PubMed] [Google Scholar]
- 17.Sonnenfeld, G., and F. Kierszenbaum. 1981. Increased serum levels of an interferon-like activity during the acute period of experimental infection with different strains of Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 30:1189-1191. [DOI] [PubMed] [Google Scholar]
- 18.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi, O., and S. Akira. 2009. Innate immunity to virus infection. Immunol. Rev. 227:75-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarleton, R. L. 1990. Depletion of CD8+ T cells increases susceptibility and reverses vaccine-induced immunity in mice infected with Trypanosoma cruzi. J. Immunol. 144:717-724. [PubMed] [Google Scholar]
- 21.Tarleton, R. L., M. J. Grusby, M. Postan, and L. H. Glimcher. 1996. Trypanosoma cruzi infection in MHC-deficient mice: further evidence for the role of both class I- and class II-restricted T cells in immune resistance and disease. Int. Immunol. 8:13-22. [DOI] [PubMed] [Google Scholar]
- 22.Tarleton, R. L., B. H. Koller, A. Latour, and M. Postan. 1992. Susceptibility of beta 2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature 356:338-340. [DOI] [PubMed] [Google Scholar]
- 23.Thompson, L. J., G. A. Kolumam, S. Thomas, and K. Murali-Krishna. 2006. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J. Immunol. 177:1746-1754. [DOI] [PubMed] [Google Scholar]
- 24.Vaena de Avalos, S., I. J. Blader, M. Fisher, J. C. Boothroyd, and B. A. Burleigh. 2002. Immediate/early response to Trypanosoma cruzi infection involves minimal modulation of host cell transcription. J. Biol. Chem. 277:639-644. [DOI] [PubMed] [Google Scholar]